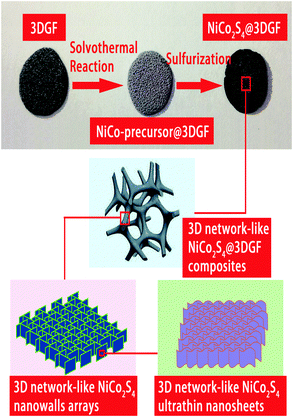
Graphene foam supported multilevel network-like NiCo2S4 nanoarchitectures for robust lithium storage and efficient ORR catalysis†
Xiaoyu
Wu
,
Songmei
Li
*,
Bo
Wang
,
Jianhua
Liu
and
Mei
Yu
Key Laboratory of Aerospace Advanced Materials and Performance of Ministry of Education, School of Materials Science and Engineering, Beihang University, Beijing 100191, China. E-mail: songmei_li@buaa.edu.cn; Fax: +86-10-82317103; Tel: +86-10-82317103
First published on 27th October 2016
Abstract
A well-designed NiCo2S4-based composite has been fabricated by growing a highly active NiCo2S4 nanoarchitecture on a three-dimensional graphene foam (3DGF) via a facile solvothermal method and performing a subsequent sulfurization reaction. The obtained NiCo2S4@3DGF composites show unique multilevel network-like structures, in which the 3DGF supports network-like building blocks of interconnected NiCo2S4 nanowalls, which are assembled with extensive interconnected ultrathin mesoporous nanosheets. Benefiting from this rational composition combining NiCo2S4 and 3DGF and the well-designed network-like morphology with a high surface area, the obtained composites exhibit excellent electrochemical activities. When used as a free-standing anode for lithium-ion batteries, the composite exhibits a high reversible capacity of 1295 mA h g−1, even after 150 cycles (at 500 mA g−1) and a remarkable rate capability. When used as a catalyst for the oxygen reduction reaction, the composite also exhibits enhanced catalytic activity (4-electron pathway), excellent tolerance to methanol, and high durability, holding great promise as an efficient non-noble-metal catalyst in practical application. In addition, a probable growth mechanism of the NiCo2S4 nanoarchitecture on the surface of 3DGF has been proposed.
Introduction
Nowadays, with the increasing global demand for energy and environmental protection, developing high-performance clean energy conventions and storage devices is vital for the sustainable development of modern society.1 Rechargeable lithium-ion batteries (LIBs) have attracted tremendous attention as the most promising power sources for electric vehicles (EVs), hybrid electric vehicles (HEVs), and portable electric devices due to their high theoretical capacity, long life span, lack of memory effect, and environmental friendliness.2,3 Although extensive efforts and significant advances have been made, further improvements on the electrode materials of LIBs to achieve excellent cycling stability, high reversible capacity and rate capability, as well as low cost, is still a great challenge to meet the increasing requirements of practical applications.4,5Meanwhile, fuel cells (FCs), as alternative typical energy devices, also have great potential as clean and efficient power sources for both EVs and portable electronics, due to their high conversion efficiency, low operation temperature, low or even zero emission, and high energy and power density.6,7 However, the sluggish kinetics of the cathodic oxygen reduction reaction (ORR) of FCs impede their commercialization. Although Pt-based materials have shown excellent ORR catalytic activity, their scarcity and high price hinder their commercial application.8,9 Therefore, it is urgently desired to exploit new low-cost non-noble-metal catalysts with comparable catalytic activities.
Recently, transition-metal chalcogenides (TMCs) have attracted extensive attention because of their high electrochemical activity, low cost, abundance, environmental compatibility, and wide-ranging potential applications, such as LIBs, optical sensors, optoelectronic devices, and oxygen catalysts.10–15 Amongst these TMCs, binary metal sulfides have received considerable attention due to their excellent electrical conductivity, mechanical and thermal stability, and higher electrochemical activities compared to their corresponding oxide counterparts.16 Especially, NiCo2S4 shows great potential as a high-performance anode material for LIBs due to its higher electrochemical activity compared to the monometal sulfides from richer redox chemistry, and its excellent electrical conductivity, which is at least two orders of magnitude higher than that of NiCo2O4 and ∼104 times higher than conventional monometal oxides.17,18 Unfortunately, the employment of NiCo2S4 in LIBs is largely hampered by sluggish ion/electron transport kinetics, a pronounced volumetric change, and severe particle aggregation associated with lithium ion insertion and deinsertion, resulting in an undesirable rate performance, rapid capacity decay, and poor cycling stability.16,19
To circumvent these issues, much effort has been focused on well-designed microstructures.20 Nano-sized structures with well-designed morphologies and high specific surface areas, especially ultrathin nanosheets assembled to form three-dimensional (3D) network-like architectures, could shorten the diffusion lengths of Li+, enlarging the electrode/electrolyte contact area, and thus accelerating the reaction kinetics of the electrochemical process, resulting in a high rate capability and an enhanced cycle performance.19,21,22 More recently, this has become a research hotspot to fabricate integrated, binder-free, and lightweight LIB electrodes by directly growing active materials on self-supported 3D porous conducting substrates.22,23 Frankly, compared to traditional binder-containing paste electrodes, without the use of any binder or conductive additives, the integrated free-standing electrodes could offer a larger electrode/electrolyte contact area and provide 3D interconnected network ion/electron pathways, resulting in efficient reaction kinetics during the charge/discharge process.18,24 Among the various conducting substrates to fabricate integrated electrodes, 3D graphene foams (3DGF) with continuously interconnected porous structures have attracted extensive interest because of their high electrical conductivity, high surface area, good flexibility, and fast mass and electron transport kinetics.22,25,26 Based on the consideration above, the 3DGF-supported network-like NiCo2S4 architectures are expected to be promising alternative anodes for high-performance LIBs.
On the other hand, the excellent electrochemical activity, high electrical conductivity, and good mechanical and thermal stability of NiCo2S4 also give it excellent potential as a high-performance catalyst for the ORR.27 The well-designed nano-sized structures with high surface areas are also of great significance for improving catalytic activity, which can facilitate the diffusion of active species, increase the surface exposure of the active sites, accelerate the surface reaction, and provide pathways for efficient mass and charge transport during the ORR procedure.8,28 Meanwhile, the further improvement in electrical conductivity resulting from the incorporation with 3DGF is also beneficial for an enhanced catalytic performance.29 Therefore, the 3DGF-supported network-like NiCo2S4 architectures are also expected to be promising catalysts for an efficient ORR.
Herein, well-designed NiCo2S4 nanoarchitectures supported on 3DGF have been developed via a facile solvothermal reaction and subsequent sulfurization. The obtained NiCo2S4@3DGF composites exhibit unique multilevel 3D network-like structures, in which network-like building blocks of NiCo2S4 nanowall arrays, assembled with extensive interconnected ultrathin mesoporous nanosheets, are supported on 3DGF substrates. Benefiting from the rational composition from the combination of NiCo2S4 with 3DGF, and the well-designed network-like morphologies with high surface areas, the obtained NiCo2S4@3DGF composites exhibit excellent lithium storage performances and enhanced catalytic activities when serving as integrated anodes for LIBs and non-noble-metal catalysts for the ORR, respectively.
Experimental
Preparation of NiCo2S4@3DGF composites
The 3DGF was synthesized via a chemical vapor deposition (CVD) method as reported previously, in which 3D nickel foams (NFs) with a porosity of ∼97% were used as templates for the growth of graphene.22 To prepare NiCo2S4@3DGF composites, 0.145 g of Ni(NO3)2·6H2O, 0.296 g of Co(NO3)2·6H2O, and 1.12 g of methenamine were dissolved in a solution of 35 mL of methanol with continous stirring. After adding four pieces of 3DGF disks (12 mm in diameter), the solution system was transferred into a 50 mL Teflon-lined stainless steel autoclave and kept at 180 °C for 12 h. After solvothermal reaction, the obtained 3DGF-supported NiCo-precursors were carefully rinsed several times with deionized water and ethanol, and dried at 60 °C overnight. After that, the NiCo-precursor/3DGF composites were immersed in 0.1 M Na2S solution and kept at 160 °C for 8 h in a 50 mL autoclave. After cooling down naturally to room temperature, the as-prepared NiCo2S4/3DGF composites were taken out and washed with deionized water and ethanol several times, then dried at 60 °C for 12 h. For comparison, the corresponding NiCo2S4 powders were also prepared by a similar method without the use of 3DGF substrates.Materials characterization
The crystallographic structures of the products were characterized by X-ray diffraction (XRD, Rigaku D/max-2200PC) in the diffraction angle range 10–80° (Cu Kα, λ = 0.15418 nm). A field-emission scanning electron microscope (FE-SEM, JEOL JSM-7500F) equipped with an energy dispersive X-ray spectrometer (EDS), and a transmission electron microscope (TEM, JEOL JEM-2100F), were used to characterize the morphology, chemical composition and microstructure of the products. Thermogravimetric analysis (TGA) was performed in a static air atmosphere with a heating rate of 10 °C min−1, using a Pyris Diamond TG/DTA instrument. Raman measurements were performed on a Renishaw spectrometer at 532 nm with a Renishaw Microscope System RM2000. The N2 adsorption/desorption was determined by Brunauer–Emmett–Teller (BET) measurements with an ASAP-2010 surface area analyzer. An X-ray photoelectron spectrometer (XPS, AXIS ULTRA DLD equipped with an Mg Kα–Al Kα anode for photoexcitation) was used to analyze the chemical compositions of the products.Electrochemical measurements
CR2025 coin-type cells were assembled in an argon-filled glove box at room temperature to investigate the lithium storage performance of the products. The obtained NiCo2S4@3DGF composites were directly used as working electrodes with no conductive additives or polymer binders; metallic lithium foil served as both counter electrode and reference electrode, polypropylene microporous film (Celgard 2400) was used as the separator, and a solution of 1 M LiPF6 dissolved in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) was used as electrolyte. The specific mass loading of the active material (NiCo2S4) for the testing electrodes (∼1.25 mg cm−2) was calculated from the average weight of 10 pieces of the samples. For comparison, the NiCo2S4 powders were also formed into working electrodes using a traditional slurry-paste method. The corresponding paste electrodes were prepared by coating N-methylpyrrolidinone (NMP) slurry composed of 80 wt% active materials, 10 wt% polyvinylidene fluoride, and 10 wt% acetylene black on steel foil, and dried in a vacuum oven at 45 °C overnight. Cyclic voltammetry (CV) and galvanostatic charge–discharge measurements were conducted at room temperature using a LAND CT2001A battery-testing instrument and a multichannel Arbin BT2000 system in the voltage range 0.005–3.0 V (vs. Li+/Li). Electrochemical impedance spectroscopy (EIS) measurements were performed using an electrochemistry system (Parstat 2273, Princeton Applied Research, USA) with an AC voltage amplitude of 5 mV in the frequency range from 10 mHz to 100 kHz.
1 (v/v) mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) was used as electrolyte. The specific mass loading of the active material (NiCo2S4) for the testing electrodes (∼1.25 mg cm−2) was calculated from the average weight of 10 pieces of the samples. For comparison, the NiCo2S4 powders were also formed into working electrodes using a traditional slurry-paste method. The corresponding paste electrodes were prepared by coating N-methylpyrrolidinone (NMP) slurry composed of 80 wt% active materials, 10 wt% polyvinylidene fluoride, and 10 wt% acetylene black on steel foil, and dried in a vacuum oven at 45 °C overnight. Cyclic voltammetry (CV) and galvanostatic charge–discharge measurements were conducted at room temperature using a LAND CT2001A battery-testing instrument and a multichannel Arbin BT2000 system in the voltage range 0.005–3.0 V (vs. Li+/Li). Electrochemical impedance spectroscopy (EIS) measurements were performed using an electrochemistry system (Parstat 2273, Princeton Applied Research, USA) with an AC voltage amplitude of 5 mV in the frequency range from 10 mHz to 100 kHz.
ORR catalytic performance measurements were performed in a conventional three-electrode electrochemical cell using a PARSTAT 2273 electrochemistry system (Princeton Applied Research, USA) at room temperature. A glassy carbon rotating disk electrode (RDE) with a diameter of 5 mm after loading the catalysts was used as the working electrode. A graphite rod was used as the counter electrode, an Ag/AgCl electrode was selected as the reference electrode, and 0.1 M KOH aqueous solution was used as electrolyte. It should be noted that all potential values mentioned in this work are given against the reference. To prepare the working electrode, 2 mg of obtained catalysts were ultrasonically dispersed into a mixed solvent containing 0.75 mL of deionized water, 0.25 mL of absolute ethanol, and 20 µL of Nafion solution (5 wt%) for 30 min to obtain a homogeneous ink. Then, 10 µL of the catalyst ink was dropped on the surface of a pre-polished glassy carbon RDE with a microsyringe and dried at room temperature. The final loading amount of the as-prepared catalyst was 99.80 µg cm−2. For comparison, a commercially available Pt/C (20 wt% Pt loading) catalyst was used and deposited on the surface of a RDE in the same manner to yield a catalyst loading of 19.96 µgPt cm−2. Cyclic voltammetry (CV) experiments were conducted in N2 or O2-saturated 0.1 M KOH electrolyte at a scanning rate of 50 mV s−1 in the voltage range 0.2 to −1.2 V (vs. Ag/AgCl). Linear sweep voltammetry (LSV) was performed using the RDE technique at a scanning rate of 5 mV s−1 under different electrode rotation rates (100, 400, 900, 1600, 2500, and 3600 rpm). Chronoamperometry tests were conducted in O2-saturated 0.1 M KOH at −0.8 V with a rotation rate of 1600 rpm.
Results and discussion
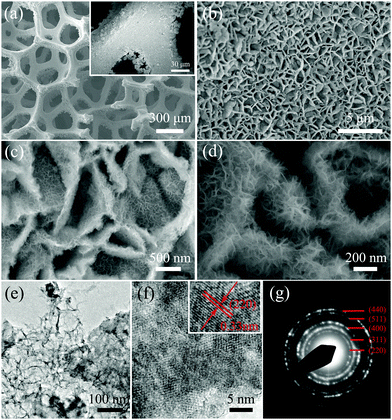
The preparation process of the multilevel network-like NiCo2S4@3DGF composites is illustrated in Scheme 1. Typically, the NiCo2S4@3DGF composites are synthesized via a facile two-step method, involving the solvothermal growth of NiCo-precursors on 3DGF substrates and a subsequent sulfurization reaction for the conversion from NiCo-precursors into NiCo2S4. In the solvothermal reaction stage, the CVD-synthesized 3DGF substrates were immersed into an Ni2+/Co2+-containing methanol solution, and then the pink NiCo-precursors were uniformly grown on the skeletons of 3DGF substrates. During the process of sulfurization, based on the Kirkendall effect, the NiCo-precursors were sulfurized using Na2S as a sulfur source to obtain the black NiCo2S4@3DGF composites.30,31 The obvious color change during the procedure and the final uniform color of the composites indicate the uniform coating of the active materials on the 3DGF substrate, as shown in the above optical image of Scheme 1. And, as illustrated in Scheme 1, the obtained NiCo2S4@3DGF composites exhibit unique multilevel 3D network-like structures, in which the network-like NiCo2S4 nanowall arrays assembled with extensive interconnected ultrathin nanosheets are supported on 3DGF substrates.To clarify the morphologies and microstructure of the 3DGF substrate and NiCo2S4@3DGF composites in detail, the samples were investigated by scanning electron microscopy (SEM). As shown in Fig. S1(a) (ESI†), the obtained graphene foam shows a porous interpenetrating 3D network-like structure, consisting of interconnected flexible graphene skeletons as fast transport channels of charge carriers for high electrical conductivity. Fig. 1(a–d) presents typical SEM images of the NiCo2S4@3DGF composites at different magnifications. As shown in Fig. 1(a), the NiCo2S4 architectures are grown on the whole 3DGF skeleton completely without spalling, fabricating 3D network-like NiCo2S4@3DGF composites. And, as seen in the inset of Fig. 1(a), numerous nanowalls uniformly cover the whole surface of the 3DGF skeletons, demonstrating the strong adhesion between NiCo2S4 and the 3DGF surface. As better revealed by the magnified SEM image in Fig. 1(b), the 3DGF-supported NiCo2S4 nanowalls form well-developed interconnected porous network-like arrays standing upright on the flexible graphene skeletons. In comparison, without the support of the 3DGF substrates, it is found that only flower-like NiCo2S4 microspheres composed of aggregated thick sheets are obtained under the same conditions (Fig. S2, ESI†). The building-block NiCo2S4 nanowalls of the composites are 100–200 nm in thickness and about 1–2 µm in lateral size, and are assembled from extensive interconnected ultrathin nanosheets, as shown in Fig. 1(c and d). Clearly, the nanosheets are curved with a lateral size of ∼100 nm, and randomly connected with adjacent nanosheets to form continuous and interconnected porous network-like architectures, thereby forming a large number of voids and electroactive sites.22 And, due to the lateral size being much larger than the thickness, the ultrathin nanosheets show bent, curled and crumpled morphologies. Such a unique multilevel 3D network with a conductive graphene support holds great promise in providing efficient electron/ion transportation pathways and offering a sufficient specific surface area to accelerate electrochemical reactions, thus delivering an excellent electrochemical performance.32,33
Transmission electron microscopy (TEM) measurements were conducted to provide further insight into the unique morphology and structure of the as-prepared NiCo2S4 nanoarchitectures and the 3DGF substrate, and the results are presented in Fig. 1(e–g) and Fig. S1(b) (ESI†), respectively. In order to conduct TEM measurements, the obtained products were dispersed in ethanol under intense ultrasonication for 30 min, and the NiCo2S4 nanoarchitectures were detached from 3DGF, while the 3DGF was scattered into pieces, and then dropped onto a 200-mesh TEM grid, followed by drying in air. As shown in Fig. S1(b) (ESI†), the TEM image of 3DGF after ultrasonication shows several interconnected transparent thin sheets with obvious wrinkles, indicating that the 3DGF is built of stacked graphene films. In addition, Raman scattering was conducted to further characterize the electronic structure of 3DGF. As shown in Fig. S3 (ESI†), both the D band around 1360 cm−1 and the G band around 1580 cm−1 are present. In contrast to the low intensity of the D band in traditional carbonaceous materials reported previously, the 3DGF exhibits a high ID/IG ratio, which can be ascribed to the presence of graphene edges and defects in the disordered graphitic lattice, and further confirms the existence of graphene.34,35 As shown in the low-magnification TEM image of the NiCo2S4 nanosheets scratched from the 3DGF substrate (Fig. 1(e)), the network-like nanostructures built up of interconnected nanosheets can be observed clearly, which is in good agreement with the SEM results. And the transparent features of the nanosheets indicate their ultrathin quality. As shown in the high-resolution TEM (HRTEM) image in Fig. 1(f), several obvious mesopores (about 2–5 nm in size) are uniformly distributed on the nanosheets, indicating the mesoporous structures of the nanosheets. The formation of the mesopores may be ascribed to the continuous outward ion diffusion during the sulfurization reaction, which can provide sufficient free space to further ameliorate the tendency for expansion during the lithium insertion and extraction process of LIBs, and allow efficient transport of reactant species for the ORR.19,28,31,36 In addition, the inset HRTEM image in Fig. 1(f) reveals lattice fringes with a lattice spacing of 0.33 nm, corresponding to the (220) crystal planes of cubic NiCo2S4.37 And the diffraction rings in the selected area electron diffraction (SAED) pattern (Fig. 1(g)) can be well indexed to the (220), (311), (400), (511), and (440) planes of the NiCo2S4 crystal structure (JCPDF No. 43-1477), revealing the polycrystalline nature of the NiCo2S4 nanosheets.37,38
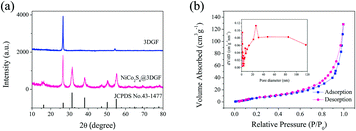
The XRD pattern of the obtained 3DGF and NiCo2S4@3DGF composites, and the standard XRD pattern of cubic NiCo2S4, are presented in Fig. 2(a). Obviously, the absence of Ni diffraction peaks indicates the complete dissolution of the NFs in the obtained 3DGF. And the only two typical diffraction peaks at 26.5° and 54.6° in the XRD patterns of 3DGF are attributed to the (002) and (004) reflections of graphitic carbon, respectively, in agreement with its standard XRD pattern (JCPDS card No. 75-1621).22,39 The sharp diffraction peaks confirm the highly crystalline structure of 3DGF, which is beneficial for efficient electron transfer and ion diffusion.22 As for the XRD patterns of the NiCo2S4@3DGF composites, except for the characteristic peaks from 3DGF, the diffraction peaks at 15.4°, 26.8°, 31.6°, 38.3°, 50.5°, and 55.3° can be well indexed to the (111), (220), (311), (400), (511), and (440) planes of the cubic NiCo2S4 phase, respectively, in agreement with the standard XRD pattern of cubic NiCo2S4 (JCPDS No. 43-1477).37,38 And the composition of the composites is further confirmed by EDX analysis under N2 atmosphere. As shown in Fig. S4 (ESI†), as well as the O peak arising from exposure to the air, the C, S, Ni, and Co peaks are observed in the EDX spectrum of the as-prepared composites, suggesting that the product is mainly composed of C, S, Ni, and Co. And the Ni/Co atomic ratio of the composite is determined to be around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.90, which is close to the stoichiometric composition of NiCo2S4. According to the TGA results (Fig. S5, ESI†), the as-prepared composites contain about 89.4 wt% graphene and 10.6 wt% NiCo2S4.
1.90, which is close to the stoichiometric composition of NiCo2S4. According to the TGA results (Fig. S5, ESI†), the as-prepared composites contain about 89.4 wt% graphene and 10.6 wt% NiCo2S4.
Full nitrogen adsorption and desorption isotherms of the NiCo2S4@3DGF composites were obtained to reveal their specific surface area and pore size distribution. As shown in Fig. 2(b), a type-IV isotherm with a distinct type-H3 hysteresis loop in the relative pressure range of 0.5–1.0 P/P0 can be observed, indicating the presence of mesoporous structures in the composites, which is consistent with the TEM results.40 Accordingly, as calculated from the adsorption data in the relative pressure (P/P0) range of 0.05–0.30, the NiCo2S4@3DGF composites possess a high Brunauer–Emmett–Teller (BET) specific surface area of 97.32 m2 g−1. Meanwhile, the Barrett–Joyner–Halenda (BJH) pore size distribution further confirms the mesoporous and macroporous features of the obtained NiCo2S4@3DGF composites. As shown in the inset of Fig. 2(b), sharp peaks in the pore size range 2–5 nm can be seen clearly, which is attributed to the existence of mesopores in the NiCo2S4 nanosheets, while the sharp peak at ∼30 nm and broad peak in the range 40–80 nm correspond to the mesopores and macropores from the interconnected NiCo2S4 nanosheets, respectively. This high specific surface area of the NiCo2S4@3DGF composites, with extensive mesopores and macropores, can facilitate the electron/ion transportation efficiently, buffer the volume change during charge/discharge, and increase the surface exposure of the active sites, thus improving the electrochemical activities of the composites.8,19,31,36
X-ray photoelectron spectroscopy (XPS) measurements were conducted to further identify the elemental composition and chemical state of the obtained NiCo2S4@3DGF composites, and the results are given in Fig. 3. As shown in the survey spectrum (Fig. 3(a)), the observed peaks corresponding to Ni 2p, Co 2p, S 2p, and C 1s confirm the presence of Ni, Co, S, and C elements in the NiCo2S4@3DGF composites, while the presence of the O element is due to exposure to the air. Using the Gaussian fitting method, the Ni 2p and Co 2p spectra can both be well fitted with two spin–orbit doublets and two shake-up satellites. As shown in Fig. 3(b), the two main peaks of the Ni 2p spectrum located at 856.2 and 873.4 eV are associated with the Ni 2p3/2 and 2p1/2 electronic configurations, respectively.41,42 The spin–orbit splitting values of Ni 2p3/2 and 2p1/2 are over 15 eV, suggesting that these are in the divalent and trivalent states.42 And the Co 2p spectrum (Fig. 3(c)) exhibits two main peaks at 779.2 and 794.3 eV, which correspond to the low energy band of Co 2p3/2 and the high energy band of Co 2p1/2, respectively, indicating the coexistence of Co2+ and Co3+.42,43 Meanwhile, the core-level spectrum of the S 2p region (Fig. 3(d)) can be divided into two main peaks at about 162.2 and 163.5 eV, and one shake-up satellite at around 168.9 eV. As reported previously, the component at ∼162.2 eV is characteristic of S2−, while the peak at ∼163.5 eV can be attributed to the sulfur ion in low coordination at the surface.42,44,45 These results indicate that the chemical composition of NiCo2S4 in the composites contains Co2+, Co3+, Ni2+, Ni3+, and S2.
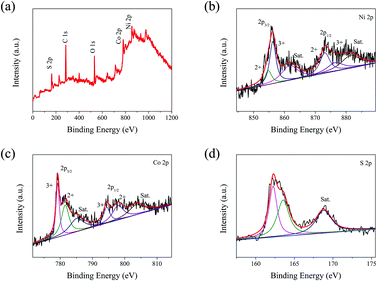 | ||
| Fig. 3 (a) XPS survey spectra and high-resolution XPS spectra of (b) Ni 2p, (c) Co 2p, and (d) S 2p for the NiCo2S4@3DGF composites. | ||
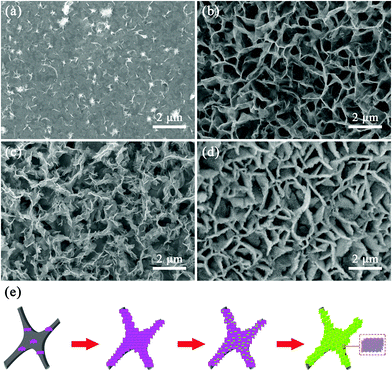
To explore the formation process and reveal the corresponding mechanism of the NiCo2S4 architectures supported on the 3DGF substrate, a series of time-dependent experiments with different solvothermal reaction times were conducted, with the same subsequent sulfurization treatment. It should be noted that the time-dependent experiments were carried out during the first solvothermal process, because we found that the basic multilevel network-like structures of the NiCo2S4@3DGF composites were formed during the first solvothermal process, according to the SEM results shown in Fig. S6 (ESI†). Fig. 4 shows the SEM images of the products obtained with different reaction times (1, 3, 6, and 12 h). Obviously, there were no architectures formed on the surface of 3DGF before the reactions, as confirmed in Fig. S2 (ESI†). As shown in Fig. 4(a), after solvothermal reaction for 1 h and the subsequent sulfurization, some small aggregated NiCo2S4 nanosheets appeared on the surface of 3DGF. When the reaction time was set as 3 h, the well-distributed interconnected NiCo2S4 nanosheet arrays were formed vertically on the surface of 3DGF (Fig. 4(b)). And when the reaction time was further prolonged to 6 h, the secondary structure of ultrathin nanosheets began to emerge on the surface of the initial NiCo2S4 nanosheets (Fig. 4(c)). Along with the increasing reaction time, the small ultrathin nanosheets gradually increased in size, resulting in the formation of more void spaces between each nanosheet and higher surface areas. Finally, the 3DGF surface was uniformly covered by extensive interconnected ultrathin nanosheets, forming NiCo2S4 nanowall arrays after 12 h, forming a multilevel network-like structure (Fig. 4(d)). Based on these experimental observations, a possible growth mechanism of the multilevel network-like NiCo2S4 nanoarchitectures on 3DGF has been proposed, and the results are illustrated in Fig. 4(e).46 For convenience of expression, we chose to illustrate the formation process of the NiCo-precursor instead of, for example, the final product of NiCo2S4 after sulfurization. Firstly, many nanoparticles appeared to aggregate on the surface of 3DGF, which corresponds to the nucleation process of numerous NiCo-precursor (Ni2/3Co4/3(CO3)(OH)2) nucleation centers arising from the combination of Ni2+, Co2+ and CO32−, OH− ions from the hydrolysis of raw materials.41,47,48 After nucleation, the derived nucleation centers grew to small NiCo-precursor nanosheets, and gradually grew into larger and thicker nanosheets, forming network-like nanosheet arrays, in which the crystal growth stage transferred to a kinetically controlled process.46 With the gradual hydrolysis of raw materials, new secondary nucleation centers emerged on the surface of the nanosheet arrays and gradually grew into ultrathin nanosheets. Finally, the extensive ultrathin nanosheets interconnected with each other and assembled into network-like porous architectures, and were densely distributed on the surface of the larger and thicker nanosheet arrays, forming interconnected nanowalled building blocks uniformly supported on 3DGF. In addition, considering the gradual establishment of the well-designed NiCo2S4@3DGF nanoarchitectures, it is possible to control the architectures of NiCo2S4 by controlling the solvothermal reaction time.
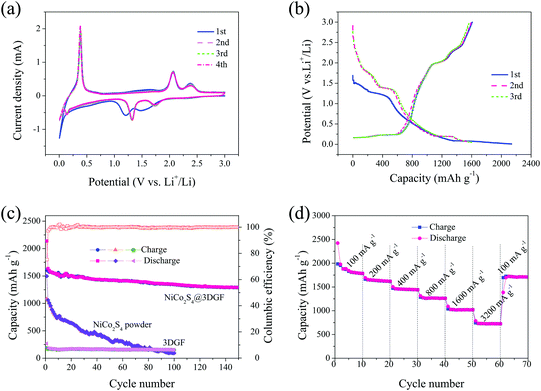
Due to the unique multilevel 3D network-like nanoarchitectures with favorable mesoporous features and high specific surface areas, as well as the rational incorporation of NiCo2S4 with 3DGF, the obtained NiCo2S4@3DGF composites are expected to act as high-performance integrated free-standing anodes for LIBs. The lithium storage performance of the composites as anodes for LIBs was evaluated directly without binder or conductive additives. As a control experiment for comparison, the electrochemical performances of traditional slurry-coated electrodes of pure NiCo2S4 powders were also evaluated, and the results are all presented in Fig. 5. Cyclic voltammetry (CV) measurements were performed to identify the electrochemical reactions occurring in the electrodes during the lithiation/delithiation process. Fig. 5(a) presents the initial four consecutive CV curves for the NiCo2S4@3DGF electrode at a scan rate of 0.1 mV s−1 in the voltage range 0.005–3.0 V (vs. Li+/Li). It is clear that the CV curve of the first cycle is significantly different from that of the subsequent cycles. In the first cathodic scan, two obvious broad reduction peaks, located at around 1.20 V and 1.48 V, are observed, which can be attributed to the Li+ insertion into NiCo2S4 to form LixNiCo2S4, as well as the decomposition of the organic electrolyte to form a solid–electrolyte interphase (SEI) layer at the electrode/electrolyte interphase.49–51 During the first anodic scan, the broad peaks at about 2.38 V and 2.05 V could be ascribed to reverse extraction of Li+, resulting in the formation of NiSx and CoSx.19 Meanwhile, the intense cathodic and anodic peaks in the potential range 0.005–0.38 V correspond to the insertion and extraction of Li+ into 3DGF, respectively, which is also electroactive for lithium storage.52 Due to the occurrence of certain irreversible reactions associated with the formation of a SEI layer on the electrode surface, the main cathodic peaks at about 1.20 and 1.48 V shift toward higher potentials of 1.32 and 1.74 V in the second and following cycles, which correspond to the reversible reductive reaction of NiSx and CoSx to Ni and Co metal, respectively.19,53 Obviously, the CV curves of the second and subsequent cycles are well-overlapped, indicating the good reversibility of the electrochemical reactions. These results indicate that the good electrochemical reversibility of the NiCo2S4@3DGF electrode is gradually built after the first cycle.19,22
Fig. 5(b) shows the typical galvanostatic charge–discharge profiles of the NiCo2S4@3DGF electrode at a current density of 500 mA g−1 in the initial three cycles between 0.005 and 3.0 V (vs. Li+/Li). In agreement with the CV curves, two voltage plateaus at around 1.48 V and 1.20 V can be observed in the discharge process of the first cycle, corresponding to Li+ insertion into NiCo2S4 accompanying the formation of the SEI film.49–51 As shown in Fig. 5(b), the NiCo2S4@3DGF electrode delivered high initial discharge and charge capacities of 2137 and 1609 mA h g−1, respectively, giving a Coulombic efficiency of 75.29%. As reported previously, the pure 3DGF exhibits a quite low capacity, thus makes little contribution to the total specific capacity of the composite electrode.22 Therefore, the high initial lithium storage capacity of the composites might be attributed to the unique multilevel 3D network-like architectures assembled from ultrathin mesoporous nanosheets, and the synergistic effect from rational combination of NiCo2S4 with 3DGF. And the irreversible capacity loss of around 600 mA h g−1 is mainly due to the inevitable electrolyte decomposition and SEI formation in the first cycle, as confirmed in the above CV results.54 In the second cycle, the discharge and charge capacities of the NiCo2S4@3DGF electrode are 1635 and 1591 mA h g−1, respectively, with the Coulombic efficiency rapidly rising to 97.27%. And the potential plateaus at 1.74 V and 1.32 V in the discharge profile of the second cycle are also in agreement with the two cathodic peaks of the CV curves in Fig. 5(a). Moreover, the second and third discharge profiles almost coincide with each other, suggesting an excellent cycling performance. Fig. 5(c) compares the cycling performances of the NiCo2S4@3DGF electrode, the NiCo2S4 powder-paste electrode and the pure 3DGF electrode. Noticeably, the capacities of the pure 3DGF electrodes are much lower than those of the NiCo2S4/3DGF electrodes, indicating that the 3DGF substrates contribute little to the capacity of the composite electrode. As shown in Fig. 5(c), after 50 cycles, the discharge capacities of the NiCo2S4@3DGF electrode are still maintained at 1424 mA h g−1. Even after 150 cycles, the reversible capacity of the NiCo2S4@3DGF electrode remains as high as 1292 mA h g−1, corresponding to a capacity retention ratio of 79.02% with respect to the second discharge specific capacity. It is noteworthy that the reversible capacity of the NiCo2S4@3DGF electrode is much higher than that of the previously reported MSs electrodes,3,55,56 and superior to the reported 3D-networked NiCo2S4 nanosheet array/carbon cloth anodes (∼1137 mA h g−1 after 100 cycles)19 and nanowire interwoven NiCo2S4 nanowall array anodes (∼713 mA h g−1 after 100 cycles).57 These results are in stark contrast to those for the NiCo2S4 powder-paste electrode (Fig. 5(c)), which show continuous and progressive capacity fading along with cycling under the same testing conditions, only retaining a discharge capacity of ∼100 mA h g−1 after 100 cycles. And, as shown in Fig. 5(c), the Coulombic efficiency of the NiCo2S4@3DGF electrode increases rapidly from 75.29% to nearly 100%, indicating that a stable SEI layer has already formed during the initial cycles.19 In addition, the morphology and structure of the 3D network-like NiCo2S4 retained almost intact after 150 cycles (Fig. S7, ESI†), which demonstrated the structural stability of as-obtained NiCo2S4 nanoarchitectures and the strong adhesion of NiCo2S4 on the graphene foam.
Moreover, the NiCo2S4@3DGF composites also show remarkable rate capability, which was examined at different current densities ranging from 100 mA g−1 to 3200 mA g−1. As presented in Fig. 5(d), the discharge capacities remain stable and decrease regularly with increasing rate, indicating good kinetic features and a facile charge transport process for the NiCo2S4@3DGF composites. The average discharge capacity of the NiCo2S4@3DGF electrode decreases from 1899 to 1641, 1456, 1269, and 1027 mA h g−1 when the current density is increased from 100 to 200, 400, 800, and 1600 mA g−1, respectively. Even when cycled at a high current of 3200 mA g−1, an average capacity of ∼733 mA h g−1 can be maintained. Notably, after deep cycling at 3200 mA g−1, the specific capacity is recovered to almost the same levels as the previous measurements when the testing current is returned to 100 mA g−1, manifesting excellent high-rate lithium storage performance and good reversibility. And as shown in Fig. S8 (ESI†), the charge–discharge profiles at various current densities in the rate capability tests almost maintain the same shapes, with stable voltage plateaus in agreement with the CV curves, further indicating the excellent high-rate lithium storage performance of the composite electrodes. Therefore, the obtained NiCo2S4@3DGF composites exhibit a remarkable lithium storage performance with high reversible capacity, good cycling stability, and excellent rate capability, holding great promise for application as free-standing integrated anodes for LIBs. Additionally, EIS measurements were performed to further clarify the superior electrochemical performance of the NiCo2S4@3DGF composites. As shown in the obtained Nyquist plots in Fig. S9 (ESI†), the diameter of the semicircle for the NiCo2S4@3DGF electrode in the high-middle frequency region is smaller than that of the NiCo2S4 powder-paste electrode, indicating that the composite electrodes possess lower contact and electron-transfer resistances.50,58 Meanwhile, the more vertical line in the low-frequency region for the NiCo2S4@3DGF electrode, compared to the NiCo2S4 powder-paste electrode, indicates the faster Li+ diffusion behavior of the composites, thereby resulting in better electrode reaction kinetics during the charge/discharge process and superior cycling performance.59
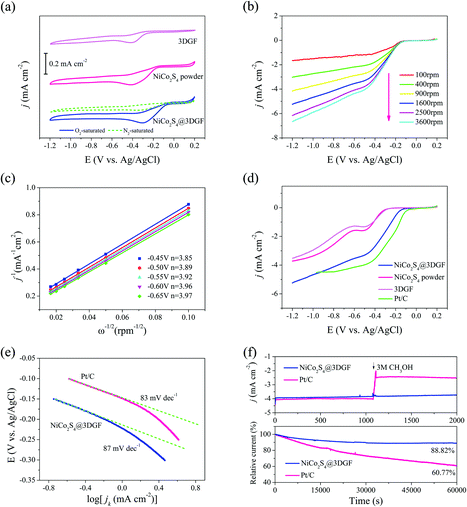
Benefiting from the high conductivity of graphene foam, and the high surface area and mesoporous features of the unique multilevel 3D network-like structure, the obtained NiCo2S4@3DGF composites hold great promise as non-noble-metal high-activity catalysts for efficient ORRs. The ORR catalytic activities of the obtained composites were confirmed using cyclic voltammetry (CV) measurements in a conventional three-electrode system with N2 or O2-saturated 0.1 M KOH aqueous electrolyte at a scan rate of 50 mV s−1, and the results are presented in Fig. 6(a). The CV curves of pure 3DGF and NiCo2S4 powder were investigated for comparison. As shown in Fig. 6(a), no obvious peak appears in the potential range 0.2 to −1.2 V for the NiCo2S4@3DGF in N2-saturated 0.1 M KOH solution. In stark contrast, prominent cathodic peaks centered at about −0.29 V are observed in O2-saturated electrolyte solution, which is attributed to the electrocatalytic reduction of oxygen on the electrode in alkaline media, indicating that the composites exhibit catalytic activity toward the ORR.9 Although the CV curves of the pure 3DGF and NiCo2S4 powder have exhibited a visible ORR peak as expected, the peak potentials of the composites are more positive than those of the pure 3DGF (−0.41 V) and NiCo2S4 powders (−0.40 V), indicating the improved catalytic activities of the composites arising from the synergistic effect between 3DGF and NiCo2S4, and the well-designed microstructures.
Linear sweep voltammetry (LSV) measurements were performed using a RDE in O2-saturated 0.1 M KOH with a scan rate of 5 mV s−1 to further reveal the catalytic performance and ORR kinetics of the samples. Typical LSV curves of NiCo2S4@3DGF composites at various rotating speeds from 100 to 3600 rpm were recorded in Fig. 6(b). Obviously, the current density increases gradually as the rotation rates increase due to the faster oxygen flux to the electrode surface.60–62 Meanwhile, the corresponding Koutecky–Levich (K–L) plots (j−1vs. ω−1/2) derived from the LSV curves of the NiCo2S4@3DGF composites are presented in Fig. 6(c). As shown in Fig. 6(c), the K–L plots at various potentials for the NiCo2S4@3DGF composites exhibit good linearity and parallelism, indicating first-order reaction kinetics for the ORR with respect to the concentration of dissolved oxygen, and a similar electron transfer number for the ORR at different potentials.10,28,60 According to the K–L equations, the transferred electron numbers n of the NiCo2S4@3DGF composites in the potential range −0.45 to −0.65 V were calculated to be in the range 3.85–3.97, indicating that the composites favor a four-electron oxygen reduction pathway.28
Fig. 6(d) compares LSV curves of the pure 3DGF, NiCo2S4 powder, NiCo2S4@3DGF composites, and commercial Pt/C (20 wt%) at a rotation rate of 1600 rpm. It is clear that the polarization curves of the NiCo2S4@3DGF composites exhibit the characteristic S-shape of RDE measurements, with no defined diffusion plateau.60 As shown in Fig. 6(d), the NiCo2S4@3DGF composites possess an onset potential of −0.11 V, which is much more positive of that of the pure 3DGF (−0.27 V) and NiCo2S4 powder (−0.24 V). Meanwhile, the composites exhibit the highest current density of −5.25 mA cm−2 at −1.2 V among the obtained three samples. These results further illustrated that the ORR activities of the NiCo2S4@3DGF composites are significantly improved due to the synergistic effects between NiCo2S4 and 3DGF, and the well-designed microstructures with efficient electron/ion transportation pathways and high surface exposure of active sites. More importantly, the ORR current densities of the NiCo2S4@3DGF composites are even higher than those of the commercial Pt/C catalyst in the potential range −0.9 to −1.2 V. In addition, the intrinsic ORR activity of the samples is also revealed from the Tafel plots. As shown in Fig. 6(e), the Tafel plots of the NiCo2S4@3DGF and Pt/C catalyst are constructed from the kinetic currents.9 The NiCo2S4@3DGF composites demonstrate a Tafel slope of 87 mV dec−1 in the low overpotential region, which is close to that of commercial Pt/C (83 mV dec−1), further suggesting the enhanced reaction dynamics and high intrinsic catalytic activity toward ORR of the composites.
Chronoamperometric measurements were conducted to present the methanol crossover effect of the products at −0.8 V in O2-saturated 0.1 M KOH at a rotation rate of 1600 rpm with the addition of 3.0 M methanol, and the results are shown in Fig. 6(f). As shown in the chronoamperometric curves of the Pt/C catalyst, a severe current drop appears to occur with the addition of methanol. In contrast, no noticeable response to methanol oxidation could be observed for the NiCo2S4@3DGF composites, verifying that the composites possess higher ORR selectivity and excellent tolerance to methanol compared to the commercial Pt/C catalyst. The figure below in Fig. 6(f) shows the durability of the composites, which was evaluated at −0.8 V in O2-saturated 0.1 M KOH for 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s. In contrast to the 39.23% current loss of Pt/C, the NiCo2S4@3DGF composites retain a higher relative current of 88.82% within 60
000 s. In contrast to the 39.23% current loss of Pt/C, the NiCo2S4@3DGF composites retain a higher relative current of 88.82% within 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s under a constant anodic voltage of −0.8 V, indicating the superior stability of the active reaction sites on the composites compared to on commercial Pt/C. Considering the considerable catalytic activity, remarkable electrochemical stability, and excellent catalytic selectivity, the NiCo2S4@3DGF composites are highly promising as superior ORR catalysts in practical application.
000 s under a constant anodic voltage of −0.8 V, indicating the superior stability of the active reaction sites on the composites compared to on commercial Pt/C. Considering the considerable catalytic activity, remarkable electrochemical stability, and excellent catalytic selectivity, the NiCo2S4@3DGF composites are highly promising as superior ORR catalysts in practical application.
The excellent electrochemical performance in lithium storage and ORR catalysis of the NiCo2S4@3DGF composites can be ascribed to the appropriately combined material compositions, and rationally designed multilevel 3D network-like architectures assembled with mesoporous ultrathin nanosheets. With regard to composition design, the combination of binary-metal sulfide NiCo2S4 with 3DGF retains the inherently high electrochemical activity and high capacity of NiCo2S4, and further enhances the electronic conductivity from 3DGF, which is of great significance for facilitating lithium storage and increasing catalytically active sites.16–18,27,29 As regards the morphology and microstructure, for lithium storage, the unique multilevel 3D network-like architectures ensure that NiCo2S4 is in close contact with the electrolyte, minimize transport distances between the composites and electrolyte, and further facilitate fast transportation of lithium ions and electrons, resulting in an excellent rate performance.18,22 Meanwhile, the ultrathin nanosheets forming 3D interconnected networks relieve the volumetric expansion during the lithium insertion and extraction process, and the existence of mesopores in the nanosheets also provides sufficient free space to ameliorate the expansion tendency, thus improving the cycling stability.19 Furthermore, the integrated free-standing architectures as binder-free anodes play a significant role in relieving the aggregation and mechanical failure of the NiCo2S4 during repeated charge–discharge processes, further enhancing the lithium storage performance.22 As for the catalytic activity, the unique multilevel 3D network-like architectures with high surface area and mesoporous features could provide a higher surface density of catalytic active sites exposed to oxygen molecules, and provide efficient pathways for the transport of reactant species, resulting in a promising catalytic performance for an efficient ORR.8,10,28
Conclusions
In summary, a well-designed composite of NiCo2S4 supported on three-dimensional graphene foam (3DGF) was fabricated via a facile solvothermal method and a subsequent sulfurization reaction. The obtained NiCo2S4@3DGF composites show unique multilevel 3D network-like structures, in which the network-like NiCo2S4 nanowall arrays assembled with extensive interconnected ultrathin mesoporous nanosheets are supported on 3DGF substrates. When used as LIB anodes, the composites exhibit an excellent lithium storage performance with a high reversible capacity of 1635 mA h g−1 at a high current density of 500 mA g−1, an enhanced cycling performance with the capacity maintained at 1292 mA h g−1, even after 150 cycles, and a remarkable high-rate capability. Meanwhile, when used as oxygen reduction reaction catalysts, the composites display enhanced catalytic activity (4-electron pathway), excellent tolerance to methanol crossover, and high durability. The excellent electrochemical performances of the composites could be attributed to the well-designed multilevel 3D network-like architectures with high surface areas and mesoporous features, and the rational combination between NiCo2S4 of high capacity/activity and 3DGF of high conductivity.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 51271012) and the Academic Excellence Foundation of BUAA for PhD Students.Notes and references
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928 CrossRef CAS PubMed.
- J. Cabana, L. Monconduit, D. Larcher and M. R. Palacín, Adv. Mater., 2010, 22, E170 CrossRef CAS PubMed.
- K. S. Kang, Y. S. Meng, J. Breger, C. P. Grey and G. Ceder, Science, 2006, 311, 977 CrossRef CAS PubMed.
- B. L. Ellis, P. Knauth and T. Djenizian, Adv. Mater., 2014, 26, 3368 CrossRef CAS PubMed.
- N. Mahmood, C. Z. Zhang, J. Jiang, F. Liu and Y. L. Hou, Chem. – Eur. J., 2013, 19, 5183 CrossRef CAS PubMed.
- H. Wang, Y. Liang, Y. Li and H. Dai, Angew. Chem., 2011, 123, 11161 CrossRef.
- C. Jin, F. L. Lu, X. C. Cao, Z. R. Yang and R. Z. Yang, J. Mater. Chem. A, 2013, 1, 12170 CAS.
- R. Li, Z. D. Wei and X. L. Gou, ACS Catal., 2015, 5, 4133 CrossRef CAS.
- J. W. Xiao, X. W. Zeng, W. Chen, F. Xiao and S. Wang, Chem. Commun., 2013, 49, 11734 RSC.
- H. Long, T. L. Shi, S. L. Jiang, S. Xi, R. Chen, S. Y. Liu, G. L. Liao and Z. R. Tang, J. Mater. Chem. A, 2014, 2, 3741 CAS.
- M. R. Wang, Y. Q. Lai, J. Fang, F. R. Qin, Z. A. Zhang, J. Li and K. Zhang, Catal. Sci. Technol., 2016, 6, 434 CAS.
- L. Yu, J. F. Yang and X. W. Lou, Angew. Chem., Int. Ed., 2016, 55, 1 CrossRef.
- Y. M. Chen, X. Y. Yu, Z. Li, U. Paik and X. W. Lou, Sci. Adv., 2016, 2, e1600021 Search PubMed.
- H. Hu, L. Han, M. Z. Yu, Z. Y. Wang and X. W. Lou, Energy Environ. Sci., 2016, 9, 107 CAS.
- X. Y. Yu, L. Yu and X. W. Lou, Adv. Energy Mater., 2016, 6, 1501333 CrossRef.
- H. C. Chen, J. J. Jiang, L. Zhang, H. Z. Wan, T. Qi and D. D. Xia, Nanoscale, 2013, 5, 8879 RSC.
- L. F. Shen, J. Wang, G. Y. Xu, H. S. Li, H. Dou and X. G. Zhang, Adv. Energy Mater., 2015, 5, 1400977 CrossRef.
- R. J. Zou, Z. Y. Zhang, M. F. Yuen, M. L. Sun, J. Q. Hu, C. S. Lee and W. J. Zhang, NPG Asia Mater., 2015, 7, e195 CrossRef CAS.
- J. Liang, X. Y. Yu, H. Zhou, H. B. Wu, S. Ding and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 12803 CrossRef CAS PubMed.
- M. Zhang, X. Yang, X. Kan, X. Wang, L. Ma and M. Jia, Electrochim. Acta, 2013, 112, 727 CrossRef CAS.
- B. Wang, S. M. Li, X. Y. Wu, W. M. Tian, J. H. Liu and M. Yu, J. Mater. Chem. A, 2015, 3, 13691 CAS.
- J. Liang, H. Hu, H. Park, C. Xiao, S. Ding, U. Paik and X. W. Lou, Energy Environ. Sci., 2015, 8, 1707 CAS.
- X. Yu, B. Lu and Z. Xu, Adv. Mater., 2014, 26, 1044 CrossRef CAS PubMed.
- J. Wang, J. Liu, D. Chao, J. Yan, J. Lin and Z. X. Shen, Adv. Mater., 2014, 26, 7162 CrossRef CAS PubMed.
- Z. L. Wang, D. Xu, H. G. Wang, Z. Wu and X. B. Zhang, ACS Nano, 2013, 7, 2422 CrossRef CAS PubMed.
- Q. Liu, J. T. Jin and J. Y. Zhang, ACS Appl. Mater. Interfaces, 2013, 6, 5002 Search PubMed.
- Y. H. Su, H. L. Jiang, Y. H. Zhu, X. L. Yang, J. H. Shen, W. J. Zou, J. D. Chen and C. Z. Li, J. Mater. Chem. A, 2014, 2, 7281 CAS.
- J. Liang, Y. Zheng, J. Chen, J. Liu, D. Hulicova-Jurcakova, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2012, 51, 3892 CrossRef CAS PubMed.
- H. P. Cong, S. Xin and S. H. Yu, Nano Energy, 2015, 13, 482 CrossRef CAS.
- R. J. Zou, Z. Y. Zhang, M. F. Yuen, J. Q. Hu, C. S. Lee and W. J. Zhang, Sci. Rep., 2015, 5, 7862 CrossRef PubMed.
- G. X. Gao, H. B. Wu, B. T. Dong, S. J. Ding and X. W. Lou, Adv. Sci., 2015, 2, 1400014 CrossRef.
- X. Li, D. Li, Z. Wei, X. Shang and D. He, Electrochim. Acta, 2014, 121, 415 CrossRef CAS.
- H. Tao, J. Moser, F. Alzina, Q. Wang and C. M. Sotomayor-Torres, J. Phys. Chem. C, 2011, 115, 18257 CAS.
- L. Qie, W. M. Chen, Z. H. Wang, Q. G. Shao, X. Li, L. X. Yuan, X. L. Hu, W. X. Zhang and Y. H. Huang, Adv. Mater., 2012, 24, 2047 CrossRef PubMed.
- D. U. Lee, Bae. J. Kim and Z. W. Chen, J. Mater. Chem. A, 2013, 1, 4754 CAS.
- M. Sun, J. J. Tie, G. Cheng, T. Lin, S. M. Peng, F. Z. Deng, F. Ye and L. Yu, J. Mater. Chem. A, 2015, 3, 1730 CAS.
- H. Z. Wan, J. J. Jiang, J. W. Yu, K. Xu, L. Miao, L. Zhang, H. C. Chen and Y. J. Ruan, CrystEngComm, 2013, 15, 7649 RSC.
- B. B. Zhan, C. B. Liu, H. P. Chen, H. X. Shi, L. H. Wang, P. Chen, W. Huang and X. C. Dong, Nanoscale, 2014, 6, 7424 RSC.
- Y. S. Lin and J. G. Duh, J. Power Sources, 2011, 196, 10698 CrossRef CAS.
- D. P. Cai, D. D. Wang, C. X. Wang, B. Liu, L. L. Wang, Y. Liu, Q. H. Li and T. H. Wang, Electrochim. Acta, 2015, 151, 35 CrossRef CAS.
- Z. Peng, D. S. Jia, A. M. Al-Enizi, A. A. Elzatahry and G. F. Zheng, Adv. Energy Mater., 2015, 5, 1402031 CrossRef.
- L. L. Hu, B. H. Qu, C. C. Li, Y. J. Chen, L. Mei, D. N. Lei, L. B. Chen, Q. H. Li and T. H. Wang, J. Mater. Chem. A, 2013, 1, 5596 CAS.
- Q. H. Wang, L. F. Jiao, H. M. Du, Y. C. Si, Y. J. Wang and H. T. Yuan, J. Mater. Chem., 2012, 22, 21387 RSC.
- Y. Liu, J. N. Zhang, S. P. Wang, K. X. Wang, Z. M. Chen and Q. Xu, New J. Chem., 2014, 38, 4045 RSC.
- B. Wang, S. M. Li, X. Y. Wu, J. H. Liu, W. M. Tian and J. Chen, New J. Chem., 2016, 40, 2259 RSC.
- G. Zhang and X. W. Lou, Adv. Mater., 2013, 25, 976 CrossRef CAS PubMed.
- L. Shen, Q. Che, H. Li and X. Zhang, Adv. Funct. Mater., 2014, 24, 2630 CrossRef CAS.
- Y. C. Du, X. S. Zhu, X. S. Zhou, L. Y. Hu, Z. H. Dai and J. C. Bao, J. Mater. Chem. A, 2015, 3, 6787 CAS.
- B. Wang, S. Li, J. Liu, M. Yu, B. Li and X. Wu, Electrochim. Acta, 2014, 146, 679 CrossRef CAS.
- S. F. Kong, Z. T. Jin, H. Liu and Y. Wang, J. Phys. Chem. C, 2014, 118, 25355 CAS.
- B. M. Goh, Y. Wang, M. V. Reddy, Y. L. Ding, L. Lu, C. Bunker and K. P. Loh, ACS Appl. Mater. Interfaces, 2014, 6, 9835 CAS.
- R. H. Wang, C. H. Xu, J. Sun, Y. Q. Liu, L. Gao, H. L. Yao and C. H. Lin, Nano Energy, 2014, 8, 183 CrossRef CAS.
- B. S. Li, J. K. Feng, Y. T. Qian and S. L. Xiong, J. Mater. Chem. A, 2015, 3, 10336 CAS.
- M. L. Mao, L. Jiang, L. C. Wu, M. Zhang and T. H. Wang, J. Mater. Chem. A, 2015, 3, 13384 CAS.
- D. Zhang, Y. J. Mai, J. Y. Xiang, X. H. Xia, Y. Q. Qiao and J. P. Tu, J. Power Sources, 2012, 217, 229 CrossRef CAS.
- F. Zhu, H. Xia and T. Feng, Mater. Technol., 2015, 30, A53 CrossRef CAS.
- D. Aurbach, J. Power Sources, 2000, 89, 206 CrossRef CAS.
- K. M. Shaju, F. Jiao, A. Débart and P. G. Bruce, Phys. Chem. Chem. Phys., 2007, 9, 1837 RSC.
- Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Nat. Mater., 2011, 10, 780 CrossRef CAS PubMed.
- S. M. Jeong, M. K. Kim, G. P. Kim, T. Y. Kim and S. H. Baeck, Chem. Eng. J., 2012, 198–199, 435 CrossRef CAS.
- Y. P. Huang, Y. E. Miao, H. Y. Lu and T. X. Liu, Chem. – Eur. J., 2015, 21, 10100 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nj02184k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |