Screening of deep eutectic solvents (DESs) as green CO2 sorbents: from solubility to viscosity†
Shokat
Sarmad
 *a,
Yujiao
Xie
a,
Jyri-Pekka
Mikkola
bc and
Xiaoyan
Ji
a
*a,
Yujiao
Xie
a,
Jyri-Pekka
Mikkola
bc and
Xiaoyan
Ji
a
aDepartment of Engineering Science and Mathematics, Division of Energy Science, Luleå University of Technology, 971 87 Luleå, Sweden. E-mail: shokat.sarmad@ltu.se
bTechnical Chemistry, Department of Chemistry, Chemical-Biological Centre, Umeå University, SE-90871, Umeå, Sweden
cIndustrial Chemistry & Reaction Engineering, John Gadolin Process Chemistry Centre, Åbo Akademi University, Biskopsgatan 8, Fl-20500, Åbo-Turku, Finland
First published on 29th November 2016
Abstract
Deep eutectic solvents (DESs) as ionic liquid (IL) analogues show great potential for CO2 capture. They exhibit favorable solvent properties and are considered to be economical alternatives to conventional ILs. In this study, we prepare 35 DESs and screen them in terms of their CO2 solubility and viscosity, both crucial factors to be considered when designing efficient CO2 sorbents. The influence of salt and HBD type and structure, as well their molar ratio on the CO2 solubility and viscosity of the DESs is investigated. The viscosity and CO2 solubility of the DESs are compared with those of other DESs and conventional ILs. 15 DESs, which exhibit comparable CO2 absorption capacity to choline chloride–urea DESs, glycerol DESs and fluorinated ILs, are chosen as the promising ones. The viscosities of the selected DESs are below 200 mPa s and are lower than those of choline chloride-based DESs. Since the viscosity of the DESs is relatively high, on a par with those of conventional ILs, the effect of water as a co-solvent is investigated in order to decrease the viscosity. The addition of water to the glycerol-based DESs improves the kinetics of absorption by decreasing the viscosity, thus increasing the CO2 absorption capacity. Dry or aqueous DESs that demonstrate a high sorption capacity and low viscosity are chosen for additional analysis and characterization, and further functionalization will be carried out in the future to improve their sorption capacity.
1. Introduction
The anthropogenic emission of CO2 results in global warming and remains a matter of great concern in our time. Carbon capture and storage (CCS) is one feasible solution to reduce CO2 emissions from fossil fuel combustion, which is the largest human source of CO2. Developing new technologies to inexpensively and efficiently capture CO2 is of great importance.1–4 Current technologies for CO2 capture from power plants are mainly based on aqueous amine solutions. Generally, the amine-based technologies result in high energy penalties in the regeneration of sorbent. Due to the volatile and corrosive nature of amine sorbents, they are considered as potential threats to the environment and humans.5–7Using ionic liquids (ILs) for reversible CO2 capture is proposed as one of the most promising methods, due to the inherent advantages of ILs over traditional aqueous amine solutions. ILs are well known as physical CO2 sorbents with high CO2 solubility, and their CO2-philicity can be tuned by choosing an appropriate anion or cation. Taking the advantages of the tunability of ILs into consideration, task specified ILs can be designed to chemically react with CO2.8,9 For example, task-specific ILs that contain amine groups are capable of reversibly bonding CO2 molecules via the formation of carbamate salts. However, employing ILs for CO2 capture in large scale industrial applications is hindered due to several drawbacks such as high viscosity and complicated and high-cost synthesis and purification processes. Furthermore, there are growing concerns in regard to the toxicity of several ILs.10,11
Deep eutectic solvents (DESs) are a new class of ILs containing asymmetric and large ions with low lattice energy and hence low melting points.12 DESs are typically formed by mixing a hydrogen bond acceptor (HBA) (quaternary ammonium or phosphonium salts) and a hydrogen bond donor (HBD) such as a carboxylic acid, amide, amine, alcohol or metal halide. An appropriate molar ratio is required to form a eutectic mixture with a lower melting point. HBAs and HBDs are able to interact with each other via hydrogen bonds.13,14 The hydrogen bonding results in charge delocalization between the HBA and HBD and consequently, the freezing point of the eutectic mixture is much lower compared to the individual compounds.15 Meanwhile, their physicochemical properties depend on the nature of HBA and HBD, as well as the molar ratio of HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD. These compounds have acquired remarkable attention due to their inherent advantages, such as high thermal and chemical stability, negligible vapour pressure, non-flammability and broad adjustability, making them promising alternatives for common organic solvents in industrial applications. These new solvents have been employed in different areas of chemistry like metal extraction,12 nanotechnology,16 separation processes,17 stabilization of DNA,18 material chemistry,19 organic synthesis20 and so on. DESs can be easily prepared at a low cost and in high purity without further purification. Many inexpensive quaternary salts are available, in addition to a wide choice of cheap, biodegradable and non-toxic HBDs. Therefore, it is possible to form many novel and inexpensive DES systems.15,21 Indeed, DESs are considered to be a green and environmentally benign CO2 capture medium, and they have been extensively explored during the last few decades due to their similarities with ILs.
HBD. These compounds have acquired remarkable attention due to their inherent advantages, such as high thermal and chemical stability, negligible vapour pressure, non-flammability and broad adjustability, making them promising alternatives for common organic solvents in industrial applications. These new solvents have been employed in different areas of chemistry like metal extraction,12 nanotechnology,16 separation processes,17 stabilization of DNA,18 material chemistry,19 organic synthesis20 and so on. DESs can be easily prepared at a low cost and in high purity without further purification. Many inexpensive quaternary salts are available, in addition to a wide choice of cheap, biodegradable and non-toxic HBDs. Therefore, it is possible to form many novel and inexpensive DES systems.15,21 Indeed, DESs are considered to be a green and environmentally benign CO2 capture medium, and they have been extensively explored during the last few decades due to their similarities with ILs.
The first DES was introduced by Abbott et al.22 It was made up of choline chloride (ChCl) and urea in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Both of these components are non-toxic, readily available and biodegradable.22 Recently, the possibility of applying DESs as CO2 separation media has been extensively surveyed. For the first time, Li et al. reported on the solubility of CO2 in eutectic mixtures of ChCl and urea in molar ratios of 1
2. Both of these components are non-toxic, readily available and biodegradable.22 Recently, the possibility of applying DESs as CO2 separation media has been extensively surveyed. For the first time, Li et al. reported on the solubility of CO2 in eutectic mixtures of ChCl and urea in molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1
1.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, as a function of pressure (up to 13 MPa) and temperature (313.15, 323.15 and 333.15 K).23 Their studies revealed that the CO2 solubility in the DESs depends on the molar ratio of salt-to-HBD, pressure and temperature. The CO2 solubility increased with increasing pressure and decreasing temperature. Among the investigated DESs, ChCl–urea (1
2.5, as a function of pressure (up to 13 MPa) and temperature (313.15, 323.15 and 333.15 K).23 Their studies revealed that the CO2 solubility in the DESs depends on the molar ratio of salt-to-HBD, pressure and temperature. The CO2 solubility increased with increasing pressure and decreasing temperature. Among the investigated DESs, ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) exhibited the highest solubility. The CO2 solubilities in the studied DESs were in the same order as those of ammonium-based ILs, but were lower than those of imidazolium-based ILs under similar conditions. The nature of HBDs has a significant influence on the CO2 solubility in DESs. For example, by choosing lactic acid as the HBD, the CO2 absorption capacity is lower than that of urea-based DESs with an identical molar ratio (1
2) exhibited the highest solubility. The CO2 solubilities in the studied DESs were in the same order as those of ammonium-based ILs, but were lower than those of imidazolium-based ILs under similar conditions. The nature of HBDs has a significant influence on the CO2 solubility in DESs. For example, by choosing lactic acid as the HBD, the CO2 absorption capacity is lower than that of urea-based DESs with an identical molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).24 On the contrary, using glycerol or ethylene glycol as the HBD results in the promotion of the CO2 absorption capacity up to the level of that of imidazolium-based ILs.25,26 All these DESs are capable of physically absorbing CO2. Sze and coworkers reported the first task-specific DES system composed of ChCl, glycerol, and a superbase.27 The superbase was responsible for the deprotonation of the OH groups in glycerol and ChCl, resulting in the formation of active alkoxide anions. This species facilitated the reversible capture of CO2 under ambient conditions. However, compared to conventional ILs, research on DESs as CO2 sorbents is still limited, and the CO2 absorption capacities of the available DESs are still relatively low.
