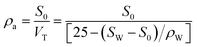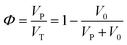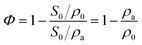One-step modification of PU sponges for selective absorption of oil–water mixtures
Jiahong
Guo
a,
Jikui
Wang
*a,
Sai
Zhang
a,
Xiangyan
Ma
a,
Zhoutong
Qiu
a,
Xing
Peng
a,
Jie
Ying
a,
Yuming
Wang
a and
Genhua
Wu
b
aShanghai Key Laboratory of Advanced Polymeric Materials, Key Laboratory for Ultrafine Materials of Ministry of Education, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, P. R. China. E-mail: wang326@ecust.edu.cn
bCollaborative Innovation Centre for Petrochemical New Materials, Anqing, Anhui 246011, P. R. China
First published on 15th November 2016
Abstract
A porous PDMS–PU sponge with excellent wettability was employed in this investigation for the fast absorption of oils and organic solvents. In this study, a robust superhydrophobic sponge was based on a simple dipping–coating method and HCl corrosion. The as-prepared PDMS–PU sponge possesses the advantages of convenient adsorbent collection and good recyclability. By combining the special wettability and high roughness, the sponges exhibited high absorption capacity and selectivity when used as absorptive materials for separate oil–water mixtures. Thus, these sponges show great potential for the large-scale removal of organic contaminants or oil spills from water.
Introduction
With the expansion of oil production and transportation, massive marine oil spills have occurred frequently which pose a detrimental effect on the environment owing to its toxicity, carcinogenicity and bioaccumulation.1–3 As a result, there is a requirement to develop new materials for the collection and separation of large amounts of oils and organic pollutants from water.4–6 The technologies for removing organic pollutants from real samples include skimmers,7 solidifiers,8 burning,9 bioremediation,10 absorption,11etc. Among the above mentioned methods, absorption has been considered to be an eco-friendly and competitive approach, owing to its flexibility, fast absorption rate, the simplicity of operation, recyclability and synthesis method.Recently, several types of porous materials with excellent surface superhydrophobicity and superoleophilicity have been reported and used for the separation and absorption of organic solvents and oils from water.12–14 Nevertheless, their practical application is still limited by expensive raw materials and complicated synthesis procedures.
PDMS (polydimethylsiloxane) is one of the most widely used polymers in the field of biomedical science and material engineering. The Si–O–Si backbone of the PDMS endows intriguing properties, such as non-toxicity, nonflammability, low bulk density and high flexibility.15 The backbone nature and the intermolecular forces between the side chains attribute to PDMS low surface energy and superhydrophobicity. Porous PDMS materials have also attracted attention due to their low cost fabrication, high porosity, and absorption capacity compared to other polymers, which makes it applicable for use in oil remediation.16–18 Si et al. controlled morphology and improved pore interconnectivity to PDMS sponges for oil sorption by partially fusing the sugar particles (400–1600 μm) together prior to creation of a continuous PDMS matrix.19 The modification of polydopamine/octadecylamine endows the PDMS sponge with a self-cleaning property.20 By introducing the ln(OH)3 nanoparticles, the sponges can be used for ammonia detection and absorption.21
Environmentally-friendly polyurethane (PU) sponge which is a type of commercially available 3D porous material has served as a host material for oil–water separation.22–24 For example, Liu et al. designed rGPU sponges coated with graphene oxide on polyurethane (PU) sponges, and proved them to be very promising adsorbents for the treatment of oil spills and for oil–water separation.25 Wang et al. fabricated a robust superhydrophobic and superoleophilic carbon nanotube/poly(dimethylsiloxane)-coated polyurethane sponge for continuous absorption and expulsion of oils and organic solvents from water surfaces.26 Zhu et al. fabricated sponges which were initially coated with a film of copper via electroless deposition and subsequently modified with superhydrophobic and superoleophilic coatings through a simple solution–immersion step.27
The aim of this work is to develop a porous, robust 3D eco-friendly PDMS–PU sponge by a simple dipping–coating method and HCl corrosion. The combination of these two procedures endow the sponges with good properties, and exhibited high absorption capacity and excellent selectively when they were employed as adsorptive materials for collecting oils and organic solvents from water.
Experimental
Materials
SYLGARD 184 Silicone Elastomer prepolymer (Sylgard 184A, Mw ≈ 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and the thermal curing agent (Sylgard184B, Mw ≈ 15
000 g mol−1) and the thermal curing agent (Sylgard184B, Mw ≈ 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) were purchased from Dow Corning. PU sponge was obtained from Co., Ltd (Nangtong, China). Carbon tetrachloride, n-octane, n-hexane, cyclohexane, ethyl acetate, methyl acrylate, trichloromethane, dichloromethane, tetrahydrofuran, concentrated hydrochloric acid (HCl), kerosene, diesel oil, petroleum ether and ethanol were brought from Lingfeng Chemical Reagent Co., Ltd (Shanghai, China). Oil red O was purchased from Maikun Chemical Reagent Co., Ltd (Shanghai, China).
000 g mol−1) were purchased from Dow Corning. PU sponge was obtained from Co., Ltd (Nangtong, China). Carbon tetrachloride, n-octane, n-hexane, cyclohexane, ethyl acetate, methyl acrylate, trichloromethane, dichloromethane, tetrahydrofuran, concentrated hydrochloric acid (HCl), kerosene, diesel oil, petroleum ether and ethanol were brought from Lingfeng Chemical Reagent Co., Ltd (Shanghai, China). Oil red O was purchased from Maikun Chemical Reagent Co., Ltd (Shanghai, China).
Preparation of PDMS–PU sponge
The sponges were ultrasonically cleaned in ethanol and distilled water, successively. After being dried in an oven, the resulting sponges were immersed in a mixed solution containing carbon tetrachloride, n-octane and PDMS (0.5–10%, w/w) for 30 min. The sponges were taken out from the solution and dried in air for a period at ambient temperature to remove diluents including carbon tetrachloride and n-octane. Then the sponges were dried at 70 °C for 2 h to obtain superhydrophobic samples. The resulting sponges were etched in HCl solution for a period of time to improve the robustness. After being washed with distilled water and immersed in boiling water for 30 minutes, the sponges dried in an oven.Oil absorbency and reusability
A piece of sponge was immersed in oil at room temperature. The sponge was taken out after absorption equilibrium, and then excess oil on the surface of the sponges was allowed to drain for 30 s. The saturated absorbent was then immediately transferred to a pre-weighed bottle and weighed. The oil absorption SR of the sponge was determined at room temperature according to the following equation from the literature:27–30| SR = (m1 − m0)/m0 × 100% |
The consequence of the repeated absorption–desorption of oils on the oil absorbency of the sponges is measured to evaluate its reusability. The sponge was immersed in ethyl acetate until absorption equilibrium, and then weighed to calculate the absorbency. The sample was squeezed and washed with ethanol, and then dried in an oven at 70 °C. The absorption–desorption procedure was repeated 20 times.
Apparent density and porosity of sponge
The apparent density could be determined by the ratio of the mass to the total volume (VT, cm3, including the solid and void volumes) of the porous sponge. Specifically, 3 g dried sponges were placed into a 25 mL volumetric flask of known weight at 20 °C. The flask was then filled with carbon tetrachloride to the mark and weighed. The apparent density of the sponge can be calculated from the following equation:where SW (g) is the total weight of the sponge and carbon tetrachloride, S0 (g) is the weight of the dry porous, ρW is the density of carbon tetrachloride.
The porosity (Φ) of a porous medium describes the fraction of void space in the material. It is defined by the ratio:
Characterization
The morphology of sponges was observed by a scanning electron microscope (SEM S-3400) under an electron beam with an accelerating voltage of 15 keV and a working distance of 4 mm. All of the samples were coated with a thin layer of gold for a better conductivity. The surface chemical composition of the sample was analyzed via X-ray photoelectron spectroscopy (XPS) using the constant pass energy mode with a value of 100 eV, and all the binding energies were calibrated using the C1s peak at 284.6 eV as the reference. The structures of the PU sponges and PDMS–PU sponges were measured by a Nicolet 6700 Fourier transform infrared spectrometer (FTIR). The mechanical properties were investigated on the PDMS–PU sponges (2 cm × 2 cm × 2 cm) by an electromechanical universal testing machine 2T CMT4204. The water CA measurement was performed to investigate the surface wettability of PDMS sponges with JC2000D3. The syringe was positioned in such a way that the liquid drop (5 μL) could contact the surface of the samples before leaving the needle. Each sample was measured at least three times.Result and discussion
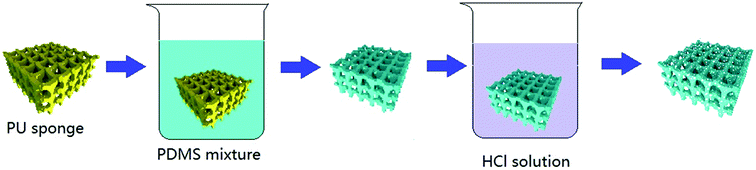
Fig. 1 shows the process of preparing the superhydrophobic sponge. The PDMS–PU sponges were obtained by modifying commercially available polymer sponges with a PDMS coating through a facial dipping–coating method. Commercially available PU sponges can be easily modified by scalable dipping–coating. The original PU sponge with an open macroporous structure is an excellent absorber for water and organic solvents. After being dipped in PDMS composite solution containing carbon tetrachloride and n-octane, the whole PU sponge was filled quickly and then pulled up and aired to remove the solvent at room temperature. During the process of curing at 70 °C, the diluents of the sites were released and left with holes to obtain the PDMS coated sponge. The coated sponges immersed in HCl solution for a period of time that roughens the sponge surface to improve the superhydrophobic property. The resulting sponges were dried at 70 °C for 2 hours to obtain superhydrophobic PDMS–PU samples.
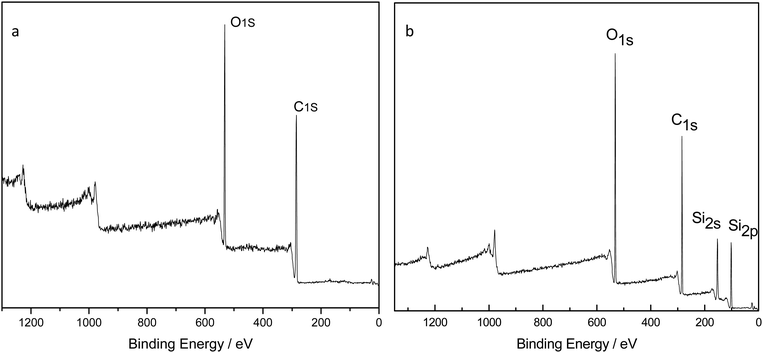
Fig. 2(a) is the FTIR comparison figure of PU, PDMS and PDMS–PU sponge after HCl corrosion. In PU FTIR spectrum, the bands at 3290 cm−1 and 1532 cm−1 correspond to the N–H vibration and deformation, and the band at 1722 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption. In the PDMS FTIR spectrum, the bands at 1072 cm−1 and 1007 cm−1 correspond to the Si–O stretching vibration, 864 cm−1 and 789 cm−1 are Si–(CH3)2 stretching vibration. In PDMS–PU FTIR spectrum, the above-mentioned characteristic absorption peaks of PU and PDMS all appeared in this spectrum, which demonstrated the existence of the PDMS coatings on PU sponge. Fig. 2(b) is the comparison figure of PDMS–PU sponge before and after HCl corrosion. From the two spectra, it can be seen that HCl treatment does not change the PDMS–PU sponges' molecule structure. Fig. 3(a) and (b) are XPS spectra of a pristine PU sponge and PDMS–PU sponge. The result shows that C and O elements all appear on these two figures, the existence of Si element on PDMS–PU spectrum corresponds to the above results. Combined with the data analysis of FTIR and XPS, it proved that PDMS has coated on to the porous PU sponge. It was believed that the crosslinked Si–O–Si bond will greatly increase the mechanical stability and the superhydrophobic property of the porous PDMS coatings on the sponges.
O absorption. In the PDMS FTIR spectrum, the bands at 1072 cm−1 and 1007 cm−1 correspond to the Si–O stretching vibration, 864 cm−1 and 789 cm−1 are Si–(CH3)2 stretching vibration. In PDMS–PU FTIR spectrum, the above-mentioned characteristic absorption peaks of PU and PDMS all appeared in this spectrum, which demonstrated the existence of the PDMS coatings on PU sponge. Fig. 2(b) is the comparison figure of PDMS–PU sponge before and after HCl corrosion. From the two spectra, it can be seen that HCl treatment does not change the PDMS–PU sponges' molecule structure. Fig. 3(a) and (b) are XPS spectra of a pristine PU sponge and PDMS–PU sponge. The result shows that C and O elements all appear on these two figures, the existence of Si element on PDMS–PU spectrum corresponds to the above results. Combined with the data analysis of FTIR and XPS, it proved that PDMS has coated on to the porous PU sponge. It was believed that the crosslinked Si–O–Si bond will greatly increase the mechanical stability and the superhydrophobic property of the porous PDMS coatings on the sponges.
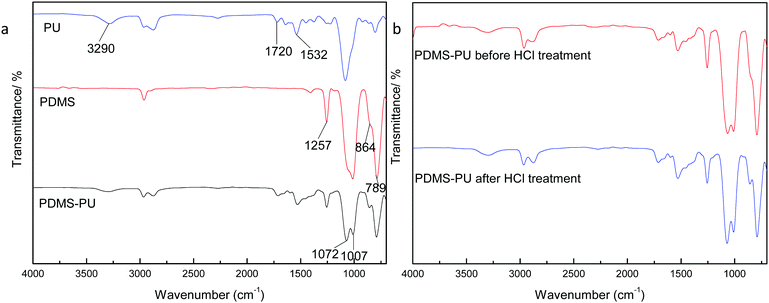 | ||
| Fig. 2 FTIR spectra of sponges. (a) Comparison of PU, PDMS, PDMS–PU sponge; (b) comparison of PDMS–PU sponge before and after HCl treatment. | ||
Fig. 4(a)–(i) are the SEM and CA figures of the PU sponge, PDMS coated PU sponge, PDMS–PU sponge after corrosion shown with different magnifications. As shown in Fig. 4(a) and (b), the PU sponge possessed the bulk three-dimensional structure, specific macroporous shape, which was beneficial for the uptake capacity. The smooth skeleton of the PU sponge contributed to the low water contact angle which is 124° ± 1.2° in Fig. 4(c). From Fig. 4(d) and (e), it can be clearly seen that the surfaces of the skeleton changed from smooth to randomly rough at the micrometer and sub-micrometer scales after the modification process. The water contact angle improved to 145° ± 1.5° due to the existence of the PDMS. From Fig. 4(g) and (h), the PDMS–PU sponge after corrosion was found to exercise a high degree of roughness with a number of holes. Combination of the impact of PDMS with HCl corrosion, the wettability of the PDMS–PU sponge became superhydrophobic with a water contact angle of 158° ± 1.3°. When organic solvent (carbon tetrachloride) dripped on these three sponges, the contact angles all closed to 0°, proving that the surfaces of these sponges are superoleophilic, compared with the pristine PU sponge.
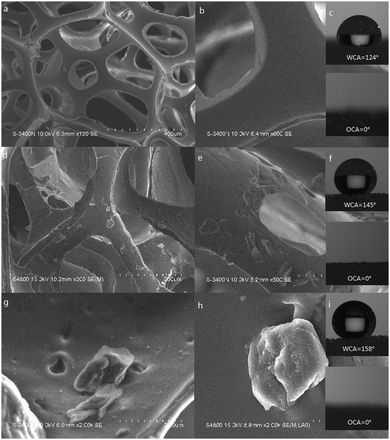 | ||
| Fig. 4 SEM images and WCA of the sponges. (a–c) PU sponges, (d–f) PDMS coated PU sponges, (g–i) PDMS–PU sponges after HCl corrosion. | ||
Fig. 5 is the TGA graph of PDMS, PU and PDMS–PU sponges. The TGA curves of the PU sponge and PDMS–PU sponge are similar due to the low PDMS concentration. With the initial temperature range (<300 °C), the weight loss was mainly ascribed to the evaporation of residual water, which was 26.53% for the PDMS–PU sponge.31 With the temperature increasing to 400 °C, significant weight losses for the PDMS–PU sponge appeared. The weight loss was 75.7%. The oil absorption experiment usually performed at room temperature which is no more than 100 °C. This shows the sponge to be stable under real service conditions.
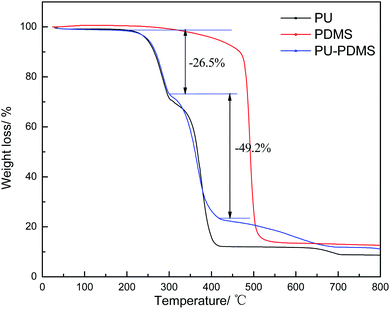 | ||
| Fig. 5 The TGA curves of PU, PDMS and PDMS–PU sponge measured at a scanning rate of 10 °C min−1 in nitrogen. | ||
Generally, the wettability of a solid surface depends on two factors: its topographical microstructure and its surface chemical composition.32–34 Combining hydrophobic PDMS with the microscale roughness of the surface of a PU sponge provided us with a superhydrophobic sponge.
It reveals hierarchical structures that exist in the form of cloud-like nanostructures. Such a structure dramatically increased the surface roughness and led to a composite interface in which air became trapped within the groove beneath the liquid, thereby including superhedrophobicity (the so-called Cassie–Baxter model).35Fig. 6(a) reveals the change in appearance of the PDMS–PU sponge after immersion in water. The surface of the PDMS–PU sponge appeared like that of a silver mirror when immersed in water under an external force, suggesting that this sponge featured Cassie–Baxter surfaces.36
Fig. 6(b) and (c) are the water and oil contact angles of the PDMS sponge. When a water droplet was placed on the surface fabricated by PDMS–PU sponges, the static water contact angle was measured to be 159.5° ± 1.1°, indicating superhydrophobic behavior. When a CCI4 droplet was dropped on surface, it could be quickly absorbed into the PDMS–PU sponge, the static water contact angle was measured to be 0°, indicating a superoleophilic behavior. The fact that the methyl groups tend to appear on the rough surface of the sponge can improve the superhydrophobic/superoleophilic property of the sponge.
Fig. 7(a) shows the absorption capacity of PDMS–PU sponge for different organic solvents. All experiments were conducted at room temperature. These sponges show excellent absorbencies (2461–8613%), depending on the density and viscosity of the oils and organic solvents. Although longer immersion times were expected to lead to greater absorption, a qualitative visual observation showed that the PDMS sponge was rapidly wetted in the oils, which did not greatly depend on time. Once a cube of PDMS sponge was put into the oil/water mixture, the sponge can be absorbed completely in 20 s. In addition, according to the water absorption capacity of the PU sponge (1011%) and PDMS–PU sponge (79%), the water absorption decreased sharply after coating, contributing to the improvement of the PDMS–PU sponge selectivity.
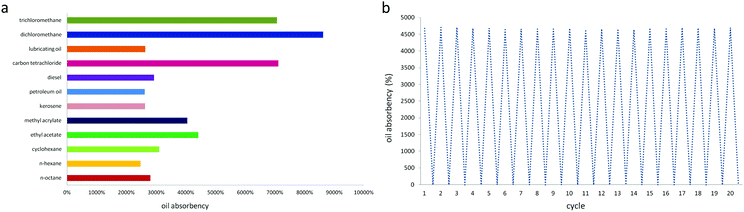 | ||
| Fig. 7 Oil absorbency and recycling performance of PDMS sponge. (a) Absorbency of the PDMS sponge. (b) Demonstration of the recyclability of the PDMS sponge. | ||
The recyclability of the PDMS–PU sponge was tested by the repeated absorption–desorption process, which is a significant feature for practical applications. After absorption equilibrium, the sponge was squeezed and immersed into ethanol solution to exclude the remaining oils in the PDMS–PU sponge completely. Finally, the resulting sponge was dried in 70 °C and absorption–desorption trails were repeated. Fig. 7(b) is the recyclability of the PDMS–PU sponge, it shows that the sponge weight is constant before absorption and after desorption. PDMS–PU sponges show little change in their absorption capacities and own weight after 20 absorbing/recovering cycles. And when the sponge was immersed in water after the reusability test, it still did not absorb any water, indicating that hydrophobicity remained.
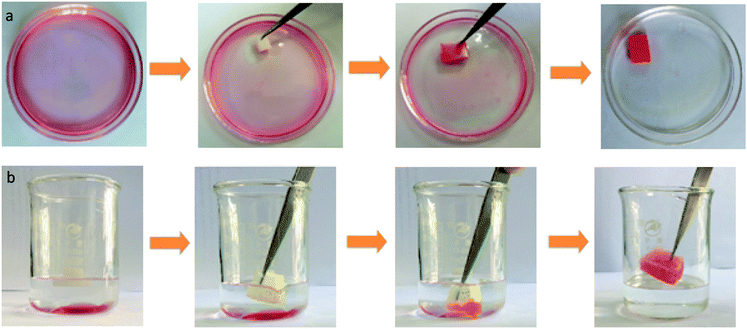
Fig. 8 is the selective absorption of the PDMS–PU sponge. When a piece of PDMS–PU sponge was put in the surface of an n-octane–water mixture, red colored n-octane (dyed with Oil Red O) could be absorbed into the sponge in a few seconds which is depicted in Fig. 8(a). Even high-density organic mixtures (e.g. carbon tetrachloride), lying below in water–organic mixtures, could also be selectively absorbed by applying the PDMS–PU sponges which are depicted in Fig. 8(b). Notably, no water uptake was observed during the absorption of organics both on the surface of water and under water, indicating an excellent selective absorption of the PDMS–PU sponges. More interestingly is that the absorbed oils were readily collected through a simple mechanical squeezing process by taking advantage of the elasticity of the sponge. The collection method reported here is more eco-friendly and time-saving than the reported burning off and heat treatment processes.
The mechanism for the high capacity and selectivity is ascribed to the following reasons. The sponge's surface is spontaneously wetted by the oils and organic solvents because of similar surface energies between the oils (e.g. ethyl acetate, 26.29 mN m−1) and PDMS coating (20.4 mN m−1). However, water is strongly repelled by the sponge's surface due to a much higher surface tension (72 mN m−1) than that of the PDMS coating. More importantly, the HCl corrosion and the oleophilic and hydrophobic properties of the PDMS coatings are greatly enhanced by the surface roughness, leading to superhydrophobicity and superolephilicity. The oils are driven through the pores of the sponges into its bulk in the presence of a capillary force, while water is completely excluded by the superhydrophobic surface, resulting in a separation of the oils from water with a high efficiency. The capillary flow further improves the possibility that the oils spread into the inner pores of the sponge due to the superoleophilic PDMS coated on the interconnected skeleton. Then the oils were stored in the pores formed by the interconnected skeleton of the sponge, exhibiting a high oil absorption capacity.
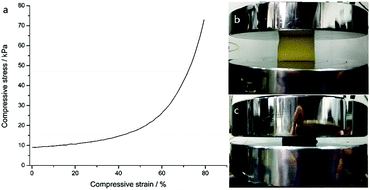
Fig. 9 shows the mechanical property of the PDMS–PU sponge which was evaluated by stress–strain measurements. This shows that the superhydrophobic sponges are able to be compressed to large strains (ε = 79%) at relatively low stress (72 kPa), owing to the high porosity and elasticity of the sponges. In addition, the PDMS–PU sponges immediately recovered to their original shapes with no plastic deformation after release of the pressure. So, the absorbed solvents can be collected through a simple mechanical squeezing process by taking advantage of the elasticity of the sponge.
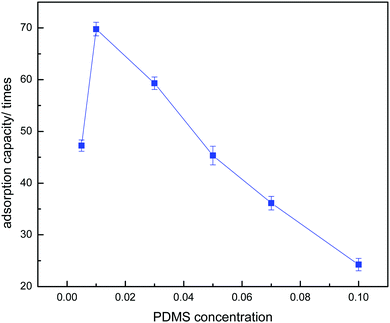
Optimization of the PDMS concentration for the absorption medium plays an important role in absorption studies. The effect of PDMS concentration on the absorption capacity of a PDMS–PU sponge is shown in Fig. 10. The absorption capacity of the PDMS–PU sponge was changed with increase in the PDMS content. It was observed that when the PDMS concentration increased from 0.5% to 1.0%, the absorption capacity increased from 4862% to 6997%. But, with continued increase in the PDMS concentration, the absorption capacity was reduced. The explanation for this is that when the PDMS concentration is 0.5%, it is too low to coat completely on the PU sponge surface and inner pore surface. But when the PDMS concentration increased to 3%, the PDMS mixture plugged up the holes of the PU sponge and reduced the porosity of the obtained sponge, so the absorption capacity was reduced with further increases in the PDMS concentration. According to the above comparison, the optimistic PDMS concentration is 1.0%. Table 1 gives the apparent density and porosity of the PU and PDMS–PU sponge. With the increase of PDMS concentration, the apparent density increased and the porosity decreased. The density of the 1% PDMS–PU sponge is also low, so it can float on the oil/water mixture during application.
| Sample | Apparent density (g cm−3) | Porosity (%) |
|---|---|---|
| PU | 0.289 | 76.88 |
| PDMS–PU (1%) | 0.343 | 72.36 |
Conclusions
In summary, we demonstrated a facile approach to fabricate robust superhydrophobic polyurethane sponges through a combination of a one-step dipping–coating process with HCl corrosion and their oil–water separation properties. The PDMS–PU sponges show excellent absorption performance (2461–8613%) for different types of oils and organic solvents, and the reusability of the sponge could be demonstrated by continuous absorption experiments. Because of the excellent properties of the PDMS–PU sponges, such as superhydrophobicity/superolephilicity, elasticity, thermostability, easy fabrication, good commercial availability of raw materials, we may provide a new way for numerous potential applications, including oil-spill cleanup, liquid–liquid separation and water treatment.Acknowledgements
The authors sincerely acknowledge ‘Shanghai university-industry collaboration program (CXY-2014-023)’ and ‘Scientific and technological achievements transformation program of Jiangsu Province (SBA2014010034)’.References
- H. Y. Shen, Z. X. Chen, Z. H. Li, M. Q. Hu, X. Y. Dong and Q. H. Xia, Colloids Surf., A, 2015, 481, 439 CrossRef CAS.
- C. Ruiz, E. Mena, P. Cañizares, J. Villaseñor and M. A. Rodrigo, I&EC Research, 2014, 53, 840 CAS.
- J. M. Pan, H. Yao, L. C. Xu, H. X. Ou, P. W. Huo, X. X. Li and Y. S. Yan, J. Phys. Chem. C, 2011, 115, 5440 CAS.
- X. Yao, Y. L. Song and L. Jiang, Adv. Mater., 2011, 23, 719 CrossRef CAS PubMed.
- T. L. Yip, W. K. Talley and D. Jin, Mar. Pollut. Bull., 2011, 62, 2427 CrossRef CAS PubMed.
- F. J. Wang, S. J. Yu, M. S. Xue, J. F. Ou and W. Li, New J. Chem., 2014, 38, 4388 RSC.
- B. Victoria and A. A. Keller, Environ. Sci. Technol., 2006, 40, 7914 CrossRef.
- S. Mukherjee and B. Mukhopadhyay, RSC Adv., 2012, 2, 2270 RSC.
- I. Buist, J. McCourt, S. Potter, S. Ross and K. Trudel, Pure Appl. Chem., 1999, 71, 43 CrossRef CAS.
- R. P. J. Wannell, K. Lee and M. McDonagh, Microbiol. Res., 1996, 60, 342 Search PubMed.
- S. D. Yang, L. C. Chen, L. Mu, B. Hao, J. T. Chen and P. C. Ma, RSC Adv., 2016, 6, 4889 RSC.
- A. Li, H. X. Sun, D. Z. Tan, W. J. Fan, S. H. Wen, X. J. Qing, G. X. Li, S. Y. Li and W. Q. Deng, Energy Environ. Sci., 2011, 4, 2062 CAS.
- C. Serre, Angew. Chem., Int. Ed., 2012, 124, 6152 CrossRef.
- Y. Chu and Q. M. Pan, ACS Appl. Mater. Interfaces, 2012, 4, 2420–2425 CAS.
- J. E. Mark, Acc. Chem. Res., 2004, 37, 946 CrossRef CAS PubMed.
- I. Park, K. Efimenko, J. Sjöblom and J. Genzer, J. Dispersion Sci. Technol., 2009, 30, 318 CrossRef CAS.
- J. P. Lewicki, P. W. Beavis, M. W. C. Robinson and R. S. Maxwell, Polymer, 2014, 55, 1763 CrossRef CAS.
- X. Zhao, L. X. Li, B. C. Li, J. P. Zhang and A. Q. Wang, J. Mater. Chem. A, 2014, 2, 18281 CAS.
- P. X. Si, J. K. Wang, C. Zhao, H. Xu, K. Yang and W. Q. Wang, Polym. Adv. Technol., 2015, 26, 1091 CrossRef CAS.
- P. X. Si, J. K. Wang, J. H. Guo, S. Z. Li, W. P. Cai and H. Xu, New J. Chem., 2015, 39, 6823 RSC.
- J. K. Wang, J. H. Guo, P. X. Si, W. P. Cai, Y. M. Wang and G. H. Wu, RSC Adv., 2016, 6, 4329 RSC.
- X. Zhao, L. Li, B. Li, J. Zhang and A. Wang, J. Mater. Chem. A, 2014, 2, 18281 CAS.
- P. Calcagnile, D. Fragouli and I. S. Bayer, ACS Nano, 2012, 6, 5413–5541 CrossRef CAS PubMed.
- J. Li, D. M. Li, W. F. Hu, J. P. Li, Y. X. Yang and Y. X. Wu, New J. Chem., 2015, 39, 9958 RSC.
- Y. Liu, J. K. Ma, T. Wu, X. R. Wang, G. B. Huang, Y. Liu, H. X. Qiu, Y. Li, W. Wang and J. P. Gao, ACS Appl. Mater. Interfaces, 2013, 5, 10018 CAS.
- C. F. Wang and S. J. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 8861 CAS.
- Q. Zhu, Q. M. Pan and F. T. Liu, J. Phys. Chem. C, 2011, 115, 17464 CAS.
- Q. Zhu, F. Tao and Q. M. Pan, ACS Appl. Mater. Interfaces, 2010, 2, 3141 CAS.
- D. N. H. Tran, S. Kabiri, T. R. Sim and D. Losic, Environ. Sci.: Water Res. Technol., 2015, 1, 298 CAS.
- H. X. Sun, A. Li, Z. Q. Zhu, W. D. Liang, X. H. Zhao, P. Q. La and W. Q. Deng, ChemSusChem, 2013, 6, 1057 CrossRef CAS PubMed.
- S. C. Liu, J. M. Pan, J. Cao, X. H. Dai, M. J. Meng, R. R. Wu, J. T. Yao and Y. S. Yan, Chem. Eng. J., 2016, 284, 10 CrossRef CAS.
- K. Liu, Y. Tian and L. Jiang, Prog. Mater. Sci., 2013, 58, 502 CrossRef.
- K. Liu and L. Jiang, Nano Today, 2011, 6, 155 CrossRef CAS.
- M. Liu, Y. Zheng, J. Zhai and L. Jiang, Acc. Chem. Res., 2010, 43, 368 CrossRef CAS PubMed.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546 RSC.
- S. Gupta and N. H. Tai, J. Mater. Chem. A, 2016, 4, 1550 CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |