DOI:
10.1039/C6NJ02569B
(Paper)
New J. Chem., 2017,
41, 97-107
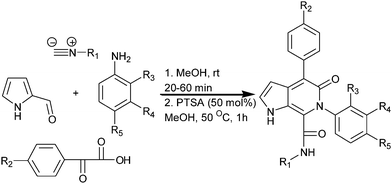
Efficient synthesis of novel pyrrolo[2,3-c]pyridone derivatives using the Ugi four-component reaction followed by condensation reaction†
Received
(in Montpellier, France)
18th August 2016
, Accepted 8th November 2016
First published on 9th November 2016
Abstract
An efficient, mild and multicomponent synthesis of novel heterocyclic compounds possessing the pyrrolo[2,3-c]pyridone backbone was achieved using the Ugi four-component reaction and post-condensation of the Ugi adduct. This method offers many advantages, such as an easy handling procedure, atom economy, short reaction time, and a huge molecular library that can be synthesized by changing the substituents on the four independent starting materials. This methodology was also successfully employed for the electron deficient anilines and phenyl glyoxylic acids having CF3 group. All the products were characterized using NMR (1H and 13C) spectroscopy and FAB mass spectrometry. Product 6g was further characterized by X-ray diffraction analysis.
1. Introduction
Synthetic protocols, involving easy handling processes, atom economy, ambient reaction conditions, short reaction times, inexpensive and less hazardous catalysts, have always been attractive. A multicomponent reaction is a chemical reaction in which three or more starting materials react to form the product with atom economy and ease of access to complex molecules in one-pot.1,2 The Ugi reaction is a multicomponent reaction involving the condensation of aldehydes, amines, carboxylic acids and isocyanides to form the corresponding diamide adducts. The Ugi multicomponent reaction has emerged as an important tool in synthetic chemistry because of its vast applications for building diverse organic molecules in one pot.3 Post-condensation transformations of the Ugi product have also emerged as an important tool for chemists due to its numerous applications in the synthesis of biologically important, fused heterocyclic systems.4–10 For example, the synthesis of pharmacologically important 1,4-benzodiazepin-2-one analogues was achieved using the Ugi four-component reaction, followed by deprotection and cyclization reactions. Also, various fused tetrazoles, peptidomimetic 3-carboxamide-1,4-benzodiazepin-5-ones, were reported using the Ugi reaction and post modification of the Ugi adduct.11–13 Pyrrolo[2,3-c]pyridone is a fused heterocyclic system possessing both pyrrole and pyridone.
Molecules possessing the pyrrolo[2,3-c]pyridone backbone have been used to arrest the cell proliferation in breast cancer and p38 kinase inhibitors.14,15 Le et al. have also reported molecules having the pyrrolo[2,3-c]pyridone backbone as BRD4 receptor inhibitors to control breast cancer.15 Inspite of having a broad range of medicinal properties, not much research has been conducted on the development of efficient methods for the synthesis of these molecules. Eycken et al. have reported the synthesis of pyrrolo[2,3-c]pyridinone with carbonyl groups at different positions using the Ugi reaction, followed by gold(I) catalyzed intramolecular hydroarylation using N-methyl-2-pyrrole carbaldehyde and N-methyl-3-pyrrole carbaldehyde.16,17 However, this methodology requires longer reaction time (72 h including two steps) and expensive gold catalysts.
As a part of our ongoing research in the development of efficient methods for the synthesis of valuable heterocyclic systems using multicomponent reactions, herein we report a simple procedure for the synthesis of pyrrolo[2,3-c]pyridones using the Ugi four-component reaction (Ugi 4CR), followed by post cyclization of the Ugi adduct under acidic conditions. Earlier, we reported the synthesis of novel spiro[indol-2,2′-pyrroles] and 3-furyl coumarin derivatives using isocyanide based multicomponent reactions.18,19
2. Results and discussion
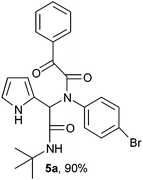
Initially, to examine the feasibility of the Ugi four-component reaction, we conducted a model reaction using 2-formyl pyrrole (1), t-butyl isocyanide (2a), phenyl glyoxylic acid (3a) and 4-bromo aniline (4a) as model substrates. Fortunately, in the initial screening stages, the Ugi four-component reaction in methanol at ambient temperature for 1 h gave the Ugi adduct, which precipitated as a white solid. The Ugi adduct was filtered off and washed with ice-cold methanol (3 mL) and used for cyclization (Scheme 1).
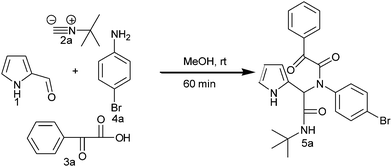 |
| | Scheme 1 Synthesis of the Ugi adduct 5a. | |
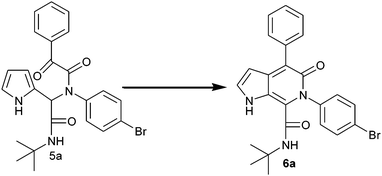
The Ugi adduct was further subjected to condensation under different reaction conditions in order to get the pyrrolo[2,3-c]pyridone analogues. The reaction was conducted in the presence of a base, such as NaOH, Na2CO3, K2CO3 and NaH, in methanol at 50 °C for 24 h (Table 1, entries 1–4); the product was obtained, but in a very low yield. However, switching over to acidic conditions gave rise to the condensation product. In the presence of PTSA as a catalyst in methanol at 50 °C for 1 h, the product yield was 96% (Table 1, entry 5). Increasing the amount of PTSA (50 mol%) did not alter the yield further (Table 1, entry 6). When the reaction time was increased to 5 h, in the presence of PTSA (10 mol%), there was a decrease in the yield (70%, Table 1, entry 7). Ugi adduct condensation also occurred in the presence of AcOH and TBAB with yields of 60% and 10%, respectively (Table 1, entries 8 and 9). From the optimization reaction conditions, 50 mol% PTSA was found to be the best catalyst to carry out the condensation of the Ugi adduct to the pyrrolo[2,3-c]pyridone derivative, with significantly short reaction time and quantitative yield.
Table 1 Optimization of the reaction solvent for condensation of the Ugi adducta
|
|
| Entry |
Catalyst |
Reaction conditions |
Time (h) |
Yieldb (%) |
|
Reaction conditions: all the reactions were performed at 50 °C, and 1 equivalent of catalyst was loaded.
Isolated yields.
Entries 6 and 7, 50 mol% and 10 mol% PTSA catalyst were loaded, respectively. ND: not determined. |
| 1 |
NaOH |
MeOH |
24 |
ND |
| 2 |
K2CO3 |
MeOH |
24 |
ND |
| 3 |
Na2CO3 |
MeOH |
24 |
ND |
| 4 |
NaH |
THF |
24 |
ND |
| 5 |
PTSA |
MeOH |
1 |
96 |
| 6 |
PTSAc (50 mol%) |
MeOH |
1 |
96 |
| 7 |
PTSAc (10 mol%) |
MeOH |
5 |
70 |
| 8 |
CH3COOH |
MeOH |
1 |
60 |
| 9 |
TBAB |
MeOH |
1 |
10 |
To explore the tolerance of these conditions, the reaction was further explored for different Ugi adducts synthesized by varying anilines, phenyl glyoxylic acids and isocyanides. The results are summarized in Table 2. Various aniline derivatives substituted at different positions with iodo, bromo, chloro, methyl, and methoxy groups, were used in the Ugi four-component reaction. Furthermore, phenyl glyoxylic acids substituted with methoxy, ethoxy, methyl and chloro groups, were used in combination with t-butyl and cyclohexyl isocyanides.
Table 2 Synthesis of pyrrolo[2,3-c]pyridone analogues
We also attempted the reaction using anilines and phenyl glyoxylic acids having electron-deficient aryl groups, such as –ArNO2 and –ArCF3. When the reaction was performed with aniline having the CF3 group and phenyl glyoxylic acid having CF3 group, the reaction proceeded smoothly to give the corresponding pyrrolo[2,3-c]pyridone derivatives in 91% (entry 25) and 81% (entry 26) yield, respectively. However, aniline substituted with NO2 failed to undergo the Ugi four-component reaction, possibly due to the presence of the NO2 group interfering with the imine formation.
On the other hand, phenyl glyoxylic acids having NO2 groups underwent Ugi condensation, but failed to cyclize. The Ugi adduct remained unchanged even after refluxing the reaction mixture for 14 h. In all the other cases, the Ugi four-component reaction and the post-condensation of the Ugi adduct proceeded smoothly, giving the corresponding Ugi adduct and pyrrolo[2,3-c]pyridone in quantitative yields (91–98%). In the first step (Ugi four-component reaction) of this protocol, all the products precipitated as white colour solids. The second step involving PTSA promoted post condensation of the Ugi adduct, and these products also precipitated on treating the reaction mixture with water. All the products were characterized by NMR (1H and 13C) spectroscopy and FAB mass spectrometry. 6g was further characterized by X-ray diffraction analysis. Good crystals, suitable for X-ray diffraction analysis, were obtained using a slow evaporation method in MeOH. The ORTEP diagram of the molecule 6g is shown in Fig. 1. One water molecule was found in the crystal structure of 6g. For clarity, the water molecule is not shown in the ORTEP diagram.
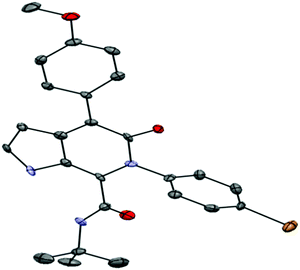 |
| | Fig. 1 ORTEP representation of compound 6g. The thermal ellipsoids were drawn at the 50% probability level. | |
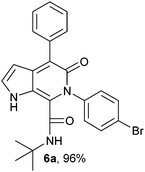
From the experimental results, the possible mechanism for the formation of pyrrolo[2,3-c]pyridone is depicted as follows. To explain the formation of pyrrolo[2,3-c]pyridone, the Ugi adduct of the final compound 6a was taken as the model substrate. The possible pathway for the formation of product 6a is shown in Scheme 2. The Ugi adduct 5a was activated by the addition of PTSA catalyst. Initially the α-keto group of the Ugi adduct was protonated and subsequently electrophilic addition on the pyrrole ring generated the intermediate I. This intermediate I, undergoes dehydration under the same catalytic conditions and yields the final product 6a.
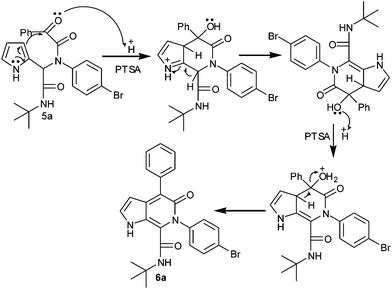 |
| | Scheme 2 Possible reaction mechanism for the synthesis of pyrrolo[2,3-c]pyridone. | |
3. Conclusion
In conclusion, we have achieved a mild, efficient and multicomponent synthesis of novel pyrrolo[2,3-c]pyridone molecules. This method offers several advantages, such as easy handling procedure, atom economy, short reaction time, and a huge molecular library that can be synthesized by changing the substituents on four independent starting materials. In previously reported methods for pyrrolo[2,3-c]pyridones, expensive metal catalysts were used, whereas in the present method, we used inexpensive PTSA as a catalyst. Considering these advantages, we hope this methodology will be useful for medicinal chemists in the drug discovery process.
4. Experimental section
General
All reagents were purchased from TCI and Sigma Aldrich and used without further purification. All the products were characterized by 1H NMR, 13C NMR, IR, and FAB-Mass analysis. The NMR spectra were recorded on a Bruker AMX-500 MHz instrument at room temperature in DMSO-d6, using TMS as an internal reference. Melting points were determined by an AS ONE instrument. X-ray data for compound 6g was collected at room temperature using a Bruker Apex II KY CCD diffractometer with graphite monochromated MoKα radiation (λ = 0.71073 Å) with the ω-scan method. CCDC 1484244 (6g). The APEX2 software was used for the preliminary determination of the unit cell.20 Integrated intensities and unit cell refinement were determined using the SAINT program.21 The structures were solved with direct methods (SHELXS-2014) and refined against F2 (SHELXL-2014).22
Experimental procedure for the synthesis of Ugi adducts (5a–5x).
Pyrrole-2-carbaldehyde (1 mmol), aniline (1 mmol), substituted phenylglyoxylic acid (1.2 mmol) and isocyanide (1 mmol) were mixed in 3 mL methanol. This mixture was stirred at room temperature for 20–60 min. During the stirring, the formation of a white precipitate was observed. The precipitated Ugi adducts were filtered off and washed with ice cold methanol (2 mL) to get pure products. No precipitate formation was observed for Ugi adducts 5c, 5d, 5m and 5q. These Ugi adducts were purified by column chromatography using 20% EtOAc/hexane. In other cases, the volatiles were removed under reduced pressure. All the products were isolated in good yields, ranging from 80% to 93%.
Experimental procedure for the PTSA promoted synthesis of pyrrolo[2,3-c]pyridone derivatives (6a–6x).
The Ugi adduct (1 mmol, 5a–5x) was taken in 5 mL methanol. To this, PTSA (50 mol%) was added and heated at 50 °C for 1 h. CH3OH was removed under reduced pressure and water was added to the reaction mixture. The yellow solid precipitate was filtered and thoroughly washed with water to completely remove the PTSA catalyst. The yellow solid was purified by column chromatography using 5% DCM/MeOH. The yields were almost quantitative ranging from 91% to 98%.
2-(2-{1-[N-(4-Bromophenyl)-2-oxo-2-phenylacetamido]-2-tert-butylamino-2-oxoethyl}-1H-pyrrol) (5a).
White solid, yield 90%; δH (500 MHz CDCl3) 1.38 (9H, s), 5.51 (1H, s), 5.79 (1H, s), 6.14 (1H, m), 6.20 (1H, m), 6.76 (1H, m), 7.04 (2H, m), 7.24 (2H, m), 7.48 (2H, t, J = 6.4 Hz), 7.59 (1H, t, J = 5.9 Hz), 7.96 (2H, m), 9.44 (1H, s); δC (125 MHz, CDCl3) 28.61, 51.91, 61.87, 108.21, 111.50, 120.13, 123.02, 123.61, 128.93, 129.79, 130.96, 132.27, 133.14, 134.67, 138.04, 167.06, 167.93, 189.84; HRMS-FAB (m/z) calcd for C24H24BrN3O3 ([M + H]+) 481.3698, found 481.3690.
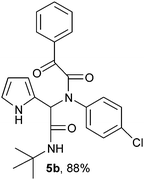
2-(2-{1-[N-(4-Chlorophenyl)-2-oxo-2-phenylacetamido]-2-tert-butylamino-2-oxoethyl}-1H-pyrrol) (5b).
White solid, yield 88%; δH (500 MHz CDCl3) 1.39 (9H, s), 5.54 (1H, s), 5.82 (1H, s), 6.13 (1H, m), 6.19 (1H, m), 6.75 (1H, m), 7.09 (4H, m), 7.48 (2H, t, J = 6.2 Hz), 7.58 (1H, m), 7.96 (2H, m), 9.45 (1H, s); δC (125 MHz, DMSO-d6) 28.61, 51.91, 61.90, 108.30, 111.49, 120.12, 123.63, 128.93, 129.28, 129.77, 130.65, 133.15, 134.65, 134.84, 137.52, 167.09, 168.00, 189.86; HRMS-FAB (m/z) calcd for C24H24ClN3O3 ([M + H]+) 436.9185, found 436.9193.
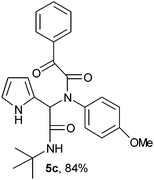
2-(2-{1-[N-(4-Methoxyphenyl)-2-oxo-2-phenylacetamido]-2-tert-butylamino-2-oxoethyl}-1H-pyrrol) (5c).
White solid, yield 84%; δH (500 MHz CDCl3) 1.39 (9H, s), 3.67 (3H, s), 5.57 (1H, s), 5.89 (1H, s), 6.12–6.14 (2H, m), 6.59 (2H, m), 6.73 (1H, m), 7.03 (2H, m), 7.45 (2H, m), 7.56 (1H, m), 7.96 (2H, m), 9.46 (1H, s); δC (125 MHz, CDCl3) 28.62, 51.79, 55.28, 61.65, 108.07, 111.21, 114.10, 119.96, 124.03, 128.79, 129.69, 130.61, 131.24, 133.32, 134.38, 159.51, 167.34, 168.29, 190.26; HRMS-FAB (m/z) calcd for C25H27N3O4([M + H]+) 432.4997, found 432.4988.
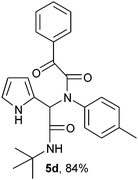
2-(2-{1-[N-(4-Methylphenyl)-2-oxo-2-phenylacetamido]-2-tert-butylamino-2-oxoethyl}-1H-pyrrol) (5d).
White solid, yield 84%; δH (500 MHz CDCl3) 1.38 (9H, s), 2.20 (3H, s), 5.50 (1H, s), 5.82 (1H, s), 6.13–6.20 (2H, m), 6.75 (1H, m), 6.91 (2H, m), 7.01 (2H, m), 7.43–7.47 (2H, m), 7.55–7.58 (1H, m), 7.98 (2H, m), 9.51 (1H, s); δC (125 MHz, CDCl3) 21.95, 28.60, 51.88, 61.75, 108.27, 111.44, 120.08, 122.95, 123.68, 129.67, 129.91, 130.76, 130.92, 132.25, 145.91, 167.13, 168.11, 189.46; HRMS-FAB (m/z) calcd for C25H27N3O3 ([M + H]+) 416.5003, found 416.5013.
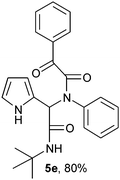
2-(2-{1-[N-Phenyl-2-oxo-2-phenylacetamido]-2-tert-butylamino-2-oxoethyl}-1H-pyrrol) (5e).
White solid, yield 80%; δH (500 MHz CDCl3) 1.39 (9H, s), 5.54 (1H, s), 5.83 (1H, s), 6.13–6.20 (2H, m), 6.74 (1H, m), 6.74–7.16 (5H, m), 7.46 (2H, m), 7.56 (1H, m), 7.98 (2H, m), 9.51 (1H, s); δC (125 MHz, CDCl3) 28.62, 51.82, 62.27, 119.97, 124.13, 128.74, 128.80, 129.09, 129.12, 129.76, 133.32, 134.41, 139.16, 167.18, 168.18, 190.05; HRMS-FAB (m/z) calcd for C24H25N3O3 ([M + H]+) 402.4737, found 402.4744.
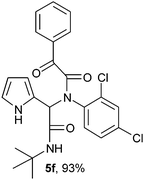
2-(2-{1-[N-(2,4-Dichlorophenyl)-2-oxo-2-phenylacetamido]-2-tert-butylamino-2-oxoethyl}-1H-pyrrol) (5f).
White solid, yield 93%; δH (500 MHz CDCl3) 1.37 (9H, s), 5.54 (1H, s), 5.97 (1H, m), 6.06 (1H, s), 6.68 (2H, m), 7.00 (2H, m), 7.11 (2H, m), 7.46 (2H, m), 7.57 (1H, m), 7.97 (2H, m); δC (125 MHz, CDCl3) 28.63, 52.03, 65.02, 115.83, 122.86, 124.81, 128.87, 129.88, 131.73, 131.82, 132.87, 133.13, 134.60, 136.05, 156.54, 167.70, 168.14, 190.41; HRMS-FAB(m/z) calcd for C25H23Cl2N3O3 ([M + H]+) 471.3632, found 471.3625.
2-(2-{1-[N-(4-Bromophenyl)-2-oxo-2-(4-methoxyphenylacetamido)]-2-tert-butylamino-2-oxoethyl}-1H-pyrrol) (5g).
White solid, yield 98%; δH (500 MHz CDCl3) 1.38 (9H, s), 3.87 (3H, s), 5.48 (1H, s), 5.78 (1H, s), 6.75 (1H, m), 6.94 (2H, m), 7.06 (2H, m), 7.26 (2H, m), 7.96 (2H, d, J = 8.05 Hz), 9.48 (1H, s); δC (125 MHz, CDCl3) 28.61, 51.87, 55.60, 62.17, 108.26, 111.45, 114.29, 120.12, 122.87, 123.82, 126.29, 130.84, 132.24, 132.32, 138.43, 164.76, 167.15, 168.36, 188.46; HRMS-FAB(m/z) calcd for C25H26BrN3O4 ([M + H]+) 511.3858, found 511.3866.
2-(2-{1-[N-(4-Bromophenyl)-2-oxo-2-(4-methlphenylacetamido)]-2-tert-butylamino-2-oxoethyl}-1H-pyrrol) (5h).
White solid, yield 90%; δH (500 MHz CDCl3) 1.38 (9H, s), 2.10 (3H, s), 5.53 (1H, s), 5.82 (1H, s), 6.13 (1H, m), 6.19 (1H, m), 6.74 (1H, m), 7.03 (2H, m), 7.26 (4H, m), 7.86 (2H, d, J = 6.2 Hz), 9.46 (1H, s); δC (125 MHz, CDCl3) 21.95, 25.60, 51.88, 61.76, 108.27, 111.44, 120.08, 122.95, 123.68, 129.67, 129.91, 130.76, 130.92, 132.25, 145.91, 167.13, 168.11, 184.46; HRMS-FAB(m/z) calcd for C25H26BrN3O3 ([M + H]+) 495.3964, found 495.3972.
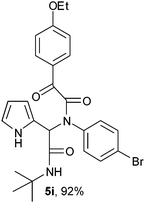
2-(2-{1-[N-(4-Bromophenyl)-2-oxo-2-(4-ethoxyphenylacetamido)]-2-tert-butylamino-2-oxoethyl}-1H-pyrrol) (5i).
White solid, yield 92%; δH (500 MHz CDCl3) 1.38 (9H, s), 1.45 (3H, t), 4.07–4.11 (2H, q), 5.53 (1H, s), 5.83 (1H, s), 6.12–6.16 (2H, m), 6.74 (1H, m), 6.92 (2H, m), 7.05 (2H, m), 7.26 (2H, m), 7.94 (2H, m), 9.49 (1H, s); δC (125 MHz, CDCl3) 14.62, 28.60, 51.86, 61.97, 63.95, 108.25, 111.42, 114.70, 120.08, 122.86, 123.77, 126.06, 130.91, 132.20, 132.31, 138.30, 164.23, 167.19, 168.39, 188.47; HRMS-FAB(m/z) calcd for C26H28BrN3O4 ([M + H]+) 525.4223, found 525.4232.
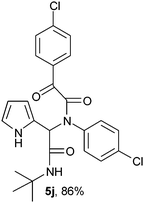
2-(2-{1-[N-(4-Chlorophenyl)-2-oxo-2-(4-chlorophenylacetamido)]-2-tert-butylamino-2-oxoethyl}-1H-pyrrol) (5j).
White solid, yield 86%; δH (500 MHz CDCl3) 1.37 (9H, s), 5.47 (1H, s), 5.74 (1H, s), 6.12–6.17 (2H, m), 6.76 (1H, m), 7.11 (4H, m), 7.45 (2H, m), 7.95 (2H, m), 9.43 (1H, s); δC (125 MHz, CDCl3) 28.59, 51.95, 62.55, 108.37, 111.63, 120.26, 123.53, 129.38, 129.41, 130.53, 131.16, 131.52, 134.97, 137.58, 141.33, 166.90, 167.67, 188.70; HRMS-FAB(m/z) calcd for C24H23Cl2N3O3 ([M + H]+) 471.3632, found 471.3645.
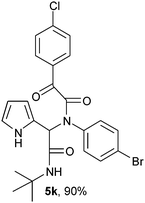
(2-{1-[N-(4-Bomophenyl)-2-oxo-2-(4-chlorophenylacetamido)]-2-tert-butylamino-2-oxoethyl}-1H-pyrrol) (5k).
White solid, yield 90%; δH (500 MHz CDCl3) 1.37 (9H, s), 5.44 (1H, s), 5.71 (1H, s), 6.13–6.17 (2H, m), 6.77 (1H, m), 7.06 (2H, m), 7.28 (2H, m), 7.46 (2H, m), 7.96 (2H, m), 9.42 (1H, s); δC (125 MHz, CDCl3) 28.59, 51.95, 62.64, 108.38, 111.65, 120.29, 123.14, 123.60, 129.39, 130.77, 131.19, 131.52, 132.44, 141.34, 166.87, 167.63, 188.65; HRMS-FAB(m/z) calcd for C24H23BrClN3O3 ([M + H]+) 515.8145, found 515.8156.
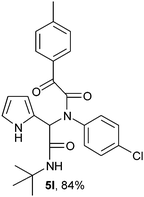
2-{1-[N-(4-Chlorophenyl)-2-oxo-2-(4-methylphenylacetamido)]-2-tert-butylamino-2-oxoethyl}-1H-pyrrol (5l).
White solid, yield 84%; δH (500 MHz CDCl3) 1.38 (9H, s), 2.40 (3H, s), 5.57 (1H, s), 5.86 (1H, s), 6.12–6.18 (2H, m), 6.76 (1H, m), 7.08 (4H, m), 7.25–7.26 (2H, m), 7.86 (2H, m), 9.46 (1H, s); δC (125 MHz, CDCl3) 21.92, 28.60, 51.88, 61.59, 108.27, 111.41, 120.03, 123.65, 129.66, 129.89, 130.69, 130.76, 134.76, 137.49, 145.90, 167.17, 168.16, 189.52; HRMS-FAB(m/z) calcd for C25H26ClN3O3 ([M + H]+) 450.9451, found 450.9442.
2-{1-[N-(4-Chlorophenyl)-2-oxo-2-phenylacetamido]-2-cyclohexylamino-2-oxoethyl}-1H-pyrrol (5m).
White solid, yield 82%; δH (500 MHz CDCl3) 1.11–1.94 (10H, m), 3.87 (1H, m), 5.58 (1H, s), 5.81 (1H, d), 6.12–6.17 (2H, m), 6.77 (1H, m), 7.09–7.14 (4H, m), 7.45–7.48 (2H, m), 7.58 (1H, m), 7.99 (2H, m), 9.51 (1H, s); δC (125 MHz, CDCl3) 24.68, 25.42, 32.74, 48.97, 61.91, 108.31, 111.65, 120.29, 123.52, 128.93, 129.33, 129.83, 130.63, 133.07, 134.68, 134.86, 137.66, 166.82, 168.15, 189.97; HRMS-FAB(m/z) calcd for C26H26ClN3O3 ([M + H]+) 462.9558, found 462.9548.
2-{1-[N-(4-Methylphenyl)-2-oxo-2-phenylacetamido]-2-cyclohexylamino-2-oxoethyl}-1H-pyrrol (5n).
White solid, yield 97%; δH (500 MHz CDCl3) 1.12–1.92 (10H, m), 2.20 (3H, s), 3.89 (1H, m), 5.49 (1H, s), 5.74 (1H, d), 6.13–6.19 (2H, m), 6.78–6.94 (3H, m), 7.05–7.07 (2H, m), 7.44–7.54 (2H, m), 7.54–7.57 (1H, m), 8.02 (2H, m), 9.55 (1H, s); δC (125 MHz, CDCl3) 21.06, 24.68, 25.45, 32.76, 48.85, 62.48, 108.08, 111.45, 120.12, 124.17, 128.76, 128.78, 129.81, 129.86, 133.29, 134.38, 136.78, 138.82, 166.95, 168.41, 190.21; HRMS-FAB(m/z) calcd for C27H29N3O3 ([M + H]+) 442.5376, found 442.5362.
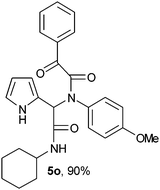
2-{1-[N-(4-Methoxyphenyl)-2-oxo-2-methylphenylacetamido]-2-cyclohexylamino-2-oxoethyl}-1H-pyrrol (5o).
White solid, yield 90%; δH (500 MHz CDCl3) 1.12–1.67 (10H, m), 3.67 (3H, s), 3.87 (1H, m), 5.55 (1H, s), 5.81 (1H, s), 6.19 (2H, m), 6.62 (2H, m), 6.77 (1H, m), 7.08 (2H, m), 7.45 (2H, m), 7.57 (1H, m), 7.98 (2H, m), 9.51 (1H, s); δC (125 MHz, CDCl3) 24.69, 25.45, 32.78, 48.84, 55.29, 62.05, 108.09, 111.40, 114.18, 120.00, 124.00, 128.80, 129.78, 130.52, 133.26, 134.40, 159.52, 167.05, 168.47, 190.36; HRMS-FAB(m/z) calcd for C27H29N3O4 ([M + H]+) 458.5370, found 458.5360.
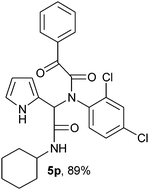
2-{1-[N-(2,4-Chlorophenyl)-2-oxo-2-phenylacetamido]-2-cyclohexylamino-2-oxoethyl}-1H-pyrrol (5p).
White solid, yield 89%; δH (500 MHz CDCl3) 1.14–1.92 (10H, m), 3.86 (1H, m), 5.54 (1H, s), 5.97 (1H, m), 6.06 (1H, d), 6.68–6.70 (2H, m), 7.00–7.02 (1H, m), 7.09–7.11 (2H, m), 7.43–7.46 (2H, m), 7.55–7.58 (1H, m), 7.97 (2H, m), 9.46 (1H, s); δC (125 MHz, CDCl3) 24.67, 25.40, 32.72, 49.04, 65.02, 115.83, 122.86, 124.81, 128.87, 129.88, 131.73, 131.82, 132.87, 133.13, 134.60, 136.05, 156.54, 167.70, 168.14, 190.41; HRMS-FAB(m/z) calcd for C26H25Cl2N3O3 ([M + H]+) 497.4005, found 497.4017.
2-{1-[N-Phenyl-2-oxo-2-phenylacetamido]-2-cyclohexylamino-2-oxoethyl}-1H-pyrrol (5q).
White solid, yield 82%; δH (500 MHz CDCl3) 1.14–1.93 (10H, m), 3.88 (1H, m), 5.53 (1H, s), 5.77 (1H, d), 6.13–6.19 (2H, m), 6.78 (1H, m), 7.13–7.20 (5H, m), 7.43–7.46 (2H, m), 7.54–7.57 (1H, m), 8.00 (2H, m), 9.57 (1H, s); δC (125 MHz, CDCl3) 24.68, 25.44, 32.75, 48.89, 62.47, 108.13, 111.54, 120.19, 124.05, 128.77, 128.81, 129.04, 129.17, 129.83, 133.23, 134.45, 139.44, 166.92, 168.34, 190.16; HRMS-FAB(m/z) calcd for C26H27N3O3 ([M + H]+) 428.5110, found 428.5122.
2-{1-[N-(3-Chlorophenyl)-2-oxo-2-phenylacetamido]-2-cyclohexylamino-2-oxoethyl}-1H-pyrrol (5r).
White solid, yield 84%; δH (500 MHz CDCl3) 1.12–1.92 (10H, m), 3.87 (1H, m), 5.48 (1H, s), 5.73 (1H, d), 6.14–6.21 (2H, m), 6.80 (1H, m), 7.05–7.16 (3H, m), 7.26 (1H, m), 7.46–7.49 (2H, m), 7.57–7.59 (1H, m), 8.02 (2H, m), 9.54 (1H, s); δC (125 MHz, CDCl3) 24.67, 25.42, 32.73, 49.00, 62.66, 108.32, 111.80, 120.40, 123.63, 127.43, 128.93, 129.12, 129.21, 129.85, 130.03, 133.09, 134.68, 140.69, 166.69, 168.21, 189.86; HRMS-FAB(m/z) calcd for C26H26ClN3O3 ([M + H]+) 462.9558, found 462.9568.
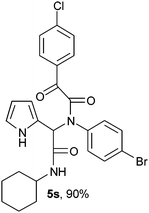
2-{1-[N-(4-Bromophenyl)-2-oxo-2-(4-chlorophenylacetamido)]-2-cyclohexylamino-2-oxoethyl}-1H-pyrrol (5s).
White solid, yield 90%; δH (500 MHz CDCl3) 1.35–1.91 (10H, m), 3.85 (1H, m), 5.48 (1H, s), 5.71 (1H, d), 6.14–6.17 (2H, m), 6.79 (1H, m), 7.07–7.08 (2H, m), 7.26–7.30 (2H, m), 7.44–7.46 (2H, m), 7.98 (2H, m), 9.47 (1H, s); δC (125 MHz, CDCl3) 24.67, 25.40, 32.72, 49.04, 62.53, 108.38, 111.82, 120.46, 123.18, 123.44, 129.41, 130.73, 131.23, 131.44, 132.50, 138.34, 141.37, 167.75, 168.65, 188.74; HRMS-FAB(m/z) calcd for C26H25BrClN3O3 ([M + H]+) 541.8158, found 541.8166.
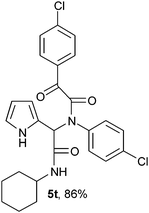
2-{1-[N-(4-Chlorophenyl)-2-oxo-2-(4-chlorophenylacetamido)]-2-cyclohexylamino-2-oxoethyl}-1H-pyrrol (5t).
White solid, yield 86%; δH (500 MHz CDCl3) 1.11–1.91 (10H, m), 3.87 (1H, m), 5.49 (1H, s), 5.73 (1H, d), 6.13–6.17 (2H, m), 6.79 (1H, m), 7.13 (4H, m), 7.46 (2H, m), 7.98 (2H, m), 9.47 (1H, s); δC (125 MHz, CDCl3) 24.67, 25.40, 32.72, 49.03, 62.51, 108.30, 111.80, 120.43, 123.45, 129.40, 129.48, 130.47, 131.22, 131.45, 135.01, 137.74, 141.36, 166.68, 167.81, 188.78; HRMS-FAB(m/z) calcd for C26H25Cl2N3O3 ([M + H]+) 497.4005, found 497.4020.
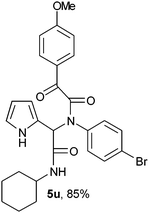
2-{1-[N-(4-Bromophenyl)-2-oxo-2-(4-methoxyphenylacetamido)]-2-cyclohexylamino-2-oxoethyl}-1H-pyrrol (5u).
White solid, yield 85%; δH (500 MHz CDCl3) 1.10–1.92 (10H, m), 3.85 (1H, m), 3.86 (3H, s), 5.51 (1H, s), 5.76 (1H, d), 6.13–6.16 (2H, m), 6.78 (1H, m), 6.93 (2H, m), 7.09 (2H, m), 7.25 (2H, m), 7.99 (2H, m), 9.53 (1H, s); δC (125 MHz, CDCl3) 24.68, 25.42, 32.72, 48.96, 55.58, 62.21, 108.27, 111.62, 114.31, 120.30, 122.90, 123.69, 126.21, 130.80, 132.30, 132.39, 138.58, 164.78, 166.90, 168.52, 188.57; HRMS-FAB(m/z) calcd for C27H28BrN3O4 ([M + H]+) 537.4330, found 537.4342.
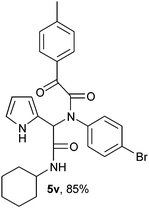
2-{1-[N-(4-Bomophenyl)-2-oxo-2-(4-methylphenylacetamido)]-2-cyclohexylamino-2-oxoethyl}-1H-pyrrol (5v).
White solid, yield 85%; δH (500 MHz CDCl3) 1.16–1.91 (10H, m), 2.40 (3H, s), 3.86 (1H, m), 5.54 (1H, s), 5.78 (1H, d), 6.13–6.18 (2H, m), 6.77 (1H, m), 7.06 (2H, m), 7.25 (4H, m), 7.89 (2H, m), 9.50 (1H, s); δC (125 MHz, CDCl3) 21.96, 24.68, 25.42, 32.72, 48.94, 61.89, 108.28, 111.60, 120.27, 122.98, 123.61, 129.69, 129.97, 130.68, 130.86, 132.32, 138.36, 145.95, 166.84, 168.27, 189.56; HRMS-FAB(m/z) calcd for C27H28BrN3O3 ([M + H]+) 521.4336, found 521.4321.
2-{1-[N-(4-Chlorophenyl)-2-oxo-2-(4-methylphenylacetamido)]-2-cyclohexylamino-2-oxoethyl}-1H-pyrrol (5w).
White solid, yield 86%; δH (500 MHz CDCl3) 1.12–1.93 (10H, m), 2.40 (3H, s), 3.86 (1H, m), 5.57 (1H, s), 5.82 (1H, d), 6.12–6.17 (2H, m), 6.76 (1H, m), 7.13 (4H, m), 7.25 (2H, m), 7.89 (2H, m), 9.51 (1H, s); δC (125 MHz, CDCl3) 21.96, 24.69, 25.43, 32.74, 48.94, 61.75, 108.27, 111.58, 120.23, 123.58, 129.29, 129.68, 129.95, 130.62, 130.68, 134.78, 137.73, 145.93, 166.88, 168.33, 189.62; HRMS-FAB(m/z) calcd for C27H28ClN3O3 ([M + H]+) 476.9823, found 476.9837.
2-{1-[N-(2-Iodophenyl)-2-oxo-2-phenylacetamido]-2-cyclohexylamino-2-oxoethyl}-1H-pyrrol (5x).
White solid, yield 82%; δH (500 MHz CDCl3) 1.11–1.95 (10H, m), 3.86 (1H, m), 5.53 (1H, s), 5.76 (1H, d), 6.13–6.18 (2H, m), 6.78 (1H, m), 7.06–7.08 (2H, m), 7.25–7.27 (2H, m), 7.45–7.48 (2H, m), 7.57–7.60 (1H, m), 7.99 (2H, m), 9.49 (1H, s); δC (125 MHz, CDCl3) 24.68, 25.42, 32.75, 48.97, 62.09, 108.32, 111.69, 120.34, 123.05, 123.56, 128.95, 129.86, 130.86, 132.38, 133.07, 134.70, 138.34, 166.77, 168.11, 184.91; HRMS-FAB(m/z) calcd for C26H26IN3O3([M + H]+) 554.4075, found 554.4087.
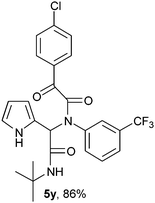
2-{1-[N-(3-Trifluorophenyl)-2-oxo-2-(4-chlorophenylacetamido)]-2-tert-butylamino-2-oxoethyl}-1H-pyrrol (5y).
White solid, yield 86%; δH (500 MHz CDCl3) 1.37 (9H, s), 5.57 (1H, s), 5.78 (1H, s), 6.11–6.17 (2H, m), 6.75 (1H, m), 7.26–7.30 (1H, m), 7.39–7.45 (5H, m), 7.94 (2H, m), 9.43 (1H, s); δC (125 MHz, CDCl3) 28.57, 52.0, 62.47, 108.52, 111.74, 120.29, 123.29, 125.59, 126.3, 129.39, 131.49, 132.73, 139.58, 141.43, 166.80, 167.62, 188.66; HRMS-FAB(m/z) calcd for C25H26ClF3N3O3 ([M + H]+) 504.9164, found 504.9151.
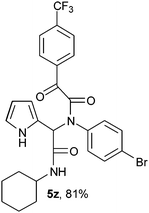
2-{1-[N-(4-Bromophenyl)-2-oxo-2-(4-trifluorophenylacetamido)]-2-cyclohexylamino-2-oxoethyl}-1H-pyrrol (5z).
White solid, yield 81%; δH (500 MHz CDCl3) 1.35–1.91 (10H, m), 3.85 (1H, m), 5.48 (1H, s), 5.71 (1H, d), 6.14–6.17 (2H, m), 6.79 (1H, m), 7.07–7.08 (2H, m), 7.26–7.30 (2H, m), 7.44–7.46 (2H, m), 7.98 (2H, m), 9.47 (1H, s); δC (125 MHz, CDCl3) 24.67, 25.40, 32.72, 49.04, 62.53, 108.38, 111.82, 120.46, 123.18, 123.44, 129.41, 130.73, 131.23, 131.44, 132.50, 138.34, 141.37, 167.75, 168.65, 188.74; HRMS-FAB(m/z) calcd for C27H25BrF3N3O3 ([M + H]+) 575.4050, found 575.4039.
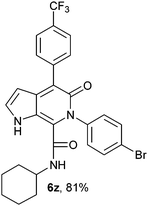
N-(tert-Butyl)-4-phenyl-6-(4-bromophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6a).
Yellow solid, yield 96%, mp 177–181 °C; δH (500 MHz DMSO-d6) 1.08 (9H, s), 6.16 (1H, s), 7.27–7.68 (10H, m), 8.34 (1H, d, J = 8.01 Hz), 10.81 (1H, s); δC (125 MHz, DMSO-d6) 28.24, 51.62, 98.81, 113.53, 121.48, 121.92, 126.84, 128.17, 128.32, 130.09, 131.68, 131.71, 137.29, 139.41, 139.82, 142.0, 157.65, 159.90; HRMS-FAB(m/z) calcd for C24H22BrN3O2 ([M + H]+) 462.0915, found 462.0925.
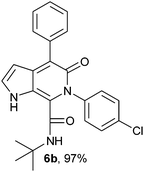
N-(tert-Butyl)-4-phenyl-6-(4-chlorophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6b).
Yellow solid, yield 97%, mp 173–177 °C; δH (500 MHz DMSO-d6) 1.07 (9H, s), 6.16 (1H, s), 7.26–7.65 (10H, m), 8.34 (1H, s), 10.80 (1H, s); δC (125 MHz, DMSO-d6) 28.24, 51.61, 98.79, 121.88, 126.83, 128.16, 128.38, 128.74, 130.08, 131.35, 133.01, 137.31, 138.97, 139.86, 141.99, 157.73, 159.91; HRMS-FAB(m/z) calcd for C24H22ClN3O2 ([M + H]+) 418.1435, found 418.1445.
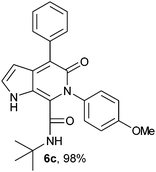
N-(tert-Butyl)-4-phenyl-6-(4-methoxyphenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6c).
Yellow solid, yield 98%, mp 162–164 °C; δH (500 MHz DMSO-d6) 1.07 (9H, s), 3.8 (3H, s), 6.25 (1H, s), 7.02–7.81 (9H, m), 8.35 (1H, d, J = 8.01 Hz), 11.34 (1H, s); δC (125 MHz, DMSO-d6); 28.33, 51.76, 55.93, 99.76, 114.06, 122.60, 125.97, 127.41, 128.54, 130.31, 130.35, 131.97, 136.30, 138.12, 140.33, 141.80, 146.14, 159.38, 159.67; HRMS-FAB(m/z) calcd for C25H25N3O3 ([M + H]+) 414.1924, found 414.1935.
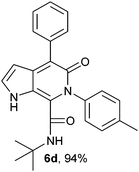
N-(tert-Butyl)-4-phenyl-6-(4-methylphenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6d).
Yellow solid, yield 98%, mp 156–160 °C; δH (500 MHz DMSO-d6) 1.05 (9H, s), 2.38 (3H, s), 6.23 (1H, s), 6.23–7.76 (10H, m), 8.31 (1H, d, J = 8.05 Hz), 11.16 (1H, s); δC (125 MHz, DMSO-d6) 21.25, 28.27, 51.67, 99.43, 113.83, 122.41, 125.97, 127.21, 128.40, 128.53, 129.04, 129.21, 129.72, 130.14, 136.66, 137.0, 138.10, 138.21, 140.08, 141.79, 156.02, 159.55; FAB: HRMS-FAB(m/z) calcd for C25H25N3O2 ([M + H]+) 398.1942, found 398.1950.
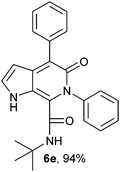
N-(tert-Butyl)-4-phenyl-6-phenyl-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6e).
Yellow solid, yield 97%, mp 150–154 °C; δH (500 MHz DMSO-d6) 1.04 (9H, s), 6.30 (1H, s), 7.27–7.66 (11H, m), 8.26 (1H, d, J = 8.15 Hz), 10.94 (1H, s); δC (125 MHz, DMSO-d6); 28.27, 51.50, 98.69, 113.40, 121.78, 126.72, 128.12, 128.32, 128.67, 129.54, 130.09, 137.53, 139.49, 140.12, 141.80, 157.90, 160.02; FAB: HRMS-FAB(m/z) calcd for C25H25N3O2 ([M + H]+) 384.1838, found 384.1849.
N-(tert-Butyl)-4-phenyl-6-(2,4-dichlorophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6f).
Yellow solid, yield 97%, mp 182–184 °C; δH (500 MHz DMSO-d6) 1.14 (9H, s), 6.20 (1H, s), 7.26–7.79 (9H, m), 8.43 (1H, d, J = 8.10 Hz), 10.80 (1H, s); δC (125 MHz, DMSO-d6); 28.24, 51.82, 98.84, 113.53, 121.96, 126.91, 127.81, 127.94, 128.19, 129.49, 130.03, 132.35, 133.98, 134.64, 137.09, 137.29, 140.21, 142.44, 157.17, 159.46; HRMS-FAB(m/z) calcd for C24H21ClN3O2 ([M + H]+) 353.3480, found 353.3488.
N-(tert-Butyl)-4-(4-methoxyphenyl)-6-(4-bromophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6g).
Yellow solid, yield 98%, mp 165–168 °C; δH (500 MHz DMSO-d6) 1.08 (9H, s), 3.79 (3H, s), 6.16 (1H, s), 6.96–7.68 (9H, m), 8.30 (1H, d, J = 8.01 Hz), 10.72 (1H, s); δC (125 MHz, DMSO-d6) 28.24, 51.58, 55.52, 98.84, 113.62, 121.38, 122.04, 127.55, 129.48, 131.21, 131.68, 139.45, 139.54, 141.60, 157.80, 158.25, 160.04; HRMS-FAB(m/z) calcd for C25H24BrN3O3 ([M + H]+) 493.3805, found 493.3816.
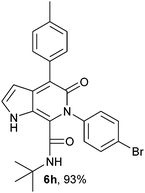
N-(tert-Butyl)-4-(4-methylphenyl)-6-(4-bromophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6h).
Yellow solid, yield 96% mp 190–194 °C; δH (500 MHz DMSO-d6) 1.07 (9H, s), 2.36 (3H, s), 6.26 (1H, s), 7.20–7.67 (9H, m), 8.31 (1H, d, J = 8.01 Hz), 10.74 (1H, s); δC (125 MHz, DMSO-d6) 21.37, 28.21, 51.59, 98.85, 113.68, 121.41, 121.96, 127.90, 128.75, 129.94, 131.68, 134.34, 135.94, 139.50, 139.58, 141.83, 157.76, 159.99; HRMS-FAB(m/z) calcd for C25H24BrN3O3 ([M + H]+) 477.3805, found 477.3811.
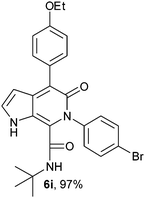
N-(tert-Butyl)-4-(4-ethoxyphenyl)-6-(4-bromophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6i).
Yellow solid, yield 97%, mp 163–167 °C; δH (500 MHz DMSO-d6) 1.07 (9H, s), 1.36 (2H, t, J = 6.95 Hz), 4.08 (2H, q, J = 5.55 Hz), 6.16 (1H, s), 6.94–7.69 (9H, m), 8.30 (1H, d, J = 8.05 Hz), 10.71 (1H, s); δC (125 MHz, DMSO-d6) 15.20, 28.24, 51.57, 98.86, 113.63, 114.08, 121.37, 122.04, 127.51, 129.35, 131.21, 131.67, 139.42, 139.55, 141.57, 157.51, 157.80. 160.04; HRMS-FAB(m/z) calcd for C25H24BrN3O3([M + H]+) 507.4071, found 507.4082.
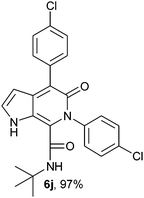
N-(tert-Butyl)-4-(4-chlorophenyl)-6-(4-chlorophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6j).
Yellow solid, yield 97%, mp 239–242 °C; δH (500 MHz DMSO-d6) 1.07 (9H, s), 6.21 (1H, s), 7.39–7.69 (9H, m), 8.39 (1H, s), 10.94 (1H, s); δC (125 MHz, DMSO-d6) 28.24, 51.68, 98.73, 111.89, 121.87, 125.97, 128.23, 128.51, 128.99, 131.32, 131.80, 133.16, 136.11, 138.75, 140.24, 141.92, 157.41, 159.72; HRMS-FAB(m/z) calcd for C24H21ClN3O2([M + H]+) 453.3480, found 453.3492.
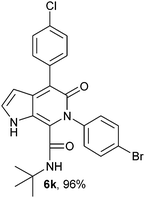
N-(tert-Butyl)-4-(4-chlorophenyl)-6-(4-bromophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6k).
Yellow solid, yield 96%, mp 266–270 °C; δH (500 MHz DMSO-d6) 1.07 (9H, s), 6.20 (1H, s), 7.30–7.69 (9H, m), 8.35 (1H, d, J = 8.05 Hz), 10.90 (1H, s); δC (125 MHz, DMSO-d6) 28.23, 51.66, 98.66, 111.86, 121.58, 121.83, 128.21, 128.85, 131.07, 131.66, 131.74, 136.18, 139.25, 140.20, 141.91, 157.51, 159.76; HRMS-FAB(m/z) calcd for C24H21BrClN3O2([M + H]+) 497.7993, found 497.7999.
N-(tert-Butyl)-4-(4-methylphenyl)-6-(4-chlorophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6l).
Yellow solid, yield 97%, mp 129–132 °C; δH (500 MHz DMSO-d6) 1.07 (9H, s), 2.51 (3H, s), 6.15 (1H, s), 7.20–7.61 (9H, m), 8.31 (1H, d, J = 8.05 Hz), 10.74 (1H, s); δC (125 MHz, DMSO-d6) 21.35, 28.24, 51.59, 98.85, 113.66, 121.94, 128.72, 128.75, 129.94, 131.35, 132.96, 134.34, 135.95, 139.04, 139.59, 140.12, 141.83, 157.81, 159.99; HRMS-FAB(m/z) calcd for C25H24ClN3O2 ([M + H]+) 432.9298, found 432.9291.
N-Cyclohexyl-4-phenyl-6-(4-chlorophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6m).
Yellow solid, yield 96%, mp 190–194 °C; δH (500 MHz DMSO-d6) 0.95–1.57 (10H, m), 3.49 (1H, sex), 6.15 (1H, d, J = 1.81 Hz), 7.26–7.65 (10H, m), 8.65 (1H, d, J = 8.05 Hz), 10.94 (1H, s); δC (125 MHz, DMSO-d6) 24.59, 25.54, 31.80, 48.91, 98.73, 113.47, 121.89, 126.83, 128.03, 128.16, 128.76, 130.08, 131.28, 133.11, 137.30, 138.77, 139.75, 141.88, 157.78, 159.63; HRMS-FAB(m/z) calcd for C26H24ClN3O2 ([M + H]+) 444.9405, found 444.9413.
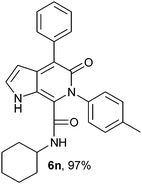
N-Cyclohexyl-4-phenyl-6-(4-methylphenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6n).
Yellow solid, yield 97%, mp 169–173 °C; δH (500 MHz DMSO-d6) 0.94–1.56 (10H, m), 2.35 (3H, s), 3.47 (1H, m), 6.15 (1H, d, J = 1.81 Hz), 7.19–7.65 (9H, m), 8.57 (1H, d, J = 8.05 Hz), 10.86 (1H, s); δC (125 MHz, DMSO-d6) 21.19, 24.61, 25.56, 31.83, 48.22, 98.61, 113.30, 121.80, 126.69, 128.11, 128.53, 129.10, 129.11, 130.09, 137.34, 137.59, 139.33, 141.61, 157.96, 159.75; HRMS-FAB(m/z) calcd for C27H27N3O2 ([M + H]+) 424.5223, found 424.5231.
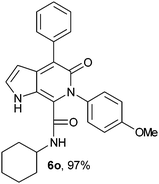
N-Cyclohexyl-4-phenyl-6-(4-methoxyphenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6o).
Yellow solid, yield 97%, mp 150–154 °C; δH (500 MHz DMSO-d6) 0.95–1.59 (10H, m), 3.47 (1H, m), 3.79 (3H, s), 6.15 (1H, d, J = 1.81 Hz), 6.99–7.65 (10H, m), 8.58 (1H, d, J = 8.05 Hz), 10.98 (1H, s); δC (125 MHz, DMSO-d6) 24.61, 25.56, 31.90, 48.22, 55.91, 98.70, 113.24, 113.91, 121.79, 126.74, 128.14, 128.91, 130.09, 130.39, 132.53, 137.49, 139.58, 141.59, 157.85, 159.72; HRMS-FAB(m/z) calcd for C27H27N3O3 ([M + H]+) 440.5217, found 440.5226.
N-Cyclohexyl-4-phenyl-6-(2,4-dichlorophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6p).
Yellow solid, yield 98%, mp 139–142 °C; δH (500 MHz DMSO-d6) 0.95–1.56 (10H, m), 3.52 (1H, m), 6.25 (1H, d, J = 1.81 Hz), 7.31–7.84 (9H, m), 8.40 (1H, d, J = 8.05 Hz), 10.98 (1H, s); δC (125 MHz, DMSO-d6) 24.74, 25.57, 31.82, 48.63, 98.81, 122.09, 126.93, 127.46, 127.92, 128.20, 129.55, 130.02, 132.44, 134.53, 137.04, 137.06, 140.19, 142.33, 157.20, 159.14; HRMS-FAB(m/z) calcd for C26H23Cl2N3O2 ([M + H]+) 479.3854, found 479.3864.
N-Cyclohexyl-4-phenyl-6-phenyl-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6q).
Yellow solid, yield 96%, mp 199–202 °C; δH (500 MHz DMSO-d6) 0.87–1.58 (10H, m), 3.43 (1H, m), 6.15 (1H, d, J = 1.81 Hz), 7.25–7.66 (11H, m), 8.58 (1H, d, J = 8.05 Hz), 10.90 (1H, s); δC (125 MHz, DMSO-d6) 24.27, 25.54, 31.82, 48.27, 98.64, 113.35, 121.84, 126.74, 128.12, 128.38, 128.42, 128.67, 129.44, 130.09, 137.50, 139.45, 139.89, 141.68, 157.89, 159.70; HRMS-FAB(m/z) calcd for C26H25N3O2 ([M + H]+) 410.4957, found 410.4966.
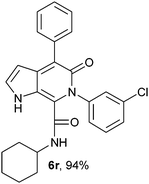
N-Cyclohexyl-4-phenyl-6-(3-chlorophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6r).
Yellow solid, yield 96%, mp 199–203 °C; δH (500 MHz DMSO-d6) 0.95–1.59 (10 H, m), 3.48 (1H, m), 6.16 (1H, d, J = 1.81 Hz), 7.13–7.69 (10H, m), 8.68 (1H, d, J = 8.05 Hz), 10.97 (1H, s); δC (125 MHz, DMSO-d6) 24.59, 25.55, 31.83, 48.32, 98.76, 113.55, 122.02, 126.87, 127.86, 128.16, 128.30, 128.56, 129.60, 130.08, 130.39, 132.94, 137.23, 139.88, 141.13, 141.95, 157.74, 159.57; HRMS-FAB(m/z) calcd for C26H24N3O2([M + H]+) 444.9405, found 444.9419.
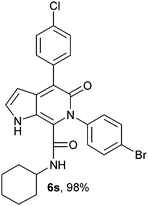
N-Cyclohexyl-4-(4-chlorophenyl)-6-(4-bromophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6s).
Yellow solid, yield 98%, mp 266–270 °C; δH (500 MHz DMSO-d6) 0.95–1.59 (10H, m), 3.49 (1H, m), 6.19 (1H, d, J = 1.81 Hz), 7.28–7.69 (9H, m), 8.68 (1H, d, J = 8.05 Hz), 11.0 (1H, s); δC (125 MHz, DMSO-d6) 24.56, 25.54, 31.78, 48.30, 98.59, 111.82, 121.69, 121.92, 128.19, 128.50, 128.19, 128.50, 131.06, 131.58, 131.76, 131.78, 136.18, 139.06, 140.12, 141.79, 157.58, 159.49;HRMS-FAB(m/z) calcd for C26H23BrClN3O2([M + H]+) 523.8365, found 523.8376.
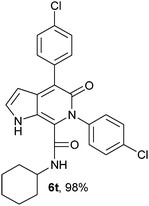
N-Cyclohexyl-4-(4-chlorophenyl)-6-(4-chlorophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6t).
Yellow solid, yield 98%, mp 272–276 °C; δH (500 MHz DMSO-d6) 0.93–1.59 (10H, m), 3.49 (1H, m), 6.19 (1H, d, J = 1.81 Hz), 7.35–7.70 (9H, m), 8.67 (1H, d, J = 8.05 Hz), 11.04 (1H, s); δC (125 MHz, DMSO-d6) 24.57, 25.53, 31.79, 48.32, 98.59, 111.81, 128.19, 128.58, 128.80, 131.06, 131.25, 131.78, 133.21, 136.19, 138.61, 140.12, 141.79, 157.63, 159.49; HRMS-FAB(m/z) calcd for C26H23Cl2N3O2([M + H]+) 479.3852, found 479.3864.
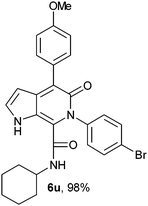
N-Cyclohexyl-4-(4-methoxyphenyl)-6-(4-bromophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6u).
Yellow solid, yield 98%, mp 162–166 °C; δH (500 MHz DMSO-d6) 0.95–1.56 (10H, m), 3.40 (1H, m), 6.15 (1H, d, J = 1.81 Hz), 6.96–7.66 (9H, m), 8.61 (1H, d, J = 8.05 Hz), 10.85 (1H, s); δC (125 MHz, DMSO-d6) 24.60, 25.55, 31.80, 48.30, 98.80, 113.56, 113.62, 121.51, 129.45, 131.21, 131.60, 131.70, 139.33, 139.42, 141.49, 157.81, 158.26, 159.73; HRMS-FAB(m/z) calcd for C27H26BrN3O3 ([M + H]+) 519.4178, found 519.4189.
N-Cclohexyl-4-phenyl-6-(4-bromophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6v).
Yellow solid, yield 97%, mp 180–184 °C; δH (500 MHz DMSO-d6) 0.95–1.59 (10H, m), 2.35 (3H, s), 3.47 (1H, m), 6.23 (1H, d, J = 1.81 Hz), 7.20–7.66 (9H, m), 8.62 (1H, d, J = 8.05 Hz), 10.88 (1H, s); δC (125 MHz, DMSO-d6) 21.35, 24.59, 25.55, 31.80, 48.29, 98.81, 113.67, 121.54, 122.07, 127.55, 128.75, 129.93, 131.60, 131.71, 134.31, 135.97, 139.29, 141.72, 157.77, 159.69; HRMS-FAB(m/z) calcd for C27H26BrN3O2 ([M + H]+) 503.4184, found 503.4193.
N-Cyclohexyl-4-(4-methylphenyl)-6-(4-chlorophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6w).
Yellow solid, yield 98%, mp 166–170 °C; δH (500 MHz DMSO-d6) 0.93–1.59 (10H, m), 2.34 (3H, s), 3.48 (1H, m), 6.14 (1H, d, J = 1.81 Hz), 7.21–7.81 (8H, m), 8.61 (1H, d, J = 8.05 Hz), 10.88 (1H, s); δC (125 MHz, DMSO-d6) 21.35, 24.59, 25.54, 31.80, 48.30, 98.80, 113.65, 122.05, 127.63, 128.74, 129.93, 131.27, 133.06, 134.32, 135.96, 138.83, 139.54, 141.71, 157.82, 159.69; HRMS-FAB(m/z) calcd for C27H26ClN3O2 ([M + H]+) 458.9671, found 458.9681.
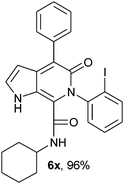
N-(Cyclohexyl)-4-phenyl-6-(2-iodophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6x).
Light yellow solid, yield 95%, mp 162–164 °C; δH (500 MHz DMSO-d6) 0.91–1.80 (10H, m), 3.48 (1H, m), 6.28 (1H, d, J = 1.81 Hz), 7.12–7.95 (11H, m), 8.67 (1H, d, J = 8.01 Hz), 11.34 (1H, s); δC (125 MHz, DMSO-d6); 14.45, 21.26, 22.54, 48.58, 99.45, 114.19, 122.73, 125.97, 127.31, 128.46, 128.57, 128.99, 130.03, 130.62, 136.51, 138.21, 139.34, 140.55, 142.04, 142.59, 145.99, 155.49, 158.58; HRMS-FAB(m/z) calcd for C26H24IN3O2 ([M + H]+) 536.3912, found 536.3924.
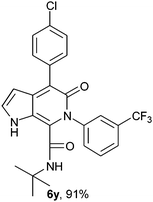
N-(tert-Butyl)-4-(4-chlorophenyl)-6-(3-trifluoromethylphenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6y).
Yellow solid, yield 91%, mp 125–129 °C; δH (500 MHz DMSO-d6) 0.94 (9H, s), 6.14 (1H, d, J = 5 Hz), 7.37–7.74 (9H, m), 8.26 (1H, s), 10.85 (1H, s); δC (125 MHz, DMSO-d6); 28.10, 51.59, 98.70, 111.99, 121.96, 125.44, 126.47, 128.20, 128.62, 130.18, 131.13, 131.81, 133.84, 136.07, 140.41, 140.59, 142.10, 157.67, 159.72; HRMS-FAB(m/z) calcd for C25H21ClF3N3O2 ([M + H]+) 486.9012, found 486.9025.
N-(Cyclohexyl)-4-(4-trifluoromethylphenyl)-6-(4-bromophenyl)-5-oxo-1H-pyrrolo[2,3-c]pyridone-7-carboxamide (6z).
Yellow solid, yield 87%, mp 145–149 °C; δH (500 MHz DMSO-d6) 0.97–1.57 (10H, m), 3.48 (1H, m), 6.25 (1H, d, J = 1.81 Hz), 7.29–7.91 (9H, m), 8.69 (1H, d, J = 8.01 Hz), 11.13 (1H, s); δC (125 MHz, DMSO-d6); 24.55, 25.52, 31.76, 48.32, 98.55, 111.22, 121.78, 121.84, 125.04, 129.29, 130.58, 131.58, 131.81, 138.92, 140.54, 141.80, 142.03, 157.61, 159.38; HRMS-FAB(m/z) calcd for C27H23BrF3N3O2 ([M + H]+) 557.3897, found 557.3888.
Acknowledgements
We are very thankful to the Kyushu Institute of Technology for their kind support and encouragement. We also thank Dr Kenji Yoza (Bruker AXS Japan) for experimental assistance during final stages of the X-ray analysis. We are very thankful to rotary Yoneyama memorial foundation (Japan, Fukuoka) for providing scholarship to JVP.
Notes and references
- B. H. Rotstein, S. Zaretsky, V. Rai and A. K. Yudin, Chem. Rev., 2014, 114, 8323–8359 CrossRef CAS PubMed.
- A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 CrossRef PubMed.
- K. S. Upendra, S. Nandini, D. V. Dipak and V. E. Erik, Chem. Soc. Rev., 2015, 44, 1836–1860 RSC.
- L. D. Miranda and E. Hernández-Vázquez, J. Org. Chem., 2015, 80, 10611–10623 CrossRef CAS PubMed.
- Y.-M. Yan, Y. Rao and M.-W. Ding, J. Org. Chem., 2016, 81, 1263–1268 CrossRef CAS PubMed.
- E. Hernández-Vázquez and L. D. Miranda, Org. Biomol. Chem., 2016, 14, 4875–4884 Search PubMed.
- D. Kröger, M. Franz, M. Schmidtmann and J. Martens, Org. Lett., 2015, 17, 5866–5869 CrossRef PubMed.
- K. Irfan, K. Shahnawaz, V. Tyagi, C. Pradeep Singh and M. S. C. Prem, RSC Adv., 2015, 5, 102713 RSC.
- M. Mahdavi, R. Hassanzadeh-Soureshjan, M. Saeedi, A. Ariafard, R. BabaAhmadi, P. RashidiRanjbar and A. Shafiee, RSC Adv., 2015, 5, 101353 RSC.
- M. Lisa, B. Luca, B. Andrea, B. Alice and R. Renata, Beilstein J. Org. Chem., 2014, 10, 209–212 CrossRef PubMed.
- J. Azuaje, J. M. Pérez-Rubio, V. Yaziji, A. El Maatougui, J. C. González-Gomez, V. M. Sańchez-Pedregal, A. Navarro-Vaźquez, C. F. Masaguer, M. Teijeira and E. Sotelo, J. Org. Chem., 2015, 80, 1533–1549 CrossRef CAS PubMed.
- P. Patil, K. Khoury, E. Herdtweck and A. Dömling, Org. Lett., 2014, 16, 5736–5739 CrossRef CAS PubMed.
- P. Pertejo, N. Corres, T. Torroba and M. García-Valverde, Org. Lett., 2015, 17, 612–615 CrossRef CAS PubMed.
-
K. Kossen, S. D. Seiwert, V. Serebryany, D. Ruhrmund, L. Beigelman, L. F. M. Raveglia, S. Vallese, I. Bianchi and T. Hu, WO patent, WO 2009149188 A1, Dec 10, 2009 Search PubMed.
-
L. Wang, Y. Dai, J. Holms, D. Liu, W. McClellan, K. McDaniel, L. Hasvold, S. D. Fidanze, G. Sheppard and J. Marjanovic, WO patent, WO 2014206150A1, Dec 31, 2014 Search PubMed.
- G. M. Sachin, K. Amit, D. V. Dipak, K. S. Sunil, S. P. Virinder and E. V. Van der Eycken, Chem. Commun., 2012, 48, 10916–10918 RSC.
- L. Zhenghua, K. Amit, K. S. Sunil, S. P. Virinder and E. V. Van der Eycken, Tetrahedron, 2015, 71, 3333–3342 CrossRef.
- V. Jalli, S. Krishnamurthy, H. Kawasaki, T. Moriguchi and A. Tsuge, Synth. Commun., 2015, 45, 2216–2226 CrossRef CAS.
- V. Jalli, S. Krishnamurthy, T. Moriguchi and A. Tsuge, J. Chem. Sci., 2016, 128, 217–226 CrossRef CAS.
- APEX2 Version 2009.9 (Bruker AXS Inc.).
- SAINT Version 7.68A (Bruker AXS Inc., 2009).
- Sheldrick, G. M. SHELX Version 2014/7, http://shelx.uni-ac.gwdg.de/SHELX/index.php.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1484244 (6g). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6nj02569b |
|
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |
Click here to see how this site uses Cookies. View our privacy policy here.  ,
Tetsuji
Moriguchi
and
Akihiko
Tsuge
,
Tetsuji
Moriguchi
and
Akihiko
Tsuge