2).24 On the contrary, using glycerol or ethylene glycol as the HBD results in the promotion of the CO2 absorption capacity up to the level of that of imidazolium-based ILs.25,26 All these DESs are capable of physically absorbing CO2. Sze and coworkers reported the first task-specific DES system composed of ChCl, glycerol, and a superbase.27 The superbase was responsible for the deprotonation of the OH groups in glycerol and ChCl, resulting in the formation of active alkoxide anions. This species facilitated the reversible capture of CO2 under ambient conditions. However, compared to conventional ILs, research on DESs as CO2 sorbents is still limited, and the CO2 absorption capacities of the available DESs are still relatively low.
The main goal of our study is to develop novel DESs with high CO2 sorption capacity and low viscosity for CO2 separation. To achieve this, 35 DESs are synthesized as the first step, in order to understand how the HBD, HBA and other factors affect the properties of DESs. The synthesized DESs are screened based on their viscosity and CO2 absorption capacity to find appropriate DESs for CO2 separation. Following this study, the performance of the promising DESs will be improved by functionalization or the addition of appropriate co-solvents.
2. Experimental section
2.1. Chemicals
CO2 (mole fraction ≥99.9%) was received from AGA AB (Linde group). N-Benzyl-2-hydroxy-N,N-dimethyl ethanaminium choride (BHDE) and octanoic acid (OCT) were delivered by Sigma-Aldrich. Acetic acid (AC), ethanolamine (EA) and urea were purchased from Shanghai Shenbo Chemical Company, VWR, and Merck, respectively. Lactic acid (LA) and 1,2-propanediol (1,2-PDO) were obtained from Shanghai Lingfeng chemical reagent company. Choline chloride (ChCl), benzyltimethylammonium chloride (BTMA) and glycerol (Gly) were purchased from the Simopharm chemical reagent company. Benzyltriethylammonium chloride (BTEA), guanidinium hydrochloride (Gua), methyltriphenyl phosphonium bromide (MTPP), tetrabutylammonium bromide (TBAB), tetrabutylammonium chloride (TBAC), tetraethylammonium chloride (TEAC), triethylmethylammonim chloride (TEMA), tetramethylammonium chloride (TMAC), tetrapropylammonium chloride (TPAC), ethylene glycol (EG) and levulinic acid (LV) were purchased from the Aladdin company. All chemicals were analytical grade reagents and were used as received.2.2. Preparation of DESs
In the present work, we synthesized 35 novel DESs composed of ammonium or phosphonium salts and different HBDs. The eutectic mixtures were prepared by mixing the appropriate molar ratio of salt and HBD under vigorous stirring at 333–353 K. The resulting clear homogeneous and colourless solution was stirred for an additional 2 hours and then left to cool down to room temperature. At this stage, the DESs were ready to use and no purification step was needed. The reason for this is that during preparation of the DESs, no solvents were employed and the obtained mixture was pure. The melting points of all synthesized DESs are lower than room temperature, i.e. all DESs are in the liquid phase at room temperature. Table 1 shows the abbreviations for the synthesized DESs and lists their compositions.| DES | Salt | HBD | Molar ratio (salt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD) HBD) |
|---|---|---|---|
| BHDE–AC | N-Benzyl-2-hydroxy-N,N-dimethyl ethanaminium chloride | Acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| BHDE–LA | N-Benzyl-2-hydroxy-N,N-dimethyl ethanaminium chloride | Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| BDDE–GLY–H2O | N-Benzyl-2-hydroxy-N,N-dimethyl ethanaminium chloride | Glycerol–H2O | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11 0.11 |
| BTEA–AC | Benzyltriethylammonium chloride | Acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| BTMA–AC | Benzyltrimethylammonium chloride | Acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| BTMA–GLY | Benzyltrimethylammonium chloride | Glycerol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| BTMA–GLY–H2O | Benzyltrimethylammonium chloride | Glycerol–H2O | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05 |
| BTMA–GLY–H2O | Benzyltrimethylammonium chloride | Glycerol–H2O | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11 0.11 |
| ChCl–EA | Choline chloride | Ethanolamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| ChCl–GLY–AC | Choline chloride | Glycerol–acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Gua–EA | Guanidinium hydrochloride | Ethanolamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| MTPP–1,2-PDO | Methyltriphenyl phosphonium bromide | 1,2-Propanediol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| MTPP–AC | Methyltriphenyl phosphonium bromide | Acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| MTPP–EG | Methyltriphenyl phosphonium bromide | Ethylene glycol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| MTPP–GLY | Methyltriphenyl phosphonium bromide | Glycerol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| MTPP–LV | Methyltriphenyl phosphonium bromide | Levulinic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| MTPP–LV–AC | Methyltriphenyl phosphonium bromide | Levulinic acid–acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
| TBAB–AC | Tetrabutylammonium bromide | Acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| TBAB–EA | Tetrabutylammonium bromide | Ethanolamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| TBAB–EA | Tetrabutylammonium bromide | Ethanolamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| TBAC–AC | Tetrabutylammonium chloride | Acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| TEAC–AC | Tetraethylammonium chloride | Acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| TEAC–AC | Tetraethylammonium chloride | Acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| TEAC–OCT | Tetraethylammonium chloride | Octanoic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| TEMA–AC | Triethylmethylammonium chloride | Acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| TEMA–EG | Triethylmethylammonium chloride | Ethylene glycol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| TEMA–GLY | Triethylmethylammonium chloride | Glycerol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| TEMA–GLY–H2O | Triethylmethylammonium chloride | Glycerol–H2O | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05 |
| TEMA–GLY–H2O | Triethylmethylammonium chloride | Glycerol–H2O | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11 0.11 |
| TEMA–LA | Triethylmethylammonium chloride | Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| TEMA–LV | Triethylmethylammonium chloride | Levulinic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| TMAC–AC | Tetramethylammonium chloride | Acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| TPAC–AC | Tetrapropylammonium chloride | Acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| TPAC–EA | Tetrapropylammonium chloride | Ethanolamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| TPAC–EA | Tetrapropylammonium chloride | Ethanolamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
2.3. CO2 solubility measurements
CO2 solubility measurements were performed on a vapor–liquid equilibrium set-up. The employed set-up used in this work is described in previous works.28,29 The apparatus contains a gas reservoir, an equilibrium cell, a magnetic stirrer and two pressure transducers with a precision of 0.075%. During measurements, the equilibrium cell and gas reservoir were placed in a water bath where the temperature was kept constant using a thermostat with an accuracy of ±0.1 K. An adequate amount of the DES sample was loaded into the equilibrium cell. After that, an appropriate amount of gas was introduced into the equilibrium cell and dissolved in the solvent. The pressure of the equilibrium cell was then recorded. When the pressure became constant, this point was indicated as the equilibrium state and the corresponding pressure was measured. Using the pressure changes in the gas reservoir, the equilibrium pressure and the volume of the equilibrium cell, the number of moles of dissolved gas in the liquid phase was determined by assuming that only CO2 existed in the vapour phase. This is a reasonable assumption, as the vapour pressures of DESs are negligible. In this work, the CO2 solubility was measured at pressures of up to 2 MPa at 298.15 K. The overall uncertainty of the CO2 solubility measurement, which includes system errors in the pressure (0.075%), temperature (0.1 K) and volumes of the gas reservoir and the equilibrium cell (±0.5 mL), was estimated to be within ±0.01.2.4. Viscosity measurements
The viscosity of the DESs was measured using a Bohlin CVO 100 rheometer. The lower plate had a diameter of 60 mm. A cone-on-plate geometry was used with a 1° cone angle and a 20 mm cone diameter. The viscosity measurements were carried out in the temperature range of 293.15–333.15 K.3. Results and discussion
3.1. CO2 solubility
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
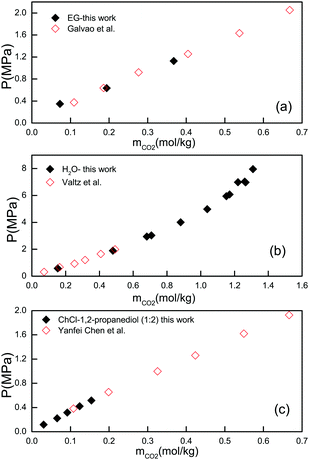
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, at 308.15 K), and in water (at 308.15 K). The measured CO2 solubilities in these three samples were compared with the experimental data reported in the literature. The comparison is presented in Fig. 1. As can be seen, the results obtained in this work agree well with those reported in the literature, confirming the validity of our experimental method as well as the reliability of our reported data.30–32
2, at 308.15 K), and in water (at 308.15 K). The measured CO2 solubilities in these three samples were compared with the experimental data reported in the literature. The comparison is presented in Fig. 1. As can be seen, the results obtained in this work agree well with those reported in the literature, confirming the validity of our experimental method as well as the reliability of our reported data.30–32
| P/MPa | m CO2 | P/MPa | m CO2 |
|---|---|---|---|
BHDE–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
BHDE–LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||
| 0.210 | 0.064 | 0.283 | 0.016 |
| 0.533 | 0.199 | 0.516 | 0.043 |
| 0.857 | 0.313 | 0.866 | 0.122 |
| 1.167 | 0.463 | 1.134 | 0.179 |
| 1.440 | 0.621 | 1.458 | 0.279 |
| 1.771 | 0.775 | 1.722 | 0.391 |
| 2.026 | 0.843 | 2.086 | 0.498 |
BTMA–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
BTMA–GLY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||
| 0.219 | 0.078 | 0.394 | 0.037 |
| 0.530 | 0.271 | 0.672 | 0.056 |
| 0.886 | 0.713 | 0.999 | 0.096 |
| 1.141 | 0.937 | 1.345 | 0.177 |
| 1.563 | 1.108 | 1.711 | 0.227 |
| 1.874 | 1.308 | 2.026 | 0.259 |
| 2.037 | 1.454 | ||
ChCl–EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
ChCl–GLY–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
||
| 0.182 | 0.784 | 0.262 | 0.052 |
| 0.346 | 1.902 | 0.542 | 0.112 |
| 0.651 | 2.700 | 0.833 | 0.177 |
| 1.010 | 2.829 | 1.134 | 0.289 |
| 1.365 | 3.183 | 1.426 | 0.342 |
| 1.741 | 3.511 | 1.746 | 0.385 |
| 2.035 | 3.584 | 2.011 | 0.433 |
MTPP–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
MTPP–EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
||
| 0.173 | 0.073 | 0.192 | 0.045 |
| 0.380 | 0.213 | 0.437 | 0.090 |
| 0.652 | 0.390 | 0.710 | 0.137 |
| 0.938 | 0.710 | 1.134 | 0.192 |
| 1.134 | 1.008 | 1.528 | 0.244 |
| 1.524 | 1.760 | 2.018 | 0.352 |
| 1.843 | 2.257 | ||
| 2.014 | 3.022 | ||
MTPP–LV–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03) 0.03) |
TBAB–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||
| 0.287 | 0.175 | 0.388 | 0.137 |
| 0.516 | 0.327 | 0.715 | 0.380 |
| 0.850 | 0.505 | 1.302 | 0.665 |
| 1.312 | 0.777 | 1.730 | 0.885 |
| 1.633 | 1.070 | 2.011 | 1.130 |
| 2.061 | 1.316 | ||
TBAC–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
TEAC–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||
| 0.348 | 0.184 | 0.281 | 0.144 |
| 0.631 | 0.393 | 0.530 | 0.284 |
| 0.943 | 0.606 | 0.822 | 0.482 |
| 1.319 | 0.869 | 1.304 | 0.683 |
| 1.673 | 1.188 | 1.699 | 0.929 |
| 2.002 | 1.411 | 2.018 | 1.177 |
TEMA–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
TEMA–EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||
| 0.198 | 0.081 | 0.138 | 0.062 |
| 0.413 | 0.192 | 0.314 | 0.199 |
| 0.806 | 0.419 | 0.543 | 0.381 |
| 1.155 | 0.612 | 0.802 | 0.474 |
| 1.410 | 0.810 | 1.041 | 0.575 |
| 1.624 | 0.985 | 1.345 | 0.626 |
| 1.837 | 1.176 | ||
TEMA–GLY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
TEMA–GLY–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05) 0.05) |
||
| 0.150 | 0.017 | 0.226 | 0.009 |
| 0.420 | 0.059 | 0.544 | 0.032 |
| 0.833 | 0.126 | 0.854 | 0.098 |
| 1.238 | 0.264 | 1.253 | 0.329 |
| 1.648 | 0.433 | 1.622 | 0.561 |
| 1.982 | 0.656 | ||
TPAC–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
TPAC–EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
||
| 0.350 | 0.251 | 0.481 | 0.338 |
| 0.554 | 0.481 | 0.784 | 0.636 |
| 0.826 | 0.722 | 1.057 | 0.935 |
| 1.220 | 1.010 | 1.317 | 1.148 |
| 1.652 | 1.373 | 1.700 | 1.294 |
| 2.030 | 1.721 | 2.009 | 1.427 |
BHDE–GLY–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11) 0.11) |
BTEA–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||
| 0.233 | 0.037 | 0.325 | 0.127 |
| 0.542 | 0.048 | 0.551 | 0.265 |
| 0.854 | 0.083 | 0.957 | 0.417 |
| 1.214 | 0.119 | 1.377 | 0.595 |
| 1.636 | 0.144 | 1.664 | 0.785 |
| 2.016 | 0.206 | 2.054 | 0.974 |
BTMA–GLY–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05) 0.05) |
BTMA–GLY–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.011) 0.011) |
||
| 0.208 | 0.044 | 0.255 | 0.016 |
| 0.530 | 0.089 | 0.616 | 0.062 |
| 0.831 | 0.139 | 1.041 | 0.087 |
| 1.121 | 0.177 | 1.442 | 0.140 |
| 1.547 | 0.225 | 1.746 | 0.255 |
| 1.767 | 0.260 | 2.031 | 0.325 |
| 2.018 | 0.285 | ||
Gua–EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
MTPP–1,2-PDO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
||
| 0.226 | 0.306 | 0.220 | 0.022 |
| 0.563 | 0.827 | 0.528 | 0.095 |
| 0.836 | 1.183 | 0.861 | 0.228 |
| 1.129 | 1.357 | 1.120 | 0.296 |
| 1.480 | 1.433 | 1.547 | 0.383 |
| 1.787 | 1.531 | 1.834 | 0.487 |
| 2.025 | 1.663 | 2.026 | 0.549 |
MTPP–GLY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
MTPP–LV (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
||
| 0.161 | 0.009 | 0.301 | 0.024 |
| 0.443 | 0.033 | 0.698 | 0.072 |
| 0.875 | 0.111 | 0.994 | 0.161 |
| 1.225 | 0.203 | 1.209 | 0.272 |
| 1.696 | 0.258 | 1.526 | 0.400 |
| 2.026 | 0.289 | 1.759 | 0.572 |
| 2.068 | 0.688 | ||
TBAB–EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
TBAB–EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
||
| 0.351 | 0.439 | 0.381 | 0.533 |
| 0.654 | 1.036 | 0.637 | 1.208 |
| 0.918 | 2.104 | 0.940 | 2.142 |
| 1.172 | 2.445 | 1.251 | 2.650 |
| 1.645 | 2.665 | 1.627 | 2.888 |
| 2.021 | 2.779 | 2.040 | 3.009 |
TEAC–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
TEAC–OCT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
||
| 0.397 | 0.126 | 0.353 | 0.157 |
| 0.654 | 0.315 | 0.624 | 0.342 |
| 0.957 | 0.506 | 0.940 | 0.600 |
| 1.230 | 0.731 | 1.277 | 0.850 |
| 1.634 | 0.982 | 1.619 | 1.102 |
| 2.016 | 1.230 | 2.018 | 1.390 |
TEMA–LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
TEMA–LV (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||
| 0.143 | 0.047 | 0.136 | 0.057 |
| 0.418 | 0.109 | 0.409 | 0.163 |
| 0.938 | 0.265 | 0.735 | 0.310 |
| 1.265 | 0.369 | 1.043 | 0.439 |
| 1.500 | 0.453 | 1.617 | 0.613 |
| 1.863 | 0.532 | ||
TEMA–GLY–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11) 0.11) |
TMAC–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
||
| 0.135 | 0.025 | 0.294 | 0.120 |
| 0.427 | 0.202 | 0.519 | 0.296 |
| 0.731 | 0.322 | 0.882 | 0.518 |
| 1.015 | 0.383 | 1.297 | 0.810 |
| 1.312 | 0.458 | 1.718 | 1.218 |
| 1.741 | 0.663 | 2.096 | 1.560 |
TPAC–EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
|||
| 0.357 | 1.714 | ||
| 0.645 | 2.051 | ||
| 0.952 | 2.683 | ||
| 1.232 | 3.157 | ||
| 1.673 | 3.441 | ||
| 2.019 | 3.525 | ||
Pressure. For all the studied systems, the solubility increased with increasing pressure, as is typically expected for gas solubility in liquids. Generally, the CO2 solubility in the DESs follows Henry's law, i.e., the CO2 solubility is proportional to its partial pressure.33
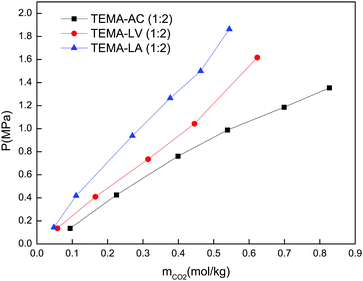
Nature of the HBD. The type of HBD used affected the CO2 solubility of the DESs. As an example, the CO2 solubility in the DESs composed of tetraethylammonium chloride as the HBA, as well as acetic acid, lactic acid or levulinic acid as the HBD, is depicted in Fig. 2. As can be seen, the studied systems follow the trend: TEMA–LA < TEMA–LV < TEMA–AC. These differences are attributed to the interactions between the groups in the HBDs and CO2.
Based on the molecular structure of the HBDs in lactic acid, due to the vicinity of the hydroxyl group to the carboxylic group, the intermolecular hydrogen bonds are stronger compared to those of levulinic acid or acetic acid, and thus it is difficult to break intermolecular hydrogen bonds to interact with CO2 molecules. Consequently, the CO2 solubility in the lactic acid-based DES is the lowest. In the case of acetic acid, the intermolecular hydrogen bonds are the weakest.
Consequently, acetic acid molecules can interact with CO2 easily, and thus the CO2 solubility in the acetic acid-based DES is higher than that of the other DESs.
Alkyl chain length in the HBA or HBD. The comparison of CO2 solubility in the acetic acid-based DESs indicates that the CO2 solubility increases with increasing alkyl chain length of the HBA. For example, the CO2 solubility increased from 1.177 to 1.411 mol kg−1 when the alkyl chain length increased from ethyl to butyl (i.e. from tetraethylammonium to tetrabutylammonium).
Similarly, the increase in the number of carbon atoms in the alkyl chain of HBD also leads to an increase of CO2 solubility. For example, by increasing the alkyl chain length from acetic acid to octanoic acid in TEAC–AC and TEAC–OCT, the CO2 solubility increased from 1.230 to 1.390 mol kg−1. It is plausible that the length of the alkyl chain had a significant effect on the free volume within the DESs.34,35 By increasing the alkyl chain length, the molar volume and the free volume consequently increase, and the increased free volume results in higher CO2 solubility.36,37 Therefore, we can conclude that the underlying reason for the observed trend is related to the free volume mechanism, as the DESs with a longer alkyl chain exhibit a lower density and a larger free volume.35
Nature of the salt. The CO2 solubilities in the DESs that were made up of three different salts and ethanolamine as the HBD in a molar ratio of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 were compared. The CO2 solubilities in TPAC–EA, TBAB–EA and ChCl–EA under identical conditions were 3.525, 3.009 and 3.584 mol kg−1, respectively, and they followed the trend: TBAB–EA < TPAC–EA < ChCl–EA.
7 were compared. The CO2 solubilities in TPAC–EA, TBAB–EA and ChCl–EA under identical conditions were 3.525, 3.009 and 3.584 mol kg−1, respectively, and they followed the trend: TBAB–EA < TPAC–EA < ChCl–EA.
The symmetry of the salt. The CO2 solubility in the DES composed of tetraethylammonium chloride–acetic acid (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 298.15 K and 2 MPa was 1.235 mol kg−1. By introducing one benzyl group instead of one ethyl group, the CO2 solubility was changed to 0.974 mol kg−1 in benzyltriethylammonium chloride–acetic acid (1
2) at 298.15 K and 2 MPa was 1.235 mol kg−1. By introducing one benzyl group instead of one ethyl group, the CO2 solubility was changed to 0.974 mol kg−1 in benzyltriethylammonium chloride–acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) due to the change in salt symmetry.
2) due to the change in salt symmetry.
Using water as a co-solvent. The DESs consisting of glycerol as the HBD exhibited high viscosity. Meanwhile, their viscosities increased considerably with an increase in the amount of dissolved CO2. To cope with this problem, we chose H2O as a co-solvent to decrease the viscosity of these DESs in order to improve the kinetics of CO2 absorption. In the studied systems, the addition of 0.11 mol of H2O to the DES of BTMA–GLY (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) resulted in a considerably reduced viscosity from 716.55 to 20.36 mPa s at 298.15 K, and an increased CO2 solubility from 0.259 to 0.325 mol kg−1. The contribution of H2O to the uptake of CO2 was very limited. By further increasing the amount of H2O, the gas solubility decreased at a constant temperature and pressure due to the low solubility of CO2 in H2O. Similar behaviour was reported for (2-hydroxyethyl)-trimethyl-ammonium (S)-2-pyrrolidine-carboxylic acid salt [Cholin][Pro]-polyethylene glycol 200 (PEG200) by Li et al.38 The addition of PEG200 also resulted in a decrease in viscosity of the IL and thus improved the CO2 absorption rate. However, PEG200 exhibited a limited contribution to the CO2 absorption capacity, and the CO2 solubility decreased with increasing the amount of PEG200 due to the low solubility of CO2 in PEG200.38
2) resulted in a considerably reduced viscosity from 716.55 to 20.36 mPa s at 298.15 K, and an increased CO2 solubility from 0.259 to 0.325 mol kg−1. The contribution of H2O to the uptake of CO2 was very limited. By further increasing the amount of H2O, the gas solubility decreased at a constant temperature and pressure due to the low solubility of CO2 in H2O. Similar behaviour was reported for (2-hydroxyethyl)-trimethyl-ammonium (S)-2-pyrrolidine-carboxylic acid salt [Cholin][Pro]-polyethylene glycol 200 (PEG200) by Li et al.38 The addition of PEG200 also resulted in a decrease in viscosity of the IL and thus improved the CO2 absorption rate. However, PEG200 exhibited a limited contribution to the CO2 absorption capacity, and the CO2 solubility decreased with increasing the amount of PEG200 due to the low solubility of CO2 in PEG200.38
Based on the results presented in Table 2, 15 DESs which exhibited CO2 solubilities above 1 mol kg−1 were considered as promising candidates to carry out the comparative study detailed in Section 3.3. These promising DESs were: BTEA–AC, BTMA–AC, ChCl–EA, Gua–EA, MTPP–AC, MTPP–LV–AC, TBAB–AC, TBAB–EA, TBAC–AC, TEAC–AC, TEAC–OCT, TEMA–AC, TMAC–AC, TPAC–AC and TPAC–EA.
3.2. Viscosity
Viscosity describes the resistance of a fluid to flow and in fact, it is a measure of the internal friction of a moving fluid.39 DESs, similar to conventional ILs, have a relatively higher viscosity in comparison to other organic solvents, causing problems in handling, filtering and stirring. Investigating the viscosity of DESs for industrial applications, such as CO2 capture processes, is very important.For some DESs during the CO2 capture process, the viscosity increases with increasing amount of CO2.7,27,40,41 The increase in viscosity results in a reduction in the heat and mass transfer, which in turn results in a decreased CO2 absorption rate and higher energy utilization in the CO2 separation cycle. In order to capture CO2 with a high efficiency, it is essential to obtain a balance between the viscosity and the CO2 absorption capacity. In this work, the viscosities of the synthesized DESs were measured in the temperature range of 293.15–333.15 K. The measured results are listed in Table 3. The viscosity of the DESs is sensitive to temperature, the type of salt and HBD, the salt symmetry, the water content of the DESs and the molar ratio of salt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD.
HBD.
| 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | 323.15 K | 328.15 K | 333.15 K |
|---|---|---|---|---|---|---|---|---|
BHDE–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 334.28 | 238.16 | 166.52 | 122.72 | 94.54 | 75.82 | 59.99 | 57.86 | 41.67 |
BHDE–GLY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
||||||||
| 1434.36 | 962.20 | 619.78 | 414.50 | 297.01 | 213.60 | 160.74 | 148.30 | 93.36 |
BHDE–GLY–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11) 0.11) |
||||||||
| 32.76 | 29.98 | 27.29 | 24.35 | 21.41 | 19.79 | 18.28 | 17.52 | 17.11 |
BHDE–LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 1434.23 | 900.26 | 570.50 | 385.16 | 266.06 | 214.46 | 142.45 | 112.76 | 84.09 |
BTEA–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 341.10 | 244.04 | 173.29 | 133.86 | 112.70 | 96.15 | 84.90 | 80.89 | 74.12 |
BTMA–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 151.30 | 113.01 | 85.38 | 68.62 | 55.25 | 49.83 | 39.11 | 35.04 | 22.39 |
BTMA–GLY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 1017.67 | 716.55 | 453.89 | 319.51 | 229.15 | 172.40 | 133.51 | 103.43 | 83.20 |
BTMA–GLY–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.055) 0.055) |
||||||||
| 70.76 | 55.32 | 47.94 | 40.98 | 37.22 | 32.10 | 29.73 | 28.48 | 26.81 |
BTMA–GLY–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11) 0.11) |
||||||||
| 22.19 | 20.36 | 18.98 | 17.68 | 16.68 | 15.96 | 15.56 | 14.72 | 14.41 |
ChCl–EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
||||||||
| 50.49 | 39.58 | 32.47 | 28.14 | 23.39 | 20.18 | 18.34 | 18.43 | 17.62 |
ChCl–GLY–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
||||||||
| 138.51 | 101.44 | 78.19 | 62.66 | 51.54 | 43.77 | 38.00 | 33.92 | 28.81 |
Gua–EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 104.34 | 78.32 | 58.59 | 45.65 | 36.91 | 30.62 | 25.69 | 23.63 | 19.29 |
MTPP–1,2-PDO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
||||||||
| 163.95 | 119.53 | 86.33 | 67.43 | 52.49 | 43.48 | 36.44 | 30.66 | 21.59 |
MTPP–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
||||||||
| 142.95 | 118.49 | 97.73 | 80.56 | 67.73 | 55.87 | 49.01 | 45.23 | 43.24 |
MTPP–EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
||||||||
| 149.85 | 110.52 | 82.47 | 66.19 | 53.79 | 41.94 | 36.12 | 30.75 | 27.12 |
MTPP–LV (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
||||||||
| 1658.36 | 957.09 | 574.67 | 365.74 | 243.95 | 170.58 | 124.79 | 91.20 | 71.33 |
MTPP–LV–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.035) 0.035) |
||||||||
| 40.12 | 33.95 | 28.75 | 25.18 | 21.58 | 19.50 | 18.03 | 17.01 | 16.16 |
MTPP–GLY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
||||||||
| 2753.21 | 1748.19 | 1036.18 | 632.81 | 436.33 | 280.14 | 210.18 | 178.36 | 113.94 |
TBAB–EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
||||||||
| 70.89 | 55.66 | 43.58 | 36.01 | 30.16 | 26.54 | 24.36 | 21.76 | 18.76 |
TBAB–EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
||||||||
| 65.15 | 51.76 | 42.66 | 33.28 | 28.63 | 24.99 | 22.67 | 21.27 | 19.26 |
TBAB–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 365.26 | 256.81 | 177.91 | 134.48 | 112.46 | 92.16 | 79.55 | 72.67 | 64.07 |
TBAC–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 22.64 | 20.85 | 19.19 | 17.67 | 16.28 | 15.02 | 13.91 | 12.93 | 12.08 |
TEAC–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 31.37 | 27.52 | 23.70 | 21.18 | 19.35 | 16.59 | 14.64 | 14.05 | 13.86 |
TEAC–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
||||||||
| 24.57 | 21.50 | 19.45 | 16.99 | 15.51 | 14.56 | 13.71 | 12.39 | 11.45 |
TEAC–OCT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
||||||||
| 79.27 | 65.86 | 53.37 | 46.41 | 40.65 | 34.89 | 32.47 | 31.31 | 31.07 |
TEMA–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 32.69 | 25.97 | 22.09 | 18.93 | 17.35 | 15.49 | 13.94 | 12.61 | 11.38 |
TEMA–EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 46.78 | 41.69 | 32.89 | 27.05 | 22.59 | 20.04 | 19.31 | 16.25 | 15.40 |
TEMA–GLY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 333.02 | 236.59 | 172.68 | 128.73 | 97.59 | 76.53 | 59.78 | 52.53 | 42.57 |
TEMA–GLY–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.055) 0.055) |
||||||||
| 90.98 | 72.75 | 56.15 | 45.40 | 39.17 | 30.79 | 28.07 | 26.76 | 23.56 |
TEMA–GLY–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11) 0.11) |
||||||||
| 48.63 | 37.24 | 33.62 | 28.08 | 26.98 | 23.94 | 23.31 | 20.55 | 19.31 |
TEMA–LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 170.21 | 117.46 | 87.65 | 69.03 | 54.36 | 43.77 | 37.18 | 32.66 | 30.66 |
TEMA–LV (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||||||||
| 129.47 | 96.45 | 71.49 | 56.09 | 45.15 | 37.32 | 32.23 | 28.65 | 24.38 |
TPAC–AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
||||||||
| 32.97 | 32.66 | 32.39 | 32.12 | 31.95 | 31.60 | 31.37 | 31.07 | 30.89 |
TPAC–EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
||||||||
| 71.63 | 55.13 | 42.58 | 34.85 | 27.21 | 26.96 | 22.05 | 24.85 | 16.99 |
TPAC–EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
||||||||
| 49.32 | 38.72 | 30.91 | 25.41 | 22.87 | 19.82 | 18.12 | 16.79 | 15.71 |
The comparison of the viscosity of the DESs composed of TEMA–GLY and TEMA–EG reveals that the presence of one extra hydroxyl group in glycerol compared to ethylene glycol gives rise to higher viscosity (from 41.69 to 236.59 mPa s at 298.15 K). The increased viscosity is attributed to the establishment of an extensive hydrogen bond network. On the basis of hole theory, the DESs containing bigger HBD molecules give rise to an increased ionic radius due to the formation of a hydrogen bond network between anions and HBDs, resulting in a very small free volume. The extensive hydrogen bond network lowers the mobility of free species within the DESs, and thus results in higher viscosity.42,43
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) HBD).
By increasing the molar ratio of salt
HBD).
By increasing the molar ratio of salt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD, the viscosity of the synthesized DESs decreased. For example, the viscosity of TPAC–EA at 298.15 K decreased from 55.13 to 38.72 mPa s by increasing the molar ratio from 1
HBD, the viscosity of the synthesized DESs decreased. For example, the viscosity of TPAC–EA at 298.15 K decreased from 55.13 to 38.72 mPa s by increasing the molar ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 1
4 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. By increasing the amount of acetic acid in TEAC–AC from 1
7. By increasing the amount of acetic acid in TEAC–AC from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the viscosity decreased from 27.52 to 21.50 mPa s. Similar behaviour was observed for the choline acetate–glycerol system, as well as the tertrabutylammonium bromide-based DESs.44,45
3, the viscosity decreased from 27.52 to 21.50 mPa s. Similar behaviour was observed for the choline acetate–glycerol system, as well as the tertrabutylammonium bromide-based DESs.44,45
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the viscosity was significantly reduced from 957.09 to 33.95 mPa s at 298.15 K. Similarly, by adding glycerol as the second HBD to the DES composed of ChCl–sugar-based polyols, a solution with lower viscosity was obtained. For example, by adding glycerol at 323.15 K, the viscosity of ChCl
3), the viscosity was significantly reduced from 957.09 to 33.95 mPa s at 298.15 K. Similarly, by adding glycerol as the second HBD to the DES composed of ChCl–sugar-based polyols, a solution with lower viscosity was obtained. For example, by adding glycerol at 323.15 K, the viscosity of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose (1
glucose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) decreased from 34
1) decreased from 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 to 930 mPa s in ChCl
400 to 930 mPa s in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose
glucose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1
glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5).46
0.5).46
3.3. Comparison with other DESs
Leron et al. reported the solubility of CO2 in a variety of ChCl-based DESs at moderate pressures in the temperature range of 303.15–343.15 K. The studied DESs were composed of ChCl and urea, ethylene glycol and glycerol with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. These DESs exhibited high CO2 solubility (3.1265–3.6929 mol kg−1).25,26,47 Li and co-workers measured CO2 solubility in ChCl–urea DESs with different molar ratios (1
2. These DESs exhibited high CO2 solubility (3.1265–3.6929 mol kg−1).25,26,47 Li and co-workers measured CO2 solubility in ChCl–urea DESs with different molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1
1.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5) in the temperature range of 313.15–333.15 K and up to 13 MPa. In this study, ChCl–urea (1
2.5) in the temperature range of 313.15–333.15 K and up to 13 MPa. In this study, ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 313.15 K and 12.5 MPa exhibited a CO2 solubility of 5.1646 mol kg−1, which is the highest among all studied DESs up to now.38 In another study, a task-specific DES also exhibited a high CO2 absorption capacity (2.4 mol kg−1) under ambient conditions.27 Other ChCl-based DESs such as ChCl–phenol,48 ChCl–lactic acid, ChCl–levulinic acid,49 ChCl–furfuryl alcohol49 and ChCl–diols,31 have also been studied, but their CO2 absorption capacities are low (below 1 mol kg−1).
2) at 313.15 K and 12.5 MPa exhibited a CO2 solubility of 5.1646 mol kg−1, which is the highest among all studied DESs up to now.38 In another study, a task-specific DES also exhibited a high CO2 absorption capacity (2.4 mol kg−1) under ambient conditions.27 Other ChCl-based DESs such as ChCl–phenol,48 ChCl–lactic acid, ChCl–levulinic acid,49 ChCl–furfuryl alcohol49 and ChCl–diols,31 have also been studied, but their CO2 absorption capacities are low (below 1 mol kg−1).
Similar to ILs, DESs are capable of either physical or chemical absorption of CO2. According to the available studies performed by others and us, the reported DESs generally absorb CO2 physically, and thus they exhibit a relatively low absorption capacity. In the open literature, just one single task-specific DES system composed of ChCh–Gly–superbase has been designed to chemically capture CO2 under ambient conditions.27 Similarly, switchable ILs composed of an amidine coupled with an alcohol or amino alcohol were considered as efficient CO2 sorbents at atmospheric pressure.50 Privalova et al. reported that these switchable ILs were capable of both physical and chemical sorption of CO2.50
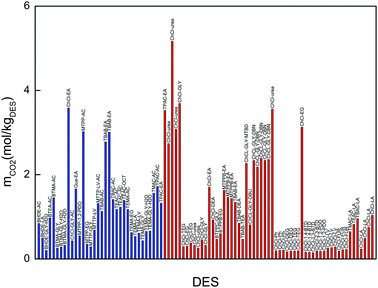
Fig. 3 compares the CO2 solubility of the promising DESs synthesized in this work with those reported in the literature. The CO2 solubility in the DESs synthesized in this work is comparable with that of other choline chloride-based DESs, such as ChCl–glycerol, ChCl–urea or ChCl–glycerol–superbase.25–27,51 Based on this comparison we can conclude that our selected DESs along with the ChCl-based DESs (with urea, glycerol, ethanolamine and task-specific DESs) and MTPP–EA can be considered as promising DESs.
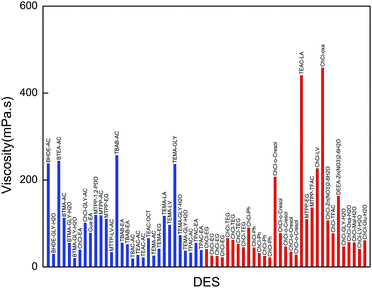
The comparison of the viscosity of our synthesized DESs with those reported in the literature is illustrated in Fig. 4. In order to show a clear and better comparison, only the DESs with a viscosity below 400 mPa s are shown. Our synthesized DESs exhibit lower viscosities (below 200 mPa s at 298.15 K) in comparison to the ChCl-based or other DESs, with the exception of BHDE–LA, BHDE–GLY, BTMA–GLY, MTPP–LV and MTPP–GLY. The viscosities of all the selected promising DESs are below 200 mPa s.
3.4. Screening and comparison with conventional ILs
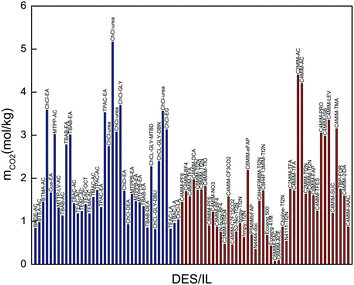 | ||
| Fig. 5 Comparison of CO2 solubilities in promising DESs with those in conventional ILs (the blue columns represent selected DESs and the red ones represent conventional ILs). | ||
It is worth noting that phosphonium-based DESs present the same problems as traditional ILs, such as toxicity and high cost. As shown in Fig. 5, the DESs exhibit comparable CO2 absorption capacity with that of fluorinated ILs. Therefore, these DESs can be considered as benign promising alternatives to expensive and toxic ILs for CO2 separation.
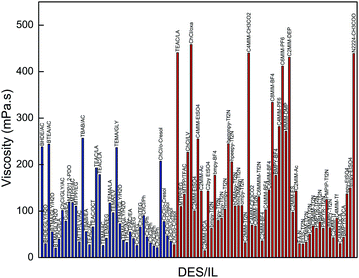 | ||
| Fig. 6 Comparison of the viscosities of promising DESs in this work with those of conventional ILs (the blue columns represent our selected DESs and the red ones represent conventional ILs). | ||
When comparing the results for the CO2 solubility and viscosity, we can conclude that the glycerol or urea-based DESs exhibit higher CO2 affinity, but their viscosity is high and results in high pumping costs as well as poor mass and heat transfer. To overcome their high viscosity, one efficient way is to use an appropriate co-solvent. In this work, water was proposed as a promising co-solvent. However, to find the optimum amount of water to decrease the viscosity of the DESs without obtaining a remarkable reduction in their CO2-philicity, a more detailed investigation is required.
4. Conclusion
In the present study, a series of deep eutectic solvents based on ammonium or phosphonium salts and different hydrogen bond donors were synthesized. Their CO2 solubilities were measured at 298.15 K and at pressures of up to 2 MPa.Their viscosities were also measured in the range of 293.15–333.15 K. The influence of the nature of the hydrogen bond donor and salt, pressure and alkyl chain length of the hydrogen bond donor and salt, were also investigated. Among the synthesized deep eutectic solvents, 15 samples exhibit higher CO2 solubilities and their viscosities are lower than those of conventional ionic liquids. These prototype deep eutectic solvents were then chosen for a more thorough investigation, and in a future study, their structures will be altered in order to improve their CO2 absorption performance. In the future, we plan to functionalize these deep eutectic solvents to reach even higher CO2 sorption capacities, surpassing the performance of the so far reported task specific ILs.
Abbreviations
| [aP4443][Ala] | (3-Ainopropyl)tributylphosphonium L-α-aminopropionic acid |
| [aP4443][Gly] | (3-Aminopropyl)tri-butylphosphonium aminoethanoic acid |
| [aP4443][Ile] | (3-Aminopropyl)tri-butylphosphonium L-α-amino-3-methylvaleric acid salt |
| [aP4443][Leu] | (3-Aminopropyl)tributylphosphonium L-α-amino-4-methylvaleric acid salt |
| [aP4443][Lys] | (3-Aminopropyl)tri-butylphosphonium L-α-Diaminocapronic acid |
| [aP4443][Met] | (3-Aminopropyl)tri-butylphosphonium L-α-amino-(methylthio)butyric acid salt |
| [aP4443][Phe] | (3-Aminopropyl)tri-butylphosphonium L-α-aminohydrocinnamic acid salt |
| [aP4443][Pro] | (3-Aminopropyl)tri-butylphosphonium 2-pyrrolidinecarboxylic acid salt |
| [aP4443][Ser] | (3-Aminopropyl)tri-butylphosphonium L-α-amino-3-hydroxypropionic acid salt |
| [aP4443][Thr] | (3-Aminopropyl)tributylphosphonium L-α-amino-3-hydroxybutyric acid salt |
| [aP4443][Val] | (3-Aminopropyl)tributylphosphonium L-α-aminoisovaleric acid salt |
| [b2-Nic][Tf2N] | 1-Butyl-nicotinic acid butyl ester bis(trifluoromethylsulfonyl)imide |
| [bmpy][BF4] | 1-Butyl-3-methylpyridinium tetrafluoroborate |
| [bmpy][Tf2N] | 1-Butyl-3-methylpyridinium bis(trifluoromethylsulfonyl)imide |
| [BMPYR][Ac] | 1-Butyl-3-methyl pyridinium acetate |
| [BMPYR][DCA] | 1-Butyl-1-methylpyrrolidinium dicyanamide |
| [BMPYR][Tf2N] | 1-Butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide |
| [C2MIM][Ac] | 1-Ethyl-3-methylimidazolium acetate |
| [C2MIM][DMP] | 1-Ethyl-3-methylimidazolium diethyl phosphate |
| [C2MIM][ES] | 1-Ethyl-3-methylimidazolium ethyl sulfate |
| [C2MIM][EtSO4] | 1-Ethyl-3-methylimidazolium ethylsulfate |
| [C2MIM][FAP] | 1-Ethyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate |
| [C2MIM][HS] | 1-Ethyl-3-methylimidazoliumhydrogen sulfate |
| [C2MIM][Tf] | 1-Ethyl-3-methylimidazolium triflate |
| [C2MIM][TFA] | 1-Ethyl-3-methylimidazolium trifluoroacetate |
| [C2MMIM][Tf2N] | 1-Ethyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)imide |
| [C2OMIM][BF4] | 1-Methoxymethyl-3-methylimidazolium tetrafluoroborate |
| [C2OMIM][DCA] | 1-Methoxymethyl-3-methylimidazolium dicyanamide |
| [C2OMIM][PF6] | 1-Methoxymethyl-3-methylimidazolium hexafluorophosphate |
| [C2OMIM][Tf2N] | 1-Methoxymethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [C2OMIM][TFO] | 1-Methoxymethyl-3-methylimidazolium trifluoromethanesulfonate |
| [C2py][EtSO4] | 1-Ethylpyridinium ethylsulfate |
| [C4MIM][Ac] | 1-Butyl-3-methylimidazolium acetate |
| [C4MIM][BETI] | 1-Butyl-3-methylimidazolium bis(perfluoroethylsulfonyl)imide |
| [C4MIM][BF4] | 1-Butyl-3-methylimidazolium tetrafluoroborate |
| [C4MIM][C7F15CO2] | 1-Butyl-3-methylimidazolium pentadecafluorooctanoate |
| [C4MIM][CF3CO2] | 1-Butyl-3-methylimidazolium trifluoroacetate |
| [C4MIM][DCA] | 1-Butyl-3-methylimidazolium dicyanamide |
| [C4MIM][FSI] | 1-Butyl-3-methylimidazolium bis(fluorosulfonyl)imide |
| [C4MIM][IAAC] | 1-Butyl-3-methylimidazolium iminoacetic acid acetate |
| [C4MIM][ISB] | 1-Butyl-3-methylimidazolium isobutyrate |
| [C4MIM][LEV] | 1-Butyl-3-methylimidazolium levulinate |
| [C4MIM][NO3] | 1-Butyl-3-methylimidazoliumnitrate |
| [C4MIM][PF6] | 1-Butyl-3-methylimidazolium hexafluorophosphate |
| [C4MIM][PRO] | 1-Butyl-3-methylimidazolium propionate |
| [C4MIM][SUC] | 1-Butyl-3-methylimidazolium succinamate |
| [C4MIM][Tf] | 1-Butyl-3-methylimidazolium triflate |
| [C4MIM][Tf2N] | 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [C4MIM][TFA] | 1-Butyl-3-methylimidazolium trifluoroacetate |
| [C4MIM][TFES] | 1-Butyl-3-methylimidazolium 1,1,2,2-tetrafluoroethanesulfonate |
| [C4MIM][TMA] | 1-Butyl-3-methylimidazolium trimethylacetate |
| [C4MIM]2[IDA] | bis(1-Butyl-3-methylimidazolium)iminodiacetate |
| [C6H4F9MIM][Tf2N] | 1-Methyl-3-(3,3,4,4,5,5,6,6,6-nonafluorohexyl)imidazoliumbis(trifluoromethylsulfonyl)imide |
| [C6MIM][ACE] | 1-Hexyl-3-methylimidazolium acesulfamate |
| [C6MIM][eFAP] | 1-Hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate |
| [C6MIM][pFAP] | 1-Hexyl-3-methylimidazolium tris(heptafluoropropyl)trifluorophosphate |
| [C6MIM][SAC] | 1-Hexyl-3-methylimidazolium saccharinate |
| [C6MIM][Tf2N] | 1-Hexyl-3-methylimidazolium bis(trifluoromethyl sulfonyl)imide |
| [C6MIM][TFSI] | 1-Hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [C8H4F13MIM][Tf2N] | 1-(3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluorooctyl)-3-methylimidazolium bis[trifluoromethylsulfonyl]amide |
| [C8MIM][BF4] | 1-Octyl-3-methylimidazolium tetrafluoroborate |
| [C8MIM][PF6] | 1-Octyl-3-methylimidazolium hexafluorophosphate |
| [C8MIM][Tf2N] | 1-Octyl-3-methylimidazolium bis(trifluoromethyl sulfonyl)imide |
| [Choline][Tf2N] | (2-Hydroxyethyl)-trimethyl-ammonium bis(trifluoromethylsulfonyl)imide |
| [EA][N] | Ethylammonium nitrate |
| Ecoeng 41M | 1-Butyl-3-methylimidazolium 2-(2-methoxyethoxy)ethylsulfate |
| Ecoeng 500 | PEG-5 cocomonium methylsulfate |
| [empy][EtSO4] | 1-Ethyl-3-methylpyridinium |
| [epy][EtSO4] | 1-Ethylpyridinium |
| [Et2Nic][EtSO4] | 1-Ethyl-nicotinic acid ethyl ester |
| [Et3S][Tf2N] | Triethylsulfonium bis(trifluoromethylsulfonyl)imide |
| [H3D3PHO][Cl] | Trihexyltetradecylphosphonium chloride |
| [hDMApy][Tf2N] | 1-Hexyl-4-(dimethylamino)pyridinium bis(trifluoromethylsulfonyl)imide |
| [hemmpy][Tf2N] | 1-Hexyl-2-ethyl-3,5-dimethylpyridinium bis(trifluoromethylsulfonyl)imide |
| [hmDMApy][Tf2N] | 1-Hexyl-3-methyl-4-(dimethylamino)pyridinium bis(trifluoromethylsulfonyl)imide |
| [hmmpy][Tf2N] | 1-Hexyl-3,5-dimethylpyridinium bis(trifluoromethylsulfonyl)imide |
| [hmpy][Tf2N] | 1-Hexyl-3-methylpyridinium bis(trifluoromethylsulfonyl)imide |
| [hpeepy][Tf2N] | 1-Hexyl-2-propyl-3,5-diethylpyridinium bis(trifluoromethylsulfonyl)imide |
| [hpy][Tf2N] | 1-Hexylpyridinium bis(trifluoromethylsulfonyl)imide |
| [MMIM][DMP] | 1,3-Dimethylimidazolium dimethyl phosphate |
| [N4111][Tf2N] | Tributylmethylammonium bis(trifluoromethanesulfonyl)imide |
| [N4444][doc] | Tetrabutylammonium docusate |
| [N-bupy][BF4] | N-Butylpyridinium tetrafluoroborate |
| [O3MN][Tf2N] | Methyltrioctylammonium bis(trifluoromethylsulfonyl)imide |
| [OMPY][Tf2N] | 1-Octyl-3-methylpyridinium bis(trifluoromethylsulfonyl)imide |
| [PMPIP][Tf2N] | 1-Methyl-1-propylpiperidinium bis(trifluoromethylsulfonyl)imide |
| [PMPYR][Tf2N] | 1-Methyl-1-propylpyrrolidinium bis(trifluoromethylsulfonyl)imide |
| [TBP][FOR] | Tetrabutylphosphonium formate |
Acknowledgements
Kempe Foundations is gratefully acknowledged for funding this research. The Swedish Energy Agency, Bio4Energy program and Wallenberg Wood Science Center under the auspices of Knut and Alice Wallenberg foundation are acknowledged. This work is also a part of the activities of the Johan Gadolin Process Chemistry Centre at Åbo Akademi University.Notes and references
- G. T. Rochelle, Science, 2009, 325, 1652–1654 CrossRef CAS PubMed.
- J. D. Figueroa, T. Fout, S. Plasynski, H. McIlvried and R. D. Srivastava, Int. J. Greenhouse Gas Control, 2008, 2, 9–20 CrossRef CAS.
- M. Wang, A. Lawal, P. Stephenson, J. Sidders and C. Ramshaw, Chem. Eng. Res. Des., 2011, 89, 1609–1624 CrossRef CAS.
- M. K. Mondal, H. K. Balsora and P. Varshney, Energy, 2012, 46, 431–441 CrossRef CAS.
- K. P. Resnik, J. T. Yeh and H. W. Pennline, Int. J. Environ. Technol. Manage., 2004, 4, 89–104 CrossRef CAS.
- R. S. Haszeldine, Science, 2009, 325, 1647–1652 CrossRef CAS PubMed.
- K. E. Gutowski and E. J. Maginn, J. Am. Chem. Soc., 2008, 130, 14690–14704 CrossRef CAS PubMed.
- X. Zhang, X. Zhang, H. Dong, Z. Zhao, S. Zhang and Y. Huang, Energy Environ. Sci., 2012, 5, 6668–6681 CAS.
- J. Huang and T. Rüther, Aust. J. Chem., 2009, 62, 298–308 CrossRef CAS.
- L. Zhang, J. Chen, J. X. Lv, S. F. Wang and Y. Cui, Asian J. Chem., 2013, 25, 23355–23358 Search PubMed.
- C. Wang, X. Luo, X. Zhu, G. Cui, D. Jiang, D. Deng, H. Li and S. Dai, RSC Adv., 2013, 3, 15518–15527 RSC.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, H. L. Munro, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2001, 2010–2011 RSC.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed.
- Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- A. Abo-Hamad, M. Hayyan, M. A. AlSaadi and M. A. Hashim, Chem. Eng. J., 2015, 273, 551–567 CrossRef CAS.
- B. Tang, H. Zhang and K. H. Row, J. Sep. Sci., 2015, 38, 1053–1064 CrossRef CAS PubMed.
- H. Zhao, J. Chem. Technol. Biotechnol., 2015, 90, 19–25 CrossRef CAS.
- F. del Monte, D. Carriazo, M. C. Serrano, M. C. Gutiérrez and M. L. Ferrer, ChemSusChem, 2014, 7, 999–1009 CrossRef CAS PubMed.
- J. García-Álvarez, Eur. J. Inorg. Chem., 2015, 5147–5157 CrossRef.
- D. Carriazo, M. C. Serrano, M. C. Gutierrez, M. L. Ferrer and F. del Monte, Chem. Soc. Rev., 2012, 41, 4996–5014 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- X. Li, M. Hou, B. Han, X. Wang and L. Zou, J. Chem. Eng. Data, 2008, 53, 548–550 CrossRef CAS.
- M. Francisco, A. van den Bruinhorst and M. C. Kroon, Angew. Chem., Int. Ed., 2013, 52, 3074–3085 CrossRef CAS PubMed.
- R. B. Leron, A. Caparanga and M. Li, J. Taiwan Inst. Chem. Eng., 2013, 44, 879–885 CrossRef CAS.
- R. B. Leron and M. Li, Thermochim. Acta, 2013, 551, 14–19 CrossRef CAS.
- L. L. Sze, S. Pandey, S. Ravula, S. Pandey, H. Zhao, G. A. Baker and S. N. Baker, ACS Sustainable Chem. Eng., 2014, 2, 2117–2123 CrossRef CAS.
- Y. Xie, H. Dong, S. Zhang, X. Lu and X. Ji, J. Chem. Eng. Data, 2014, 59, 3344–3352 CrossRef CAS.
- J. Zhang, C. Jia, H. Dong, J. Wang, X. Zhang and S. Zhang, Ind. Eng. Chem. Res., 2013, 52, 5835–5841 CrossRef CAS.
- A. C. Galvão and A. Z. Francesconi, J. Chem. Thermodyn., 2010, 42, 684–688 CrossRef.
- Y. Chen, N. Ai, G. Li, H. Shan, Y. Cui and D. Deng, J. Chem. Eng. Data, 2014, 59, 1247–1253 CrossRef CAS.
- A. Valtz, A. Chapoy, C. Coquelet, P. Paricaud and D. Richon, Fluid Phase Equilib., 2004, 226, 333–344 CrossRef CAS.
- L. Zhou, J. Fan and X. Shang, Materials, 2014, 7, 3867–3880 CrossRef.
- S. N. V. K. Aki, B. R. Mellein, E. M. Saurer and J. F. Brennecke, J. Phys. Chem. B, 2004, 108, 20355–20365 CrossRef CAS.
- L. A. Blanchard, Z. Gu and J. F. Brennecke, J. Phys. Chem. B, 2001, 105, 2437–2444 CrossRef CAS.
- A. Shariati and C. J. Peters, J. Supercrit. Fluids, 2004, 30, 139–144 CrossRef CAS.
- A. Shariati and C. J. Peters, J. Supercrit. Fluids, 2004, 29, 43–48 CrossRef CAS.
- X. Li, M. Hou, Z. Zhang, B. Han, G. Yang, X. Wang and L. Zou, Green Chem., 2008, 10, 879–884 RSC.
- C. Ru and B. Konig, Green Chem., 2012, 14, 2969–2982 RSC.
- B. F. Goodrich, J. C. de la Fuente, B. E. Gurkan, Z. K. Lopez, E. A. Price, Y. Huang and J. F. Brennecke, J. Phys. Chem. B, 2011, 115, 9140–9150 CrossRef CAS PubMed.
- B. F. Goodrich, J. C. de la Fuente, B. E. Gurkan, D. J. Zadigian, E. A. Price, Y. Huang and J. F. Brennecke, Ind. Eng. Chem. Res., 2011, 50, 111–118 CrossRef CAS.
- A. P. Abbott, R. C. Harris and K. S. Ryder, J. Phys. Chem. B, 2007, 111, 4910–4913 CrossRef CAS PubMed.
- A. P. Abbott, ChemPhysChem, 2004, 5, 1242–1246 CrossRef CAS PubMed.
- H. Zhao, G. A. Baker and S. Holmes, Org. Biomol. Chem., 2011, 9, 1908–1916 CAS.
- Y. Rizana, A. Emilia, S. Kamaliah and A. R. Mohd Basyaruddin, Molecules, 2014, 19, 8011–8026 CrossRef PubMed.
- Z. Maugeri and P. Dominguez de Maria, RSC Adv., 2012, 2, 421–425 RSC.
- R. B. Leron, A. N. Soriano and M. Li, J. Taiwan Inst. Chem. Eng., 2012, 43, 551–557 CrossRef CAS.
- G. Li, D. Deng, Y. Chen, H. Shan and N. Ai, J. Chem. Thermodyn., 2014, 75, 58–62 CrossRef CAS.
- M. Lu, G. Han, Y. Jiang, X. Zhang, D. Deng and N. Ai, J. Chem. Thermodyn., 2015, 88, 72–77 CrossRef CAS.
- E. Privalova, M. Nurmi, M. S. Marañón, E. V. Murzina, P. Mäki-Arvela, K. Eränen, D. Y. Murzin and J. P. Mikkola, Sep. Purif. Technol., 2012, 97, 42–50 CrossRef CAS.
- R. B. Leron and M. Li, J. Chem. Thermodyn., 2013, 57, 131–136 CrossRef CAS.
- A. M. Popescu, C. Donath and V. Constantin, Bulg. Chem. Commun., 2014, 46, 452–457 Search PubMed.
- B. Jibril, F. Mjalli, J. Naser and Z. Gano, J. Mol. Liq., 2014, 199, 462–469 CrossRef CAS.
- F. S. Mjalli, J. Naser, B. Jibril, S. S. Al-Hatmi and Z. S. Gano, Thermochim. Acta, 2014, 575, 135–143 CrossRef CAS.
- L. Bahadori, M. H. Chakrabarti, F. S. Mjalli, I. M. AlNashef, N. S. A. Manan and M. A. Hashim, Electrochim. Acta, 2013, 113, 205–211 CrossRef CAS.
- A. Yokozeki, M. B. Shiflett, C. P. Junk, L. M. Grieco and T. Foo, J. Phys. Chem. B, 2008, 112, 16654–16663 CrossRef CAS PubMed.
- M. J. Muldoon, S. N. V. K. Aki, J. L. Anderson, J. K. Dixon and J. F. Brennecke, J. Phys. Chem. B, 2007, 111, 9001–9009 CrossRef CAS PubMed.
- G. Cui, J. Wang and S. Zhang, Chem. Soc. Rev., 2016, 45, 4307–4339 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: [Tables S1–S4]. See DOI: 10.1039/c6nj03140d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |