Design of a three-state switchable chromogenic radical-based moiety and its translation to molecular logic systems†
Sanjoy
Mukherjee
 a and
Bryan W.
Boudouris
a and
Bryan W.
Boudouris
 *ab
*ab
aCharles D. Davidson School of Chemical Engineering, Purdue University, West Lafayette, IN-47907, USA. E-mail: boudouris@purdue.edu
bDepartment of Chemistry, Purdue University, West Lafayette, IN-47907, USA
First published on 6th April 2017
Abstract
Three distinctly different and stable colored states of phenylgalvinoxyl (i.e., neutral phenolic, radical, and anionic species) small molecule and macromolecular systems are evaluated as a function of the solution pH. A clear halochromic effect is readily observed in the design of the polymer, in a manner that is distinct from the more oft-studied small molecule analogs. This key design paradigm allows the chemical nature of the phenylgalvinoxyl moieties to be comparable to a standard AND logic function, which evolves based upon the structural constitution of the material. Moreover, this crucial change in behavior allows for the revelation that the formation of the radical polymer that bears a galvinoxyl pendant group occurs through base-promoted step-wise oxidation, and this, in turn, provides a critical handle by which to design future radical polymer archetypes and to build molecular logic systems based upon this emerging class of functional materials.
Design, System, ApplicationHere, the key design paradigm is that identical open-shell functional moieties in small molecules and polymers do not behave in a similar manner. Because small molecules are relatively independent of neighboring molecules, their cooperative behavior in a polymeric system can be distinctly different. If a functional system can have multiple accessible identities, such differences can be of fundamental importance in elucidating the chemical engineering of the system to a significant extent. In this effort, these aspects of the phenylgalvinoxyl system are brought forward in a direct manner. Although this moiety has been well known as a candidate for the formation of persistent radicals, the optical chemistry of these materials is nearly unexplored. In this work, the correlation of this system design with its optical properties is described in detail. The optical logic functions, halochromism, and dependence on the material design are correlated with the molecular chemistry of the system. The application of this chemical optical logic system in small molecules and polymeric systems also reveals the formation mechanism of the radical polymer, and thus the functioning of these molecules in applications also provides key insights into the chemistry of these emerging redox systems. |
Introduction
Stimuli- and analyte-responsive switching of optoelectronic properties in organic materials have recently been of significant interest for myriad energy conversion and logic devices.1 The inherent simplicity of a visual change upon the application of a stimulus not only broadens the scope of the application of a material (e.g., as an electrochromic switch and/or as molecular logic systems),2 but also allows for the monitoring of various kinetic processes (e.g., as an indicator in displacement assays)3 in real time. In most of these cases, the optical switching of an independent individual species (i.e., excluding mechanisms such as aggregation and π-complex formation)4 can be related to either its redox reactions (or redox states)5 or halochromism.6Moreover, stable radical systems provide an excellent handle by which to reveal the effect of redox states of organic materials on their optoelectronic properties. That is, in most cases, stable organic radicals allow reversible oxidation or reduction of the system without the involvement of any covalent bond formation or breakage.7 Because of these advantages, organic radicals have been proven to be one of the most potent classes of materials for electrochemical applications such as in organic batteries.7,8 Furthermore, cooperative and continuous redox state switching of radicals has been also found to be relevant in the development of electrically conductive materials.9
Among the commonly encountered stable radical systems, galvinoxyl is noteworthy;10 however, even after its six decade-long existence in the literature, a true understanding of its optical properties is still an elusive issue. Fortunately, with the progress of modern chemistry, a library of closely related systems, with the added versatility of specific chemical functionalization, has been developed. Notably, researchers have established a phenylgalvinoxyl system, which can form persistent radical entities and also allows for molecules that have nearly unrestricted chemical functionalization such that they are capable of undergoing polymerization.11 The degree of conjugation and consequently the optical properties of the phenylgalvinoxyl system are expected to depend strongly on the deprotonation or oxidation of the phenolic site. Here, we take advantage of this molecule in order to generate a material that provides a system with the coupled existence of the two above-mentioned phenomena (i.e., redox- and pH-induced optical switching). In turn, this molecular platform provides a means by which to engineer new molecular design criteria in an effort to elucidate the complete relationship between the conjugation and color of these organic molecules. Additionally, the pH- and chemo-responsiveness result in distinct optical switching of the phenylgalvinoxyl molecules, which clearly paves the path for their application as a molecular logic gate.
Results and discussion
Three distinct identities of phenylgalvinoxyl
The small molecule depicted in Fig. 1, 2,6-di-tert-butyl-4-((3,5-di-tert-butyl-4-hydroxyphenyl) (4-vinylphenyl)methylene) cyclohexa-2,5-dien-1-one (GStH), was synthesized according to a four-step process that utilized a 2,4-di-tert-butyl phenol starting material (ESI,† Scheme S2 and Fig. S1 and S2). The presence of the vinyl group in GStH allows it to be polymerized, forming PGStH (Fig. 1). These two chemical species can be oxidized (using K3[Fe(CN)6] in the presence of NaOH) to form their corresponding radical species.12a Thus, combining the deprotonated (GStB/PGStB) and the radical state (GSt/PGSt), the system has three unique identities that have key differences in the symmetry and the number of π-electrons in the conjugated backbone (ESI,† Scheme S1). The half-phenolic and half-quinoidal neutral structure (GStH) is asymmetric and weakly conjugated compared to the other two species. Although GStB and GSt are structurally identical in terms of molecular design, they are electronically dissimilar. Importantly, the three distinct species (i.e., closed-shell neutral, conjugate-base anion, and radical) are distinctly different in color (ESI,† Fig. S4–S8). While GStH is orange, the color of GSt is dark brown. With only one more electron in the system, the anionic GStB is clearly different, and it appears as a much darker blue material. Such a different appearance of the conjugate-base (GStB) constitutes what is known as a halochromic behavior in the material. Beyond this halochromic behavior being of fundamental chemical importance, the distinct optical signatures of the species allow for the facile monitoring of the interplay and conversion of the three different species, which provides a more detailed insight into the formation mechanism and stability of the radical species, both as a small molecule and a polymer.Effect of electronics on optical properties
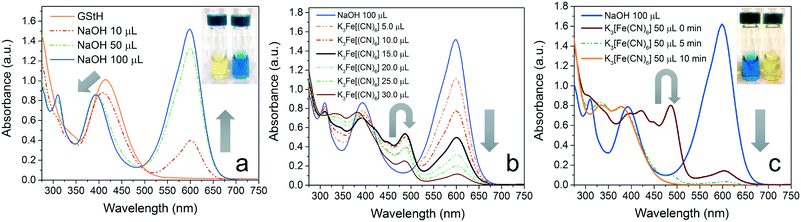
As depicted in Fig. 2, GStH shows a moderately strong and structurally broad absorption band at 415 nm (ε = 1.0 × 104 M−1 cm−1) and additional higher energy absorption bands as well. Density functional theory (DFT) computations indicate that the frontier molecular orbitals (FMOs) of the compound are dominated by the quinonoid side of the system (ESI,† Fig. S19), and the computed transition (λ = 426 nm, f = 0.614; f represents computed oscillator strength) is predominantly a HOMO–LUMO transition dominated by the quinonoid moiety (ESI,† Fig. S25). On gradual deprotonation, the formation of GStB is clearly observable due to the formation of a much stronger absorption band near 600 nm (ε = 1.5 × 104 M−1 cm−1). Computational assessment also agrees closely with the experimental observation, and predicts a HOMO–LUMO transition at λ = 632 nm (f = 0.682) (ESI,† Fig. S26). The symmetric disposition of the FMOs (ESI,† Fig. S19), coupled with the increased conjugation through the overall π-conjugated system (ESI,† Fig. S20–S22), results in a lowered band gap of the compound. Further, the higher oscillator strength of the HOMO–LUMO transition can be correlated with the stronger absorption features of GStB. In addition, due to the formation of a symmetric but rigid conjugated structure, the absorption profile of GStB is comparatively narrower than that of the neutral form. Apart from the dominant absorption band at 600 nm, GStB also shows two distinct peaks at 310 nm and 390 nm, which can be ascribed to localized π–π* transitions (Fig. S26†).Upon oxidation (in aqueous solution) of GStB to GSt, the dominant absorption peak at 600 nm disappears with the formation of a new distinct peak at 485 nm (ε = 0.9 × 104 M−1 cm−1). Additionally, a number of higher energy absorption bands were observed. The formation of the radical species also could be confirmed from the characteristic peaks of GSt observed using cyclic voltammetry (in situ). Interestingly, the removal of one electron from GStB decreases the relative energies of the FMOs (ESI,† Fig. S19). However, the filled orbital (i.e., the simultaneous HOMO/SOMO) experiences a more significant lowering (compared to the virtual LUMO), which effectively increases the HOMO–LUMO gap with a lower oscillator strength (λ = 461 nm, f = 0.533), according to computations. Considering the structure and electronic properties of the conjugate-base (GStB) and radical (GSt), it is evident that the relative torsional orientations of the three aromatic rings in these two species are comparable (ESI,† Fig. S20–S22†). However, the bond lengths in the conjugate base structure are comparatively elongated, which again supports the previously discussed argument for electronic repulsion in the system. It is notable that, similar to the FMOs, the electrostatic potential surface is also symmetric in GStB and GSt (ESI,† Fig. S23 and S24). However, in GSt, the spin density is only localized symmetrically on the two phenolic aromatic rings, which is comparable to the galvinoxyl radical. Thus, the third styryl ring has a very insignificant effect on controlling the electronic properties of GSt.
Apart from the fundamental optical properties, the distinct color signals of the radical (GSt) compared to those of the anionic species (GStB) reveal the formation mechanism of radical polymers (e.g., PGSt). The observation of a maximum intensity (at λ = 485 nm) during the course of the addition of K3[Fe(CN)6] pointed towards the relative instability of GSt in aqueous media. In fact, this can be observed more prominently via the addition of excess K3[Fe(CN)6] to GStB in one batch and the subsequent monitoring of the optical profile at 485 nm as a function of time (ESI,† Fig. S8). However, it is notable that the decomposed product is not comparable to GStH, pointing towards the reactive fate of the GSt radicals. This instability can be ascribed to the presence of excess alkali in the media, and can be confirmed using a lower equivalent of NaOH in a similar process. The partial deprotonation of the available GStH in aqueous media still leads to the formation of GSt and increase of its stability (ESI,† Fig. S9). It is notable that the decrease in the concentration of GStB is rapid relative to the formation of GSt, indicating that the deprotonation might be the rate-determining step in the formation of the radical species. This is further supported in the case of the PGSt polymer as well (vide infra). The ‘AND’ logic function towards the formation of GSt in the presence of both base and oxidant can be partially reset using a radical scavenger (e.g., ascorbic acid) (ESI,† Fig. S4 and S5). The partial regeneration of the starting component (GStB), in this case, can be ascribed to the relative instability of the radical species in aqueous medium.
Evolution of optical properties from a small molecule to a polymer
The neutral monomer (GStH) can be polymerized using free radical polymerization. However, due to the steric bulk of the system, only macromolecules with moderately low molecular weights (Mn = 4.1 kg mol−1) were obtained, which corresponds to ∼10 repeating units. In a manner akin to the small molecule (GStH), the polymer, PGStH, also shows a significant extent of deprotonation in basic solvents (ESI,† Fig. S13). In order to establish the structural orientation of the polymer, we optimized the geometry of small chain models (12 repeating units) computationally, and determined that the chains should adopt a helical shape. As demonstrated in Fig. 3c, the phenolic units are placed towards the periphery of the helix, which has a threefold screw-symmetry. Thus, it is expected that the –OH sites are solvent-accessible (unlike the π-conjugated systems of the repeating units), and the core alkyl chain is shielded by bulky aromatic substituents.Significantly, the addition of even excess base to an aqueous solution of PGStH (in THF/H2O) results in only ∼15% of the possible deprotonation, after which the material starts to precipitate. Thus, even with the pendant sites being solvent-accessible, it appears that only single deprotonation occurs in each polymer chain. After this point, the macromolecular solubility is compromised in the solvent mixture. Interestingly, such partial progress of the reaction imparts a green color to the mixture of yellow- and blue-colored materials. Comparing soaked filter paper strips of GStH and PGStH and their exposure to purely aqueous NaOH demonstrates a similar behavior (Fig. 3b). However, this observation indicates that the partial deprotonation of PGStH is probably due to the inability of the material to form poly-anionic species, which may result in significant electronic repulsions between proximal repeating units along the same macromolecular backbone. Thus, even with the presence of similar functional units, a monomer and a polymer can have distinctly distinguishable halochromic features. Such modulation of the color palettes of any material is of great interest in chromism-dependent applications.5a
Glimpse into the formation mechanism of PGSt by following the logic
As discussed earlier, the combined effect of alkali and oxidant to transform GStH to GSt can be compared to an ‘AND’ logic gate, as both of the reagents are necessary for the transformation (regardless of the order of addition) and none can singularly lead to the formation of the radical (GSt). The reversible formation of GStB in the presence of a base can be regarded as the activation step in this case. However, as noted in the previous section, this activation is only partially obtainable for the polymeric material (PGStH).Pointedly, the partial reaction of PGSt with alkali generated a key insight into the formation of similar radical polymers. Radical polymers like PGSt have been reported in the literature, where the neutral form is oxidized in the presence of a base. As demonstrated above, neither GStH nor PGStH reacts with K3[Fe(CN)6] in the absence of NaOH. Interestingly, we observed that the addition of a small amount of NaOH at this stage could move the reaction in the forward direction. As depicted in Fig. 4b, under such conditions, the relative concentration of the deprotonated species remains unaltered during this reaction. However, a steady decrease in the concentration of the phenolic form and a steady increase in the formation of the radical could be observed. In addition, unlike the monomer, the steady increase in the formation of the radical species in this case indicates that the radical moieties are much less prone to decomposition due to their inclusion in the polymer. As noted earlier, the π-conjugated system of the repeating units is shielded from solvent's influence, which supports the idea of the enhanced stability of the radical species in the polymeric motif. However, the steady concentration of the conjugate-base indicates that the two processes are comparably fast, which provides a unique example of consecutive reactions. The ionic charge balance in the media is maintained in the stepwise formation of the radical polymer, which is responsible for a steady concentration of the conjugate-base. The previously discussed structural model of the polymer chains, coupled with the mechanism depicted in Fig. 4c, can rationally explain the nature of the reaction observed in the current study. The peripheral disposition of the –OH units makes them accessible to the oxidizing agent, but the inertness of the functionalities towards the oxidizing agent prevents any reaction in the absence of a base. However, with even a partial deprotonation, the oxidizing agent reacts promptly, forming the radical species. Furthermore, in order to maintain charge neutrality in the media, the available –OH units deprotonate, and the reaction propagates in a similar manner. However, with decreasing amounts of available –OH sites, the reaction gradually slows. To the best of our knowledge, such insights into the formation mechanism of phenolic radical polymers were not investigated hitherto, and the elucidation of this mechanism provides crucial insights into the conversion of protected macromolecules to functional radical polymers. In turn, these key functionalities are known to influence the optoelectronic properties of radical polymers in energy conversion and storage applications.13
Through these molecular design rules, we have unveiled a number of crucial aspects of the phenylgalvinoxyl system (Fig. 5) with respect to its ability to be a molecular logic moiety. That is, phenylgalvinoxyl is a versatile three-state stable color switchable molecule that can be regarded as an AND logic gate system for the detection of oxidants in the presence of an alkali. Additionally, the ability to connect the conformation of the polymer to the reactivity of the pendant group sites provides a clear means by which to design future iterations of this type of molecular logic. The distinct optical features of the neutral, conjugate-base and radical species allowed for a systematic study of the conversion reactions, providing insights into the formation of radical polymers via post-synthetic oxidation. Based on the experimental results, a stepwise connected series of AND logic events lead to the formation of PGSt in solution. Thus, a broader identity of a functional polymer (PGSt) is revealed, and these insights extend the understanding regarding the fundamental aspects on the phenylgalvinoxyl formation.
Conclusions
In brief, we have shown that apart from being a redox-active polymer, PGSt can act as a visual sensory material. The combined redox and color chemistry of this polymer might open new opportunities related to non-conjugated polymers. This work also demonstrates how the structure (e.g., molecular or polymeric form) of a functional redox system influences the formation and fate of a material. Small molecules (e.g., GStH) can act as independent entities irrespective of the identical neighbors, which allows us to exploit their functional abilities (e.g., halochromism and as AND logic). However, in a polymeric system (PGStH), the independent logic function evolves to a consecutive array of events, where the initial activation step (i.e., deprotonation) remains constant throughout the conversion process and serves as a mediating influence to the reaction process. This key interplay between the small molecule and polymeric design of open-shell entities both provides a clear mechanistic insight into how to better design radical polymer systems and serves as a handle by which to generate next-generation molecular logic functions through a simple degree of polymerization control as well.Acknowledgements
We deeply thank the Air Force Office of Scientific Research (AFOSR) for the support of this work through the Organic Materials Chemistry Program (Grant Number: FA9550-15-1-0449, Program Manager: Dr. Kenneth Caster).Notes and references
- (a) C. Suksai and T. Tuntulani, Chem. Soc. Rev., 2003, 32, 192–202 RSC; (b) C. M. Lampert, Mater. Today, 2004, 7, 28–35 CrossRef CAS; (c) B. W. Boudouris, Curr. Opin. Chem. Eng., 2013, 2, 294–301 CrossRef; (d) J. Wu, B. Kwon, W. Liu, E. V. Anslyn, P. Wang and J. S. Kim, Chem. Rev., 2015, 115, 7893–7943 CrossRef CAS PubMed.
- (a) R. J. Mortimer, Annu. Rev. Mater. Res., 2011, 41, 241–268 CrossRef CAS; (b) R. Klan, Chem. Soc. Rev., 2014, 43, 148–184 RSC; (c) M. Bengisu and M. Ferrara, Materials that change color: smart materials, intelligent design, Springer, 2014 Search PubMed; (d) H. Zhu, J. Fan, B. Wang and X. Peng, Chem. Soc. Rev., 2015, 44, 4337–4366 RSC; (e) J. Andreasson and U. Pischel, Chem. Soc. Rev., 2010, 39, 174–188 RSC; (f) J. Andreasson and U. Pischel, Chem. Soc. Rev., 2015, 44, 1053–1069 RSC.
- C. V. Sapan, R. L. Lundblad and N. C. Price, Biotechnol. Appl. Biochem., 1999, 29, 99–108 CAS.
- (a) F. Wurthner, T. E. Kaiser and C. R. Saha-Moller, Angew. Chem., Int. Ed., 2011, 50, 3376–3410 CrossRef PubMed; (b) W. Sun, S. Guo, C. Hu, J. Fan and X. Peng, Chem. Rev., 2016, 116, 7768–7817 CrossRef CAS PubMed.
- (a) P. M. Beaujuge and J. R. Reynolds, Chem. Rev., 2010, 110, 268–320 CrossRef CAS PubMed; (b) S. Kumar, M. R. Ajayakumar, G. Hundal and P. Mukhopadhyay, J. Am. Chem. Soc., 2014, 136, 12004–12010 CrossRef CAS PubMed; (c) D. Schmidt, M. Son, J. M. Lim, M. J. Lin, I. Krummenacher, H. Braunschweig, D. Kim and F. Wurthner, Angew. Chem., Int. Ed., 2015, 54, 13980–13984 CrossRef CAS PubMed.
- (a) V. G. Machado, R. I. Stock and C. Reichardt, Chem. Rev., 2014, 114, 10429–10475 CrossRef CAS PubMed; (b) D. Wencel, T. Abel and C. McDonagh, Anal. Chem., 2014, 86, 15–29 CrossRef CAS PubMed; (c) T. Maeda and F. Wurthner, Chem. Commun., 2015, 51, 7661–7664 RSC; (d) H. T. Black, I. Pelse, R. M. W. Wolfe and J. R. Reynolds, Chem. Commun., 2016, 52, 12877–12880 RSC.
- (a) A. M. Janiszewska and M. Grzeszczuk, Electroanalysis, 2004, 16, 1673–1681 CrossRef CAS; (b) Z. Zhang, P. Chen, T. N. Murakami, S. M. Zakeeruddin and M. Gratzel, Adv. Funct. Mater., 2008, 18, 341–346 CrossRef CAS; (c) R. Gracia and D. Mecerreyes, Polym. Chem., 2013, 4, 2206–2214 RSC; (d) J. B. Gerken and S. S. Stahl, ACS Cent. Sci., 2015, 1, 234–243 CrossRef CAS PubMed.
- (a) T. Janoschka, M. D. Hager and U. S. Schubert, Adv. Mater., 2012, 24, 6397–6409 CrossRef CAS PubMed; (b) Z. Song and H. Zhou, Energy Environ. Sci., 2013, 6, 2280–2301 RSC; (c) T. B. Schon, B. T. McAllister, P. F. Li and D. S. Seferos, Chem. Soc. Rev., 2016, 45, 6345–6404 RSC.
- (a) K. Oyaizu and H. Nishide, Adv. Mater., 2009, 21, 2339–2344 CrossRef CAS; (b) E. P. Tomlinson, M. E. Hay and B. W. Boudouris, Macromolecules, 2014, 47, 6145–6158 CrossRef CAS; (c) A. J. Wingate and B. W. Boudouris, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 1875–1894 CrossRef CAS.
- (a) G. M. Coppinger, J. Am. Chem. Soc., 1957, 79, 501–502 CrossRef CAS; (b) H. Shi, N. Noguchi and E. Niki, Methods Enzymol., 2001, 335, 157–166 CAS; (c) S. Nagaoka, K. Nagai, Y. Fujii, A. Ouchi, K. Mukai and J. Agric, Food Chem., 2013, 61, 10054–10062 CrossRef CAS PubMed.
- (a) Y. Yonekuta, K. Susuki, K. Oyaizu, K. Honda and H. Nishide, J. Am. Chem. Soc., 2007, 129, 14128–14129 CrossRef CAS PubMed; (b) T. Suga, H. Ohshiro, S. Sugita, K. Oyaizu and H. Nishide, Adv. Mater., 2009, 21, 1627–1630 CrossRef CAS; (c) T. Suga, S. Sugita, H. Ohshiro, K. Oyaizu and H. Nishide, Adv. Mater., 2011, 23, 751–754 CrossRef CAS PubMed.
- (a) T. Kaneko, H. Abe, M. Teraguchi and T. Aoki, Macromolecules, 2013, 46, 2583–2589 CrossRef CAS; (b) T. Kaneko, Y. Umeda, T. Yamamoto, M. Teraguchi and T. Aoki, Macromolecules, 2005, 38, 9420–9426 CrossRef CAS.
- L. Rostro, S. Wong and B. W. Boudouris, Macromolecules, 2014, 47, 3713–3719 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, NMR spectra, UV-vis spectra, cyclic voltammetry data, and DFT results. See DOI: 10.1039/c7me00010c |
| This journal is © The Royal Society of Chemistry 2017 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:

