Sensitive chemiluminescence determination method for 2,4,6-trinitrotoluene based on the catalytic activity of amine-capped gold nanoparticles†
Javad
Hassanzadeh
a,
Alireza
Khataee
*ab,
Nafiseh
Bagheri
c and
Roya
Lotfi
a
aResearch Laboratory of Advanced Water and Wastewater Treatment Processes, Department of Applied Chemistry, Faculty of Chemistry, University of Tabriz, 51666-16471 Tabriz, Iran. E-mail: a_khataee@tabrizu.ac.ir; Fax: +98 41 33340191; Tel: +98 41 33393165
bDepartment of Materials Science and Nanotechnology, Near East University, 99138 Nicosia, North Cyprus, Mersin 10, Turkey
cDepartment of Chemistry, Faculty of Science, Azarbaijan Shahid Madani University, Tabriz, Iran
First published on 22nd November 2016
Abstract
A simple, sensitive and selective chemiluminescence (CL) assay for the determination of 2,4,6-trinitrotoluene (TNT) is described due to its high importance in addressing security and environmental problems. The CL system was based on the reaction of rhodamine B (RB)–KMnO4 catalyzed by gold nanoparticles (AuNPs). TNT could efficiently quench the CL emission due to its selective interaction with amine groups embedded on the AuNP surfaces. This quenching effect led to the design of a highly sensitive CL turn-off probe for the detection of TNT. Various key factors including the RB, KMnO4 and AuNP concentrations were optimized. TNT could be quantified in the concentration range of 0.27 to 20 nmol L−1, with a detection limit (3σ) of 0.045 nmol L−1 under optimal conditions. Compared to other NP-based techniques, the developed probe has a better detection limit as demonstrated by the successful analysis of environmental samples.
1. Introduction
The trace analysis of nitroaromatics (NAs) in various environmental samples has become an important challenge in analytical chemistry not only for national security, but also for environmental purposes. Because of the increasing number of terrorist acts and environmental contamination problems, there has been great attention on developing detection methods for traces of explosives in recent times.1–5 NAs can be introduced into the environment, in the form of dyes, pesticides and explosives.4 Furthermore, the mentioned compounds are a health concern due to their ability to cause mutagenic effects in humans, liver damage, gastritis, aplastic anemia, cyanosis, and dermatitis.22,4,6-Trinitrotoluene (TNT), the most commonly used NA explosive, can cause the contamination of soil and ground water. A 2 ppb limit on the concentration of TNT in drinking water has been announced by the United States Environmental Protection Agency (EPA).5,6 Some methodologies have been developed for the monitoring of TNT and other explosives based on chromatography methods,7 chromatography-mass spectrometry,8,9 X-ray imaging,10 ion mobility spectrometry,5,11 electrochemistry12 and further spectrometric procedures.7,13–18 These techniques are generally tedious and time consuming and need bulky equipment, laborious sample pretreatment, and a skilled worker.
Chemiluminescence (CL) based detection is famous with respect to its high sensitivity, simplicity, speed, good reproducibility, and wide linear dynamic range.19–21 Trace analysis involving the CL reaction of luminol has been widely exploited for the quantification of explosives.16 Also, nanoparticle-based procedures have been recently presented for the determination of NAs, which have the appropriate selectivity and sensitivity.22–33 NAs can interact with nanoparticles modified with certain groups and so change their activity.33 A cysteine-modified Au nanoparticle (AuNP) based label-free surface-enhanced Raman spectroscopic method has been developed by Dasary et al.24 for the detection of TNT in aqueous solutions, which relies on the formation of a Meisenheimer complex between the cysteine groups and TNT molecules.24 Amine modified mesoporous silica nanostructures immobilized in fluorescent polymers have been employed for the recognition of TNT based on its quenching effect.25 Fluorescence resonance energy transfer (FRET) has been reported as a method for the simple and highly sensitive detection of TNT using different semiconductor quantum dots which were functionalized with amine branches.22,23,26,27,30 Moreover, the sensitive colorimetric determination of TNT using ethylenediamine-functionalized AuNPs was described.27
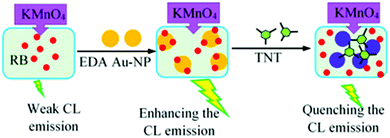
Because of their unique intrinsic properties, AuNPs have been widely used in analysis. They are unreactive and stable and can be synthesized with simple methods.34,35 In this study, a “turn-off” chemiluminescence assay was developed for the measurement of trinitrotoluene in environmental samples based on its inhibiting effect on the CL emission of an amine capped AuNP assisted rhodamine B (RB)–KMnO4 system. The interaction of NAs, especially TNT, with amine groups on AuNPs can deactivate the NPs and so quench the CL emission.
2. Experimental
2.1. Apparatus and chemicals
Fluorescence spectra were recorded by a Shimadzu spectrofluorometer (RF-5301, Japan). CL spectra were obtained using the same fluorimeter while the excitation beam was locked. UV-visible absorption spectra were obtained by a Shimadzu spectrophotometer (UV-1800, Japan). The synthesized AuNPs’ morphologies and sizes were investigated by a Mira3 FEG scanning electron microscope (SEM) (Tescan, Czech Republic) and a transmission electron microscope (TEM, Leo 906, Zeiss, Germany). Also, microstructural image processing (MIP) software (Nahamin Pardazan Asia Co., Iran) was employed for the size assessment of the AuNPs. Fourier transform infrared (FT-IR) spectra were obtained on a Bruker Tensor 27 (Germany) FT-IR spectrophotometer.Chloroauric acid (HAuCl4) was acquired from Alfa Aesar (Karlsruhe, Germany). 2-Nitrotoluene (2NT, 99%), 2,4-dinitrotoluene (DNT) (97%) and 2,4,6-trinitrotoluene (TNT, 1 mg mL−1 solution in acetonitrile) were purchased from Aldrich Co. The other used chemicals were obtained from Merck Co. (Darmstadt, Germany). Deionized water (Kasra Co., Tehran, Iran) was used for the preparation of all solutions. Stock solutions of 2,4-dinitrotoluene (2,4-DNT) and 2-nitrotoluene (2-NT) were made using an acetonitrile solvent and kept at low temperatures (4 °C).
2.2. Synthesis of amine-capped AuNPs
The synthesis of citrate–AuNPs was performed according to a previously described method based on the chemical reduction of HAuCl4 using trisodium citrate.36 Briefly, trisodium citrate 1% solution (5 mL) was quickly added into a boiling HAuCl4 solution (0.25 mmol L−1, 100 mL) while it was vigorously stirred. The heating of the solution was continued until its color changed to deep red. Then, it was stirred for 2 min and cooled to room temperature. Finally, the volume of the obtained solution was increased to 100 mL (if it had decreased by evaporation). The concentration of the synthesized AuNPs was determined to be 0.95 nmol L−1 by means of the reported extinction coefficients and Beer's law.36In the next step, the prepared AuNPs were modified with 1,2-ethylenediamine (EDA) according to Liu’s procedure.27 Specific volumes of EDA solution (1 μmol L−1) and freshly prepared AuNPs (9.5 × 10−10 mol L−1), with a volume ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, were mixed together and stirred for 12 h. Then, centrifugation (30
1, were mixed together and stirred for 12 h. Then, centrifugation (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 15 min) was carried out to remove the excess EDA. The prepared AuNPs were kept at 4 °C. TEM and SEM analysis showed that the synthesized AuNPs have an average size of about 22 nm.
000g, 15 min) was carried out to remove the excess EDA. The prepared AuNPs were kept at 4 °C. TEM and SEM analysis showed that the synthesized AuNPs have an average size of about 22 nm.
2.3. Chemiluminescence detection of nitroaromatics
The TNT determinations by CL were done in a batch system. Briefly, 1000 μL synthesized amine-capped AuNP solution (9.5 × 10−10 mol L−1), 300 μL RB solution (3 ×10−4 mol L−1), 300 μL sodium dodecyl sulfate (SDS) solution (4 × 10−4 mol L−1), 300 μL NaOH (0.2 mol L−1) solution and given volumes of a standard TNT solution or a real sample were transferred to a 3 mL cuvette. The total volume of the mixture was increased to 2.8 mL using deionized water and then 200 μL potassium permanganate solution (0.2 mmol L−1) was injected. Meanwhile, the CL signal was continuously recorded against time and its maximum was selected for the quantification investigation.3. Results and discussion
3.1. Characterization of the synthesized amine-capped AuNPs
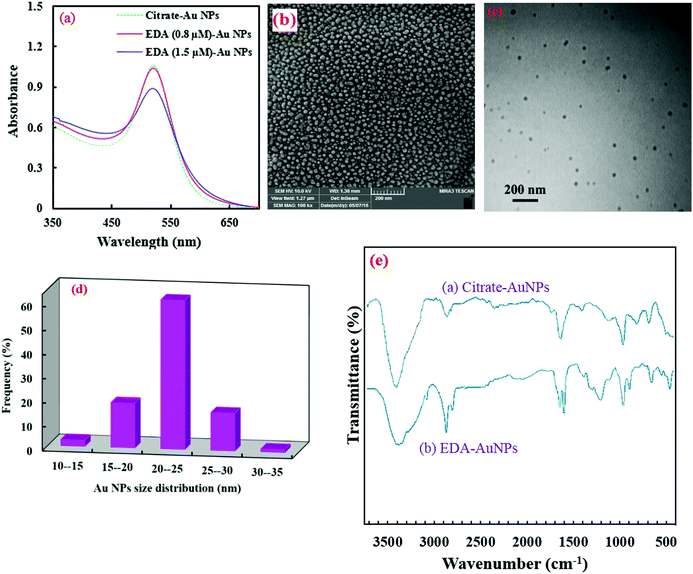
Citrate AuNPs were synthesized using a very fast, simple, reproducible and well-known reduction method. Then, the modification of their surfaces was carried out with EDA, based on the electrostatic interaction of its protonated amino groups with the negative citrate carboxyl groups on the surfaces of the AuNPs.27 The absorption spectra of the AuNPs (λmax at 518 nm) didn't show any alteration after the introduction of EDA (Fig. 1a), meaning that the AuNPs don't aggregate in the presence of EDA at lower concentrations than 1 μmol L−1. Higher amounts of EDA can aggregate AuNPs and decrease their absorption.27A complementary investigation of the morphologies and particle sizes of the synthesized AuNPs was conducted using SEM and TEM analysis, which is presented in Fig. 1(b and c). As shown in this figure, the AuNPs are spherical in shape and have good homogeneity. Also, the TEM images show no aggregation of the AuNPs. Furthermore, evaluation of the size distribution of the AuNPs was performed by employing MIP software,19 as shown in Fig. 1d. As seen in this figure, the average size of the AuNPs was calculated to be 22 nm.
Furthermore, the FT-IR spectra of the AuNPs, before and after their modification with EDA, are shown in Fig. 1e. As can be seen, the citrate capped AuNPs show some characteristic absorption bands for –OH and –COO− groups at 3424 and 1648 cm−1, respectively. The peaks at 2888, 2835 and 1405 cm−1 are related to C–H stretching and bending vibrations, respectively (spectrum a in Fig. 1e). In contrast, the EDA modified AuNPs show some additional bands (spectrum b in Fig. 1e). The peaks at 1208 and 1310 cm−1 may be related to C–N bands. The peak at 1605 cm−1 is for N–H bond bending. Also, a clear change can be observed in the ∼3400 cm−1 broad band, which may be related to N–H stretching vibrations.37
The surface potential data are also in agreement with the FT-IR data which confirms the modification of the AuNPs with amine groups (Fig. S1, ESI†). The zeta potential of the synthesized AuNPs is −29.5 mV due to the negative citrate on the surface of the NPs. However, it changes to +10.4 mV after the modification step, which demonstrates the attachment of amine groups on the surface of the AuNPs.
3.2. Enhancing effect of amine-capped AuNPs on the RB–KMnO4 CL emission
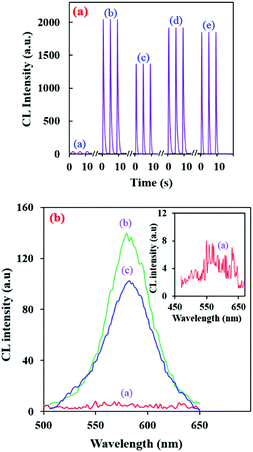
Recently, our research group presented an intrinsic CL reaction between alkaline RB–KMnO4 and AuNPs,36 and applied it for the sensitive determination of some valuable species.36,38,39 In these systems, the RB–alkaline KMnO4 reaction in the presence of AuNPs can lead to an intense CL emission. It can provide a unique analytical device with its own features; however, unexpected species can affect the AuNPs and decrease the selectivity of this determination method. Here, amine-capped AuNPs can also show this enhancing effect (Fig. 2a (curves a and b)). Furthermore, modifying the AuNPs can make the system selective for specific analytes.In order to study the developed CL system in the presence of amine-capped AuNPs, kinetic profiles of the CL emission were investigated. Interestingly, the amine capped AuNPs show a slightly greater enhancing effect than the citrate AuNPs under the same conditions (Fig. 2a (curves b and d)). This can be ascribed to the presence of the amine groups. The gold nanoparticles have two different effects on the RB molecules. They can quench the RB emission due to the inner filter effect. On the other hand, they can enhance the RB CL emission by locating RB molecules in close proximity.36,38,39 Here, in the modified AuNPs, the citrate carboxylic groups are replaced by –NH2 groups, which can reduce the interaction of the RB molecules with the AuNPs and increase the distance between the RB molecules and AuNPs. The mentioned phenomenon can relatively decrease the quenching effect of the AuNPs and give rise to slightly higher CL emission.
Also, the CL spectrum plotted in the presence of amine–AuNPs (Fig. 2b) was almost in good accordance with the RB fluorescence spectrum at λem = 585 nm (λex = 558 nm).36,38,39 Therefore, it can be concluded that the CL luminophores are the RB molecules and modification of the AuNPs doesn't affect the CL mechanism.
So, it can be stated that KMnO4 oxidizes the RB molecules, leading to the production of an excited intermediate of RB ([RB]ox*, eqn (1)).36 This intermediate was reported by previous studies to be the luminophore product of RB oxidation and its structure was investigated.40,41 Energy transfer from [RB]ox* to RB free molecules can produce the excited state of RB (RB*) (eqn (2)), which can create CL emission (eqn (3)). AuNPs can also improve the RB oxidation and energy transfer efficiency due to RB molecules’ adsorption on the AuNPs.
 | (1) |
 | (2) |
| RB* → RB + hν (λmax ≅ 580 nm) | (3) |
It should be mentioned that the alkaline medium applied in this work has some advantages in comparison with acidic conditions. The reactions of acidic KMnO4 suffer from a large amount of interference. Also, NPs can be rapidly oxidized by KMnO4 under acidic conditions, so their catalytic effect would be eliminated.
3.3. Inhibition effect of TNT
Alkaline permanganate–RB–AuNPs is an attractive and innovative CL system with high applicability in analysis.36,38,39 Here, AuNPs modified with amine groups were employed in order to establish a sensitive system which is selective for explosive NAs. Amine–NA interaction is an efficient technique for the selective analysis of NAs, and has been frequently applied with colorimetric (and some fluorometric) determination methods.22–32,42,43 Coupling this technique with a CL detection system can provide a selective and highly sensitive method for the analysis of NAs. It is found that the emission intensity of the RB–KMnO4–amine capped AuNP CL system can be affected by the presence of some NAs (Fig. 3).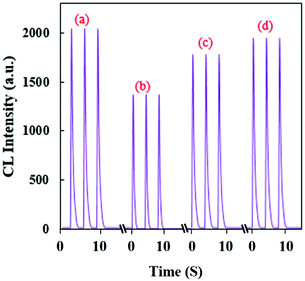 | ||
| Fig. 3 CL profiles of the RB–KMnO4–EDA capped AuNPs (a) in the absence and (b–d) in the presence of various NAs at optimum conditions [b: 10 nmol L−1 TNT, c: 100 nmol L−1 DNT, d: 100 nmol L−1 2NT]. | ||
NA molecules, as Bronsted–Lowry acids with a high affinity for electrons, can interact with the amine groups of EDA27 to form ion pairs between the NAs and the NH3+–R groups on the AuNPs. As a result, the RB molecules are isolated from the NP surface and the enhancement of the CL emission is removed. Also, the vast surface area of the NPs can improve the adsorption efficiency. Electron-rich amino groups on the surface of the AuNPs can act as recognition antennas for the electron deficient NAs and a strong electrostatic interaction takes place between them. However, the citrate–AuNPs’ interactions with TNT are very weak (Fig. 3b). This phenomenon has been frequently used for the quantification of NAs.27,42Fig. 4a shows the effect of TNT on the UV-visible absorption spectra of the amino-capped AuNPs.
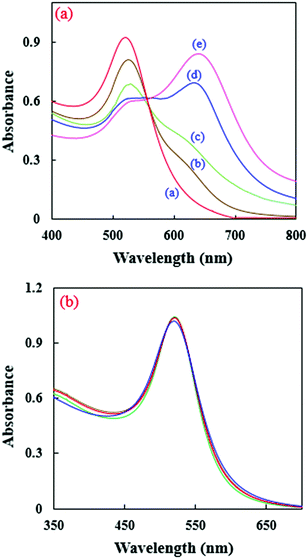 | ||
| Fig. 4 UV-visible absorption spectra for (a) EDA-modified and (b) citrate-capped AuNPs in the presence of different concentrations of TNT [a–e: 0, 1, 2, 5, and 10 μmol L−1, respectively]. | ||
As can be seen in Fig. 4a, the presence of TNT can broaden the absorption peak of the AuNPs at λmax = 518 nm and shift it to high wavelengths, which is because of AuNP aggregation. This effect leads to a change in the color of the AuNP solution from red to blue. However, the absorption band of the citrate capped AuNPs was not practically altered by the addition of TNT (Fig. 4b). Scheme 1 shows a schematic image of the presented sensor. The CL intensity decreases at high concentrations of TNT.
3.4. Optimization
The amine groups on the surfaces of the AuNPs can deprotonate the NAs and produce anion–cation pairs (NA–RNH3+). The alkalinity of the reaction solution is one of the important parameters that can affect the formation of these ion pairs. Consequently, the effect of the NaOH concentration on the developed CL system was investigated. It is clear from Fig. S2a (ESI†) that an increase in the concentration of NaOH up to 0.02 M improves the response of the sensor (ΔI = I0 − I, with I0, and I representing the CL signal in the absence and presence of TNT, respectively), because it can increase the rate of the redox reaction between RB and KMnO4. However, higher concentrations of NaOH decrease the response of the probe, due to the inhibiting effect of a high OH− concentration on the protonation of the amine groups.Increasing the RB concentration up to 0.03 mmol L−1 beneficially enhances the CL emission intensity (Fig. S2b, ESI†), because this can increase the production of excited RB. However, higher concentrations of RB quench the CL emission, perhaps due to its self-absorption effect. Thus, 0.03 mmol L−1 of RB was selected as the best concentration.
The effect of the AuNP concentration on sensor response was also studied (Fig. S2c, ESI†). Low concentrations of the AuNPs improved ΔI, however, higher concentrations than 0.33 nmol L−1 reduced it. This reducing effect can be assigned to excess AuNPs causing a greater inner-filter effect. Also, excess AuNPs can react and consume some of the KMnO4.
As revealed in previous reports, the addition of SDS to the presented CL system enhanced the CL emission efficiency. Therefore, the concentration of SDS was investigated as an effective factor in the range of 0.01–0.08 mmol L−1 (Fig. S2d, ESI†). The data showed that the optimum CL emission was achieved at 0.04 mmol L−1 of SDS. The addition of SDS is helpful for the enhancement of the CL intensity. However, it is reported that the electrostatic interaction between SDS anions and amine groups might influence the properties of amine-capped AuNPs.43 Therefore, the UV-visible absorption spectrum of the amine-capped AuNPs was taken after adding SDS. The performed experiments showed that SDS has no clear effect on the structure of the amine-capped AuNPs, maybe due to the low concentration of SDS.
Finally, the concentration of KMnO4 was changed in the range of 0.003–0.03 mmol L−1 and its effect was studied (Fig. S2e, ESI†). It is observed that the response of the designed probe for TNT becomes better upon increasing the concentration of KMnO4 up to 0.013 mol L−1 and then remains constant.
3.5. Application and selectivity
TNT has a greater quenching effect on the RB–KMnO4 CL system containing amine capped NPs (Fig. 2a). This is as a result of the high affinity of amine for the NA compounds leading to an increase in the probe’s sensitivity. Also, the acidity of TNT is stronger compared to the other NAs and it can interact with amine groups more powerfully. Therefore, TNT shows a superior quenching efficiency compared to the others (Fig. 3). Several reports have demonstrated the aforementioned great affinity of TNT for amine groups.23,26,27,30,44 In contrast, DNT and 2NT, as relatively weak Lewis acids, have weaker interactions with amines. Therefore, they weakly affect the CL intensity (Fig. 3). Nitrobenzene (NB), with a very low acidity, cannot alter the CL emission. Thus, the order of the magnitudes of the responses caused by the examined analytes is: TNT > DNT > 2NT ≫ NB. A comparison between the figures of merit for the detection of different NAs by the developed CL system is shown in Table 1.| Used NPs and NAs | EDA-capped AuNPs | ||
|---|---|---|---|
| TNT | DNT | 2NT | |
| Linear equation | ΔF = 6378.8C + 23.7 | ΔF = 942.3C + 25.6 | ΔF = 822.6C + 14.1 |
| Dynamic range (nmol L−1) | 0.27–20 | 15–280 | 24–380 |
| LOD (nmol L−1) | 0.045 | 6.8 | 10.4 |
| R 2 | 0.9996 | 0.9941 | 0.9941 |
Based on this observation, a highly sensitive CL assay was planned for the determination of TNT’s concentration. Fig. 5a shows a time profile for the developed CL sensor and its response to TNT. With the optimal parameters, the calibration graph was linear in the concentration range of 0.27–20 nmol L−1 with the equation ΔI = 6379C + 23.7 (R2 = 0.9996). ΔI(I0 − I) represents the difference between the CL intensity in the absence of (I0) and presence of (I) TNT, and C is the concentration of TNT (Fig. 5b).
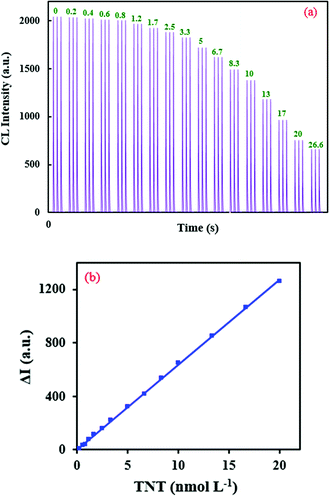 | ||
| Fig. 5 (a) CL profiles of the RB–KMnO4–AuNP system under optimum conditions with addition of different concentrations (nmol L−1) of TNT, and (b) the related calibration graph. | ||
The limit of detection was evaluated to be 0.045 nmol L−1. To study the method’s precision, the relative standard deviations (RSD%) were calculated for the determination of 0.4, 5 and 20 nmol L−1 TNT (n = 5) and found to be equal to 0.94, 1.24 and 0.06%, respectively. Therefore, this CL system shows adequate linearity, high sensitivity and appropriate precision. Compared to some other reported NP-based analytical systems, the planned technique shows relatively good features for the quantification of TNT (Table 2).
| Method | Sample | LOD (nmol L−1) | Linear range (nmol L−1) | Ref. |
|---|---|---|---|---|
| a Abbreviations: HQ: 8-hydroxyquinoline, FRET: fluorescence resonance energy transfer, QDs: quantum dots, SERS: surface-enhanced Raman spectroscopy, IMS: ion mobility spectrometry, MIP: molecularly imprinted polymers, RGO–PAMAM: electrochemically reduced graphene oxide–poly(amidoamine), ECL: electrochemiluminescence. | ||||
| LC-MS | Simulated aqueous samples | — | 25.5–1374 | 8 |
| Low temperature plasma desorption MS | — | 2.2 | — | 9 |
| CL immunoassay | Post blast residues | 880 | Up to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
16 |
| IMS | — | 31 | 88–440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
45 |
| Electrochemical | Water samples | 0.035 | 0.05–10 | 46 |
| HQ capped ZnS NPs, FRETa | — | 10 | 0–1890 | 26 |
| Creatinine–CdSe/ZnS QDs, fluorometric | Soil samples | 25 | 44–1320 | 23 |
| Amine capped AuNPs, colorimetric | — | 0.004 | 0.004–4 | 27 |
| AuNPs, spectrophotometric | Water samples | 0.027 | 0.08–1.2 | 31 |
| Graphene nanosheets/AgNPs, SERSa | Water samples | — | 0.01–104 | 32 |
| Silica NPs, fluorometric | — | 1000 | — | 25 |
| Dummy MIP-CdTe QDs, fluorometric | Soil samples | 280 | 80–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
47 |
| ERGO-PAMAM electrode, electrochemical | — | 7 | 220–5280 | 48 |
| ECL | — | 0.3 | 1–1000 | 49 |
| CL-ELISA | Spiked soils | 0.88–2.6 | — | 50 |
| Our method | Water samples | 0.045 | 0.27–20 | — |
Moreover, the selectivity of the developed CL probe was studied by testing the interference effects of some species that may be present in real samples. Certain quantities of these compounds were added to a TNT solution (2 nmol L−1). Tolerable concentration ratios for interference with a relative bias of <5% were above 3500 for Ca2+, Mg2+, and PO43−, 1500 for Fe2+, Ni2+, Zn2+, Cu2+, Fe3+, and SO42−, 500 for Co2+, Cd2+, Pb2+, As3+, Mn2+, acetone, acetonitrile, and ethanol and 250 for Hg2+. As is clear, most of these species have lower concentrations in water samples than the obtained tolerable amounts, so they wouldn't cause interference in the determination of TNT. Furthermore, investigations showed that species such as K+, Cl−, Na+ and NO3− have no interfering effect even at concentrations as high as 0.001 mol L−1.
The effects of some other explosives and TNT degradation products such as pentaerythritoltetranitrate (PETN), cyclomethylenetrinitramine (RDX) and 2-amino-4,6-dinitrotoluene were also investigated (Fig. S3, ESI†). No interferences with the assay in the analysis of TNT were detected.
3.6. Analysis of real samples
The proposed procedure was simple to exploit for the quantification of TNT in some spiked environmental water samples. The samples were filtered and diluted suitably, and then were used without any further preliminary steps. For the validation of the applied method, given amounts of a TNT standard solution were added into the samples prior to every preparation step, and then the TNT determination was performed according to the described approach in the Experimental section. As can be seen from the results (Table 3), acceptable recoveries were achieved.| Sample | Add (μmol L−1) | Founda (μmol L−1) | Recovery (%) | t-Statisticb |
|---|---|---|---|---|
| a Mean of three determinations ± standard deviation. b t-Critical = 3.18 for n = 3 and P = 0.05. | ||||
| River water (1) | 0 | ND | — | — |
| 0.20 | 0.198 ± 0.002 | 99.07 ± 0.96 | 1.71 | |
| 0.50 | 0.494 ± 0.003 | 98.89 ± 0.58 | 3.34 | |
| 2.00 | 1.9745 ± 0.015 | 98.77 ± 0.76 | 2.84 | |
| River water (2) | 0 | ND | — | — |
| 0.20 | 0.200 ± 0.007 | 99.71 ± 0.33 | 1.22 | |
| 0.50 | 0.497 ± 0.004 | 99.31 ± 0.83 | 1.45 | |
| 2.00 | 1.989 ± 0.007 | 99.42 ± 0.37 | 2.75 | |
4. Conclusions
A CL system of RB–alkaline KMnO4 enhanced by EDA-capped AuNPs was satisfactorily applied as a sensitive probe for the recognition of ultra-trace amounts of TNT in water samples. AuNPs can efficiently enhance the CL intensity of the alkaline RB–KMnO4 reaction, owing to their catalyzing effect on the reaction. Moreover, AuNPs can facilitate the energy transfer between RB molecules. The interaction of TNT with the amine groups on the surface of the AuNPs, followed by its quenching effect on the nano-assisted CL system are the basic reasons for the successful determination. The mentioned system can provide a reliable detection method for TNT in the range of 0.27–20 nmol L−1, and is more simple and rapid compared to common methods. The method is sensitive enough for the trace recognition of TNT in real samples. The selectivity of the developed CL probe for TNT is suitable and finally, it was applied for direct TNT determination in some environmental waters without pre-treatment. The recoveries were more than 98%.Acknowledgements
We thank the University of Tabriz for the support provided. We also acknowledge the support of the Iran Science Elites Federation.References
- J. S. Caygill, F. Davis and S. P. Higson, Talanta, 2012, 88, 14–29 CrossRef CAS PubMed.
- F. Fant, A. de Sloovere, K. Matthijsen, C. Marle, S. Fantroussi and W. Verstraete, Environmental pollution, Elsevier, Oxford, 2000 Search PubMed.
- A. W. Fountain, S. D. Christesen, R. P. Moon, J. A. Guicheteau and E. D. Emmons, Appl. Spectrosc., 2014, 68, 795–811 CrossRef CAS PubMed.
- B. J. Johnson, I. A. Leska, A. Medina, N. F. Dyson, M. Nasir, B. J. Melde, J. R. Taft and P. T. Charles, Sensors, 2012, 12, 14953–14967 CrossRef CAS PubMed.
- M. Mäkinen, M. Nousiainen and M. Sillanpää, Mass Spectrom. Rev., 2011, 30, 940–973 Search PubMed.
- Y. H. Lee, H. Liu, J. Y. Lee, S. H. Kim, S. K. Kim, J. L. Sessler, Y. Kim and J. S. Kim, Chem. – Eur. J., 2010, 16, 5895–5901 CrossRef CAS PubMed.
- D. S. Moore, Rev. Sci. Instrum., 2004, 75, 2499–2512 CrossRef CAS.
- L. Song and J. E. Bartmess, Rapid Commun. Mass Spectrom., 2009, 23, 77–84 CrossRef CAS PubMed.
- Y. Zhang, X. Ma, S. Zhang, C. Yang, Z. Ouyang and X. Zhang, Analyst, 2009, 134, 176–181 RSC.
- K. Wells and D. Bradley, Appl. Radiat. Isot., 2012, 70, 1729–1746 CrossRef CAS PubMed.
- R. G. Ewing, D. A. Atkinson, G. Eiceman and G. Ewing, Talanta, 2001, 54, 515–529 CrossRef CAS PubMed.
- E. G. Breijo, C. O. Pinatti, R. M. Peris, M. A. Fillol, R. Martínez-Máñez and J. S. Camino, Sens. Actuators, A, 2013, 192, 1–8 CrossRef CAS.
- J. L. Gottfried, F. C. De Lucia Jr, C. A. Munson and A. W. Miziolek, Anal. Bioanal. Chem., 2009, 395, 283–300 CrossRef CAS PubMed.
- M. R. Leahy-Hoppa, M. J. Fitch and R. Osiander, Anal. Bioanal. Chem., 2009, 395, 247–257 CrossRef CAS PubMed.
- M. S. Meaney and V. L. McGuffin, Anal. Bioanal. Chem., 2008, 391, 2557–2576 CrossRef CAS PubMed.
- M. Mirasoli, A. Buragina, L. S. Dolci, M. Guardigli, P. Simoni, A. Montoya, E. Maiolini, S. Girotti and A. Roda, Anal. Chim. Acta, 2012, 721, 167–172 CrossRef CAS PubMed.
- I. U. Mohammadzai, T. Ashiuchi, S. Tsukahara, Y. Okamoto and T. Fujiwara, J. Chin. Chem. Soc., 2005, 52, 1037–1042 CrossRef CAS.
- D. S. Moore and R. J. Scharff, Anal. Bioanal. Chem., 2009, 393, 1571–1578 CrossRef CAS PubMed.
- A. Khataee, R. Lotfi and A. Hasanzadeh, RSC Adv., 2015, 5, 82645–82653 RSC.
- A. Khataee, R. Lotfi, A. Hasanzadeh, M. Iranifam and S. W. Joo, Spectrochim. Acta, Part A, 2016, 154, 243–251 CrossRef CAS PubMed.
- A. Khataee, R. Lotfi, A. Hasanzadeh, M. Iranifam and S. W. Joo, Spectrochim. Acta, Part A, 2016, 157, 88–95 CrossRef CAS PubMed.
- M. Algarra, B. Campos, M. Miranda and J. C. E. da Silva, Talanta, 2011, 83, 1335–1340 CrossRef CAS PubMed.
- C. Carrillo-Carrión, B. M. Simonet and M. Valcárcel, Anal. Chim. Acta, 2013, 792, 93–100 CrossRef PubMed.
- S. S. Dasary, A. K. Singh, D. Senapati, H. Yu and P. C. Ray, J. Am. Chem. Soc., 2009, 131, 13806–13812 CrossRef CAS PubMed.
- J. Feng, Y. Li and M. Yang, Sens. Actuators, B, 2010, 145, 438–443 CrossRef CAS.
- L. Feng, C. Wang, Z. Ma and C. Lü, Dyes Pigm., 2013, 97, 84–91 CrossRef CAS.
- D. Lin, H. Liu, K. Qian, X. Zhou, L. Yang and J. Liu, Anal. Chim. Acta, 2012, 744, 92–98 CrossRef CAS PubMed.
- P. Marks, S. Cohen and M. Levine, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 4150–4155 CrossRef CAS.
- M. Riskin, R. Tel-Vered, O. Lioubashevski and I. Willner, J. Am. Chem. Soc., 2009, 131, 7368–7378 CrossRef CAS PubMed.
- R. Tu, B. Liu, Z. Wang, D. Gao, F. Wang, Q. Fang and Z. Zhang, Anal. Chem., 2008, 80, 3458–3465 CrossRef CAS PubMed.
- X.-D. Xia and H.-W. Huang, Chin. Chem. Lett., 2014, 25, 1271–1274 CrossRef CAS.
- M. Liu and W. Chen, Biosens. Bioelectron., 2013, 46, 68–73 CrossRef CAS PubMed.
- L. Chen, X. Wang, W. Lu, X. Wu and J. Li, Chem. Soc. Rev., 2016, 45, 2137–2211 RSC.
- T. Lou, Z. Chen, Y. Wang and L. Chen, ACS Appl. Mater. Interfaces, 2011, 3, 1568–1573 Search PubMed.
- S. Wang, Z. Chen, J. Choo and L. Chen, Anal. Bioanal. Chem., 2016, 408, 1015–1022 CrossRef CAS PubMed.
- M. Amjadi, J. Hassanzadeh and J. L. Manzoori, Microchim. Acta, 2014, 181, 1851–1856 CrossRef CAS.
- W. Zhou, Y. Cao, D. Sui and C. Lu, Angew. Chem., Int. Ed., 2016, 55, 4236–4241 CrossRef CAS PubMed.
- J. Hassanzadeh and M. Amjadi, Luminescence, 2015, 30, 439–443 CrossRef CAS PubMed.
- Y. M. Oskoei, N. Bagheri and J. Hassanzadeh, Microchim. Acta, 2015, 182, 1635–1642 CrossRef.
- Y. Ma, X. Jin, M. Zhou, Z. Zhang, X. Teng and H. Chen, Anal. Chim. Acta, 2003, 489, 173–181 CrossRef CAS.
- Y. Cao, D. Sui, W. Zhou and C. Lu, Sens. Actuators, B, 2016, 225, 600–606 CrossRef CAS.
- A. Üzer, E. Erçağ and R. Apak, Anal. Chim. Acta, 2005, 534, 307–317 CrossRef.
- L. Zhang, Y. Han, J. Zhu, Y. Zhai and S. Dong, Anal. Chem., 2015, 87, 2033–2036 CrossRef CAS PubMed.
- Q. Xiao, H. Gao, C. Lu and Q. Yuan, TrAC, Trends Anal. Chem., 2012, 40, 64–76 CrossRef CAS.
- T. Khayamian, M. Tabrizchi and M. Jafari, Talanta, 2003, 59, 327–333 CrossRef CAS PubMed.
- K. A. Mahmoud, A. Abdel-Wahab and M. Zourob, Water Sci. Technol., 2015, 72, 1780–1788 CrossRef CAS PubMed.
- S. Xu, H. Lu, J. Li, X. Song, A. Wang, L. Chen and S. Han, ACS Appl. Mater. Interfaces, 2013, 5, 8146–8154 Search PubMed.
- M. Shamsipur, M. A. Tabrizi, M. Mahkam and J. Aboudi, Electroanalysis, 2015, 27, 1466–1472 CrossRef CAS.
- W. Qi, M. Xu, L. Pang, Z. Liu, W. Zhang, S. Majeed and G. Xu, Chem. – Eur. J., 2014, 20, 4829–4835 CrossRef CAS PubMed.
- S. Girotti, S. Eremin, A. Montoya, M. Moreno, P. Caputo, M. D'Elia, L. Ripani, F. Romolo and E. Maiolini, Anal. Bioanal. Chem., 2010, 396, 687–695 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nj02324j |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |