Functionalization of SiC/SiOx nanowires with a porphyrin derivative: a hybrid nanosystem for X-ray induced singlet oxygen generation†
R.
Tatti‡
 *a,
M.
Timpel‡
a,
M. V.
Nardi
b,
F.
Fabbri
c,
R.
Rossi
*c,
L.
Pasquardini
b,
A.
Chiasera
d,
L.
Aversa
a,
K.
Koshmak
e,
A.
Giglia
e,
L.
Pasquali
*a,
M.
Timpel‡
a,
M. V.
Nardi
b,
F.
Fabbri
c,
R.
Rossi
*c,
L.
Pasquardini
b,
A.
Chiasera
d,
L.
Aversa
a,
K.
Koshmak
e,
A.
Giglia
e,
L.
Pasquali
 efg,
T.
Rimoldi
h,
L.
Cristofolini
h,
G.
Attolini
c,
S.
Varas
d,
S.
Iannotta
c,
R.
Verucchi
a and
G.
Salviati
c
efg,
T.
Rimoldi
h,
L.
Cristofolini
h,
G.
Attolini
c,
S.
Varas
d,
S.
Iannotta
c,
R.
Verucchi
a and
G.
Salviati
c
aIMEM-CNR Institute, Via alla Cascata 56/C, Povo, 38123 Trento, Italy. E-mail: tatti@fbk.eu
bDepartment of Industrial Engineering, University of Trento, via Sommarive 9, 38123 Trento, Italy
cIMEM-CNR Institute, Parco Area delle Scienze 37/A, 43124 Parma, Italy. E-mail: frossi@imem.cnr.it
dIFN – CNR CSMFO Lab. & FBK CMM, via alla Cascata 56/C Povo, 38123 Trento, Italy
eIOM-CNR Institute, Area Science Park, SS 14 Km, 163.5, 34149 Basovizza, Trieste, Italy
fUniversity of Modena e Reggio Emilia, Engineering Department, “E. Ferrari”, Via Vigolese 905, 41125 Modena, Italy
gDepartment of Physics, University of Johannesburg, PO Box 524, Auckland Park, 2006 South Africa
hDepartment of Mathematical, Physical and Computer Sciences, University of Parma, Parco Area delle Scienze 7/A, 43124 Parma, Italy
First published on 12th April 2017
Abstract
Singlet oxygen has attracted great attention in physical, chemical, as well as biological studies, mainly due to its high reactivity and strong oxidising properties. In this context, hybrid nanosystems comprised of (inorganic) X-ray absorbing nanostructures and (organic) light-sensitive material (photosensitizers) can potentially overcome the limitations of visible light penetration in matter. A deep investigation of the interface of such hybrid nanosystems for X-ray induced generation of singlet oxygen is key to better understand the processes at the hybrid interface, and to control the energy transfer from inorganic to organic counterparts, which ultimately leads to enhanced singlet oxygen generation. Here, we demonstrate that SiC/SiOx core/shell nanowires functionalized with the tetrakis(pentafluorophenyl)porphyrin can act as a highly promising and viable strategy to generate singlet oxygen, making this novel hybrid nanosystem attractive for applications in photocatalysis and nanomedical applications. Using different excitation sources (i.e., electrons, visible light, and X-rays) our findings prove that SiC/SiOx core/shell nanowires show X-ray excited optical luminescence, and that optical emission of the photosensitizer is largely enhanced by the nanowires, yielding an efficient energy transfer. A consequent singlet oxygen production of the functionalized nanowires is demonstrated after X-ray excitation in a clinical linear accelerator. These findings will provide an insight in developing an effective route to the molecular functionalization of SiC/SiOx core/shell nanowires and their potential use as singlet oxygen generators.
Design, System, ApplicationIn the manuscript, we propose a new hybrid nanosystem that is constituted by core–shell SiC/SiOx nanowires (NWs) functionalized with a porphyrin derivative, for potential applications in the biomedical field. In particular, we used the tetrakis(pentafluorophenyl)-porphyrin (H2TPPF), a molecule easily available commercially and with an interesting structural conformation, which makes it particularly suitable for our purposes. As a matter of fact, the presence of the highly reactive fluorine atoms, located in the peripheral position of the molecule, ensures a strong interaction between the organic molecule and the NWs' SiOx shell. As demonstrated by our comprehensive analysis, the obtained system is highly stable, without inserting additional linker to anchor the molecule on the inorganic nanostructures. Moreover, the developed hybrid structure has demonstrated the capability to generate singlet oxygen when irradiated by X-rays, making it attractive for applications in photocatalysis and in photodynamic therapy (PDT) for cancer. Indeed, the system can be applied as photosensitizer in X-ray excited PDT, able to treat wide and deep tumors. The in vitro evaluation of the NWs-H2TPPF nanosystem in different cell lines is currently under investigation. |
1. Introduction
Recent developments in nanoscience and nanotechnology require combining the knowledge from several fields and crossing different disciplines in order to create highly advanced hybrid nanomaterials.1–3 Most often these novel nanomaterials must exhibit multifunctionality, e.g., in the case of biomedical applications, not only excellent biocompatibility but also specific bioactivity.It has been widely proved that inorganic nanowires (NWs), either based on metal oxides,4 carbon nanotubes5 or semiconducting materials,6,7 can be used for biomedical applications. We recently focused our research on 3C-SiC/SiOx core/shell NWs, where the 3C-SiC core guarantees a lower inflammatory response and a long-term cytocompatibility.8–11 Furthermore, the amorphous silica layer is beneficial to enhance the luminescence of the crystalline 3C-SiC core,12,13 and offers a great versatility for surface functionalization by chemical methods, e.g., exploiting reactions with oxydrilic groups14 and alkyne functional groups.15 These core/shell 3C-SiC/SiOx NWs have been demonstrated to be cytocompatible over a time scale of at least 10 days,16 and suitable to create hybrid nanosystems active for biosensing applications14 and oncotherapy.15
Among the broad range of organic molecules suitable for the functionalization of the NWs, the class of large macrocycles, e.g., porphyrins, is highly appealing due to their intense optical absorption and luminescence, as well as their good interaction with biological environments. Besides applications of porphyrins in photomedicine,17 the studies on their photophysical properties have been extended to important and hot topics, such as optoelectronics,18 dye-sensitized solar cells,19 and photocatalysis.20 In particular, the selection of a partially fluorinated tetraphenyl-porphyrin to create an active inorganic–organic interface is supported by the good match between the X-ray excited optical emission of the NWs and the molecule's absorption (e.g., see Fig. 1a). This energy level matching in the hybrid nanosystem allows to have a NW-mediated porphyrin photosensitizer suitable for applications ranging from photooxidation of toxic molecules and water purification,21–24 to the use as cytotoxic agent in photodynamic therapy (PDT) of cancer.25–27 Nowadays, researchers proposed X-rays as PDT light source27 to overcome penetration limits of conventional light sources, and to extend PDT to deep tumor tissue.28–30 Since conventional photosensitizers do not directly absorb X-ray energy, the NWs can act as scintillating material to convert the X-rays to UV/visible light, which in turn can activate the photosensitizer molecule to generate singlet oxygen. Moreover, the presence of fluorine in the porphyrin makes the production of reactive oxygen species (ROS) more efficient,31,32 suggesting the potential application of our proposed system as singlet oxygen generator after visible light/X-ray irradiation.
 | ||
| Fig. 1 (a) XEOL emission spectrum (red) of bare SiC/SiOx core/shell nanowires (NWs), and room temperature absorption spectrum (blue) of tetrakis(pentafluorophenyl)-porphyrin (H2TPP(F)) used to functionalize the NWs. An enlarged view of the overlap between NWs' emission and Q band absorption is shown in the inset. (b) Schematic representation (top view) of H2TPP(F), where green (blue) spheres correspond to the F (N) atoms of the molecule. The atom numbering corresponds to the nomenclature recommended by IUPAC and adopted by Nardi et al.45 | ||
The preparation of efficient hybrid nanostructures, showing enhanced energy transfer and photodynamic properties, requires the use of controlled functionalization methods. While chemical methods14,15,24 allow anchoring a single monolayer of the specific organic molecule onto an inorganic surface, physical methods can be applied to obtain hybrid systems with a higher chemi- or physisorbed organic coverage. To this end, a novel viable and appealing approach for material growth in ultra-high vacuum (UHV) conditions is given by supersonic molecular beam deposition (SuMBD).33–39
Even though the detection of singlet oxygen generated after light irradiation is most often enough to qualify a system for any potential application, a deep understanding of the interface properties and processes that are most often related to the efficiency of the hybrid nanosystem is generally missing. In the present work, 3C-SiC/SiOx core/shell NWs have been successfully functionalized with a partially fluorinated tetraphenyl-porphyrin, namely tetrakis(pentafluorophenyl)porphyrin (in the following denoted as H2TPP(F)). The structural, optical and electronic properties of the functionalized NWs have been assessed by transmission electron microscopy (TEM), cathodoluminescence (CL) spectroscopy in the scanning electron microscope (SEM), fluorescence analysis in the confocal microscope, and X-ray photoelectron spectroscopy (XPS). Different planar substrates (i.e., high-purity quartz, native SiO2, and Au foil), have been functionalized analogously to support and/or compare the results obtained from the functionalized NWs. The orientation of the molecules on SiO2 has been studied by X-ray adsorption spectroscopy (XAS) experiments, which has assisted us to perform thorough analysis of the C 1s/F 1s core levels and its underlying chemical components. The comprehensive analysis proves that the SiC/SiOx core/shell NWs are fully functionalized with H2TPP(F), and the integrated intensity of the optical emission is two orders of magnitude higher for the functionalized NWs than for the functionalized planar substrates. This feature of the hybrid NWs/H2TPP(F) nanosystem highlights that an efficient energy transfer occurs from the SiC/SiOx core/shell NWs, acting as scintillator and energy converter, to the organic molecule. Finally, the nanosystem has been exploited to generate singlet oxygen under X-ray excitation in a clinical radiation therapy setup, in view of potential applications as PDT deep cancer treatment in nanomedicine.
2. Experimental methods
Material synthesis
Core/shell nanowires (NWs) with a crystalline 3C-SiC core covered by an amorphous SiOx shell have been grown on Si (001) substrates by a chemical vapour deposition (CVD) process at 1100 °C, as previously described elsewhere.40 The molecule tetrakis(pentafluorophenyl)porphyrin (C44H10F20N4), denoted as H2TPP(F), has been supplied by Sigma Aldrich with a nominal purity of 99.9%. Before functionalization, all NW samples have been cleaned using a series of different solvents (i.e., trichloroethylene, acetone, 2-propanol). After that, the samples have been annealed in UHV at ∼300 °C, in order to remove any traces of solvent and water. In addition to the NWs' functionalization, different planar substrates (i.e., high-purity quartz, SiO2 native oxide, Au foil) have been functionalized with H2TPP(F) to support our findings on the functionalized NWs. Similar to the NW samples, each planar substrate has been solvent-cleaned before functionalization.Functionalization via SuMBD
The functionalization of the NWs has been performed with an experimental setup devoted to thin film growth and in situ analysis in UHV conditions. The experimental setup is composed of a SuMBD apparatus, where the supersonic beam is formed, and which is directly connected to a μ-metal chamber (base pressure of 6 × 10−11 mbar), where the film deposition and in situ electronic characterization can be performed avoiding air contamination. A detailed description of the SuMBD apparatus is provided in ref. 33. The functionalization of SiC/SiOx core/shell NWs has been carried out using a H2TPP(F) supersonic seeded beam (base pressure of 1 × 10−7 mbar), using He as carrier gas and reaching a kinetic energy of ∼25 eV. The typical organic arrival rate on the substrate has been set to ∼1 Å min−1, as evaluated from a quartz microbalance, and has been kept constant during all experiments. In a first step, a thick film of H2TPP(F) has been deposited onto the NWs (nominal thickness of ∼40 nm), followed by sonication in 2-propanol in order to obtain a single monolayer of the organic molecule. The different planar substrates (i.e., high-purity quartz, SiO2 native oxide, Au foil) have been similarly functionalized with H2TPP(F) by SuMBD. Depending on the characterization technique (see discussion in the text) either a thick film of H2TPP(F) (nominal thickness of ∼40 nm, denoted as H2TPP(F) bulk material) or a monolayer has been deposited onto the corresponding planar substrate. Water contact angle measurements (see Fig. S2, ESI†) on a bare (planar) SiO2 substrate before and after functionalization with H2TPP(F) (thick film and monolayer) provide evidence of a uniform H2TPP(F) functionalization after SuMB deposition.X-ray excited optical luminescence
The X-ray excited optical luminescence (XEOL) of SiC/SiOx core/shell NWs has been performed at the BEAR end station (BL8.1 L), at the left exit of the 8.1 bending magnet of the ELETTRA synchrotron facility in Trieste (Italy).41 The NWs have been excited with a photon energy of 110 eV, and XEOL spectra have been collected by a triple grating monochromator ACTON SpectraPro 300i equipped with a liquid nitrogen-cooled CCD detector. The outcoming luminescence has been delivered from the sample into the spectrometer via an optical fiber that is positioned close to the sample surface.Electron microscopy
The morphology of the functionalized NWs has been analyzed by scanning electron microscopy (SEM) in a field-emission SUPRA40 Zeiss SEM equipped with a GEMINI FESEM detection column and using secondary electrons for imaging. Chemical mapping has been carried out by EDX microanalysis in a field-emission 2200FS JEOL TEM operated at 200 kV in scanning (STEM) mode. For TEM-EDX, the NWs have been transferred from the substrate by gentle rubbing on standard carbon-coated copper grids.The optical emission of the functionalized NWs, compared to that of H2TPP(F) deposited on different planar substrates (namely SiO2 and Au), has been studied by CL spectroscopy in a S360 Cambridge SEM equipped with a GatanMonoCL system (1800 lines per mm grating, multi-alkali photomultiplier sensitive in the range 350–830 nm). The spectra have been collected at room temperature, with an accelerating voltage of 10 kV, a beam current of 10 nA and a spectral resolution of 9 nm.
Photoluminescence analysis
In addition to cathodoluminescence analysis (i.e., optical emission induced by the electrons in the SEM), the bare and functionalized NWs have been optically excited. A Leica SP5-II confocal microscope (Leica Instruments, Wetzlar, Germany), equipped with an argon (458 nm) laser has been used for the fluorescence imaging. The bare and functionalized NWs have been observed using a 20× magnification objective, obtaining images with a total area of about 775 μm × 775 μm. For imaging, the emission bandwidth has been set from 620–740 nm. Photoluminescence spectra have been recorded using a 476.5 nm line of an Ar+ ion laser with power below 2 mW focalized on the samples. The setup was designed in order to shine the excitation beam on the sample with about 80° angle from the surface normal direction, and collect the emitted signal at an angle of 90° from the direction of the incident excitation beam. The luminescence signal was monitored with a double monochromator with a resolution of 1 nm equipped with a cooled photomultiplier in the photon counting mode.X-ray photoelectron spectroscopy
The bare and functionalized NWs (before and after the sonication process) have been characterised by XPS, using a Mg-Kα X-ray source (i.e., emission at 1253.6 eV). The photoelectrons have been collected by a VSW HA 100 electron energy analyzer, leading to a total energy resolution of 0.8 eV. All core level BEs have been referred to the Au 4f7/2 core level signal (at BE = 84.0 eV), obtained from a sputtered Au surface. The C 1s and F 1s core levels of the organic molecule and the (bare and functionalized) NWs have been analyzed through Voigt line shape deconvolution, after background subtraction of a Shirley function. The typical precision for each energy peak position is ±0.05 eV.Singlet oxygen generation
The production of singlet oxygen (1O2) in water solution has been detected by a commercial reagent, the singlet oxygen sensor green (SOSG, Life Technologies),42 based on fluorescein bound to a dimethylanthracene derivative. The functionalized NWs (after being removed from the substrate, as previously reported)15,16 have been dispersed in 500 μL of a SOSG 5 μM water solution. The dispersed NWs have been exposed to X-rays in a clinical 6 MV linear accelerator (operation parameters: gantry rotated upside down, irradiation field 10 × 10 cm2, source-skin distance (SSD) 1 m, 30 × 30 × 1.5 cm plastic water slab for dose build-up, dose rate 600 monitor units per minute, i.e., 6 Gy min−1). After irradiation, the functionalized NWs have been separated through ultra-centrifugation. The centrifugation and trustful separation of the functionalized NWs from the SOSG marker was ensured by measuring the dry product before and after ultracentrifugation (10 min at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm). A volume of 200 μL of the remaining solution has been sampled for fluorescence analysis in a high-sensitivity Varian Cary Eclipse spectrophotometer (xenon flash lamp, signal collection in backscattering geometry, acquisition with a photomultiplier detector in the range 520–620 nm). The excitation wavelength has been set at 505 nm, accordingly to SOSG data sheets, and the sample has been illuminated only during the acquisition time (about 1 min). The fluorescence spectra have been acquired in the same run on the as-prepared solution (i.e., reference signal of the SOSG marker), the irradiated solution (i.e., background without NWs), and the irradiated solution treated with functionalized NWs.
000 rpm). A volume of 200 μL of the remaining solution has been sampled for fluorescence analysis in a high-sensitivity Varian Cary Eclipse spectrophotometer (xenon flash lamp, signal collection in backscattering geometry, acquisition with a photomultiplier detector in the range 520–620 nm). The excitation wavelength has been set at 505 nm, accordingly to SOSG data sheets, and the sample has been illuminated only during the acquisition time (about 1 min). The fluorescence spectra have been acquired in the same run on the as-prepared solution (i.e., reference signal of the SOSG marker), the irradiated solution (i.e., background without NWs), and the irradiated solution treated with functionalized NWs.
3. Results and discussion
The XEOL spectrum of the bare SiC/SiOx core/shell NWs is reported in Fig. 1a (red), together with the absorption spectrum of the partially fluorinated tetraphenyl-porphyrin H2TPP(F) bulk material (blue). The bare SiC/SiOx core/shell NWs emit bright luminescence after X-ray excitation, shown as a broad peak at a wavelength of ∼460 nm (2.7 eV), in good agreement with previous XEOL studies on similar NWs.43,44 Overlapping the XEOL spectrum of the NWs with the absorption spectrum of H2TPP(F) highlights the spectral interval, where the optical emission of the inorganic donor matches the absorption bands (Soret band tail and Q bands) of the organic acceptor.A schematic representation of the chemical species of H2TPP(F) is illustrated in Fig. 1b. As can be seen, four fluorinated phenyl rings are connected to the meso-carbon atoms (i.e., positions 5, 10, 15, 20) of the porphyrin macrocycle. The free molecule has a large steric volume, due to the presence of the four phenyl rings rotated by ∼90° with respect to the main molecular plane.
After NWs' surface functionalization using H2TPP(F), the hybrid NWs/H2TPP(F) nanosystem has been comprehensively characterised by electron microscopy techniques, see Fig. 2. The SEM image in Fig. 2a shows the typical dense network of bare NWs,15,46 which proves that the morphology of the NWs is not altered by our functionalization approach.
To investigate the influence of the NWs on the luminescence behavior of the hybrid nanosystem, we carried out CL spectroscopy, a technique where the excitation source is the highly energetic (10 keV) electron beam of the SEM. Note that the CL spectrum of the bare NWs (see Fig. S1a, ESI†) exhibits the same lineshape and peak position than the corresponding XEOL spectrum in Fig. 1a. As evidenced by Montecarlo simulations,15 the energy directly released to the porphyrin layer by the impinging electrons is negligible. Therefore, direct excitation of a porphyrin layer by 10 keV electrons can be excluded, and any possible CL signal has its origin either in the underlying substrate or due to energy transfer from the substrate to the porphyrin layer. A representative CL spectrum acquired on the hybrid NWs/H2TPP(F) nanosystem is reported in Fig. 2b (red line), compared with typical CL spectra of H2TPP(F) bulk material deposited on planar SiO2 and Au substrates. A very intense luminescence is observed for H2TPP(F) on NWs, which is more than two orders of magnitude higher than on the planar substrates. This enhanced luminescence observed only for H2TPP(F) on SiC/SiOx core/shell NWs highlights the importance of the NWs' luminescence properties and the NW-mediated porphyrin excitation. A more detailed fitting analysis of the CL spectra of bare and functionalized NWs is provided in Fig. S1a (see ESI†).
The chemical mapping performed by energy dispersive X-ray (EDX) microanalysis in a TEM on single functionalized NWs (see a representative analysis in Fig. 2c) confirms that the characteristic elements of H2TPP(F), i.e., F and N are detected along the NW, and no clusters and/or inhomogeneous coverage are observed. In such a single NW analysis, the fluorine signal is rather noisy, but the detection of an intense peak at the F-K edge in the TEM-EDX spectrum acquired on a NW ensemble (see Fig. 2d) clearly proves the presence of the organic component on the NWs after the SuMBD process.
Fig. 3a and b show the fluorescence confocal images of the bare and functionalized NWs as determined for an optical bandwidth typical of the molecule's emission (i.e., 620–740 nm). The bare NWs in Fig. 3a do not show remarkable luminescence, as expected due to the negligible emission of the NWs in this wavelength range. In contrast, the hybrid NWs/H2TPP(F) nanosystem (Fig. 3b) exhibits strongly enhanced luminescence in the analyzed spectral range proving effective functionalization of the NWs.
The corresponding photoluminescence spectra of the bare and functionalized NWs are reported in Fig. 3c. The emission of the bare NWs (blue in Fig. 3c) consists of a single peak centred at a wavelength of 538 nm, which can be ascribed to the near band-edge emission (NBE) of the crystalline 3C-SiC core of the NWs.12
The intensity of the NWs' emission is largely quenched after functionalization (red in Fig. 3c). Moreover, the spectrum exhibits two peaks at 664 and 704 nm. These two emissions are characteristic for the porphyrin's Qx(0,0) and Qx(0,1) transitions.15,47 As photoluminescence analysis has been performed after optical excitation using a wavelength of 476.5 nm, i.e., inside the absorption of the NWs (3C-SiC bandgap ∼2.41 eV) but out of the main absorption bands of H2TPP(F) (see grey dashed line in Fig. S1b, ESI†), the remarkable fluorescence of the hybrid NWs/H2TPP(F) nanosystem has to be ascribed to an energy transfer from the NWs to the porphyrin, most likely by a Förster resonant energy transfer (FRET) between inorganic donor and organic acceptor.48–50
To elucidate the interaction of the H2TPP(F) with the NWs, both shape and constituents of the C 1s and F 1s core levels, before and after functionalization, have been studied via XPS fitting analysis, see Fig. 4a and b. In addition, the upper panels in Fig. 4a and b display the corresponding C 1s/F 1s core levels of the H2TPP(F) bulk material deposited on SiC/SiOx core/shell NWs, which have assisted us to distinguish the components stemming from NWs and molecule.
The Voigt analysis of the C 1s core level spectrum of bare SiC/SiOx core/shell NWs allows us to identify at least three different components (grey in Fig. 4a, lower panel). As previously reported,12 these components can be assigned to three chemical species present in the NWs, i.e., carbon of Si–C bond at a low binding energy (BE) of 287.2 eV, carbon related to C–C bonds (BE = 288.3 eV), and carbon stemming from oxycarbides (BE = 290 eV), such as Si–O–C and/or C–O compounds. Due to the high number of different carbon species in the H2TPP(F) molecule (see also schematic representation of the molecule in Fig. 1b), a complex structure of the C 1s core level after functionalization is expected (see Fig. 4a, middle panel).
In order to properly fit the C 1s/F 1s core levels of the functionalized NWs (after sonication), it is reasonable to first analyze the corresponding spectra of the H2TPP(F) bulk material (see Fig. 4a and b, upper panels). In agreement with previous reports,45 the C 1s core level of the H2TPP(F) exhibits two peaks at ∼286.5 eV and 289.5 eV, with remarkably different line shapes. Whereas the main peak at higher BE can be clearly attributed to the phenyl carbon atoms that are fluorine-saturated (CFPh),45 the broad peak at lower BE has to be fitted with more than one component. The model proposed to fit this line shape with four components is supported by theoretical calculations,45 and accounts for the different (properly weighted) carbon atoms of the molecule.
In the following, the contributions to the broad peak at lower BE are listed in order of decreasing BE and related to the C atoms in the molecule (as labelled in Fig. 1b):
• a component at 287.2 eV (brown in Fig. 4a) related to the bonds between C in α positions and pyrrolic N in the rings labelled as A and C in the macrocycle (i.e., positions 1, 4, 11, 14 in Fig. 1b), and also related to the C atoms of the phenyl rings not involved in C–F bonds,
• a component at 286.6 eV (orange in Fig. 4a) related to the bonds between C in α positions and aza-N in the pyrrolic rings labelled as B and D in the macrocycle (i.e., positions 6, 9, 16, 19), and also related to the C atoms in mesopositions (i.e., positions 5, 10, 15, 20),
• a component at 286.1 eV (violet in Fig. 4a) related to the C atoms in β positions involved in the C–C bonds of the A and C pyrrolic rings (i.e., positions 2, 3,12,13),
• a component at 285.5 eV (cyan in Fig. 4a) related to the C atoms in β positions involved in the C–C bonds of the B and D pyrrolic rings (i.e., positions 7, 8, 17, 18).
The F 1s core level of the H2TPP(F) bulk material (see Fig. 4b, upper panel) consists of a single main peak centred at BE = 689.5 eV. As expected for the H2TPP(F) bulk material,45 this single component confirms the presence of one single chemical species of fluorine atoms (despite the different locations of F atoms in the phenyl ring, as depicted for the free molecule in the upper panel of Fig. 4c).
The fitting analysis of the C 1s core level of the hybrid NWs/H2TPP(F) nanosystem has been performed as a superposition of the components stemming from the NWs and the molecule (middle panel in Fig. 4a). An additional peak at higher BE (290.5 eV) is needed to complete the fit (red in Fig. 4a), indicating a new chemical species of C atoms when the molecule is in contact with the NWs. Furthermore, the F 1s core level of the functionalized NWs (lower panel in Fig. 4b) is clearly broadened and has to be fitted with an additional component at BE = 690.5 eV (red in Fig. 4b). As in the case of the C 1s core level, this points towards a new chemical species of F atoms when the molecule is in contact with the NWs. It is noteworthy that these new C and F species are present after sonication of the functionalized NWs, i.e., physisorbed molecules are removed and a covalent functionalization of the nanowires with the porphyrin molecules is expected.
Assuming a locally flat surface of the NWs (NW radius ∼30 nm, i.e., much larger than the size of the molecule) and a mostly “flat-lying” orientation of the H2TPP(F) macrocycle on the NWs' surface (as supported by the XAS results in Fig. S3, ESI†), up to 8 of the 20 F atoms of the molecule can potentially react with the NWs' surface (see schematic illustration in the lower panel of Fig. 4c). In fact, both peaks originally stemming from CFPH and the fluorine atoms (green in Fig. 4a and b) can be split into two components, where the new peaks (red in Fig. 4a and b) are weighted with 40% of the corresponding components in the free molecule.
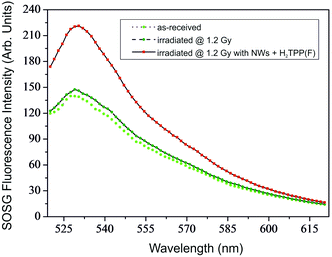
The energy transfer from the NWs to the porphyrin derivative can be exploited for key applications, mainly the singlet oxygen (1O2) generation even after X-ray irradiation. To prove the 1O2 production mediated by the energy transfer, the hybrid NWs/H2TPP(F) nanosystem has been exposed to highly energetic X-rays (6 MV, produced by a standard linear accelerator). The singlet oxygen has been revealed by an ad hoc marker (singlet oxygen sensor green, SOSG),42,51 highly selective to 1O2 against other oxygen species (such as hydroxyl radicals, superoxide, etc.). Fig. 5 shows the typical (representative) fluorescence spectra of the SOSG marker as-received, irradiated in water, and irradiated at the same dose in water with the suspended hybrid NWs/H2TPP(F) nanosystem.
A statistical analysis of the SOSG integrated fluorescence intensity of 10 repeated experiments can be found in Fig. S4 (ESI†). The comparison between the spectra highlights the enhancement of the green fluorescence, which is activated in SOSG by interaction with 1O2, in the sample treated with functionalized NWs and radiation. This proves that a photodynamic process, i.e., the singlet oxygen production by H2TPP(F), mediated by NW excitation under X-rays, has occurred.
4. Conclusions
Surface functionalization of SiC/SiOx core/shell NWs has been achieved by supersonic molecular beam deposition (SuMBD) of a partially fluorinated tetraphenyl-porphyrin H2TPP(F). Chemical analysis using EDX-TEM and luminescence characterization (CL-SEM and fluorescence after optical excitation) have confirmed the creation of a hybrid NWs/H2TPP(F) nanosystem with enhanced optical properties. The inorganic–organic interface of the nanosystem has been analyzed by XPS. A detailed analysis of the core levels (C1s, F1s) provides evidence of a strong interaction between the molecule and the inorganic NWs, i.e., the photosensitizer has been facilely linked to the nanowires leading to the formation of a stable hybrid nanosystem. We present a detailed picture of the molecules' binding to the silica shell of the NWs, together with the (average) orientation of the molecule, as measured by XAS on planar SiO2. Furthermore, we show that the hybrid NWs/H2TPP(F) nanosystem is an potential source of singlet oxygen when irradiated with 6 MeV X-rays at low dose (1.2 Gy). Therefore, the hybrid NWs/H2TPP(F) nanosystem offers potentially promising system for X-ray irradiated PDT. In vitro studies about the internalization of the hybrid NWs/H2TPP(F) nanosystem into different cancer cell lines are currently ongoing.Acknowledgements
The authors gratefully acknowledge Drs. R. Alinovi and S. Pinelli (Dept. of Clinical and Experimental Medicine – Unipr) and Dr. G. Benecchi (Dept. of Medical Physics @ A.O.U. Parma Hospital) for their help in the experiments concerning singlet oxygen production. Prof. S. Nannarone is gratefully acknowledged for the stimulating discussions concerning the XEOL measurements. The authors also acknowledge the LaBSSAH Laboratory (Bruno Kessler Foundation, Trento) for the access to the microscope facility. This study was supported by the CARITRO Foundation, project FoXIR (Progetti di ricerca scientifica svolti da giovani ricercatori, 2015). M. T. gratefully acknowledges the support by a Feodor-Lynen-Fellowship of the Alexander v. Humboldt foundation, Bonn (Germany).References
- K. Salonitis, J. Pandremenos, J. Paralikas and G. Chryssolouris, Int. J. Adv. Des. Manuf. Technol., 2010, 49, 803–826 CrossRef.
- R. F. Gibson, Compos. Struct., 2010, 92, 2793–2810 CrossRef.
- K. Varaprasad, K. Ramam, G. S. Mohan Reddy and R. Sadiku, RSC Adv., 2014, 4, 60363–60370 RSC.
- M. Curreli, C. Li, Y. Sun, B. Lei, M. A. Gundersen, M. E. Thompson and C. Zhou, J. Am. Chem. Soc., 2005, 127, 6922–6923 CrossRef CAS PubMed.
- Y. L. Bunimovich, G. Ge, K. C. Beverly, R. S. Ries, L. Hood and J. R. Heath, Langmuir, 2004, 20, 10630–10638 CrossRef CAS PubMed.
- F. Patolsky and C. M. Lieber, Mater. Today, 2005, 8, 20–28 CrossRef CAS.
- C. E. Linsmeier, C. N. Prinz, L. M. E. Pettersson, P. Caroff, L. Samuelson, J. Schouenborg, L. Montelius and N. Danielsen, Nano Lett., 2009, 9, 4184–4190 CrossRef PubMed.
- C. Coletti, M. J. Jaroszeski, A. Pallaoro, A. M. Hoff, S. Iannotta and S. E. Saddow, Proc. 29th Annu. Int. Conf. IEEE EMBS, 2007, pp. 5849–5852 Search PubMed.
- C. L. Frewin, M. Jaroszeski, E. Weeber, K. E. Muffly, A. Kumar, M. Peters, A. Oliveros and S. E. Saddow, J. Mol. Recognit., 2009, 22, 380–388 CrossRef CAS PubMed.
- S. E. Saddow, C. L. Frewin, C. Coletti, N. Schettini, E. Weeber, A. Oliveros and M. Jarosezski, Mater. Sci. Forum, 2011, 679–680, 824–830 CrossRef CAS.
- R. Yakimova, R. M. Petoral, G. R. Yazdi, C. Vahlberg, A. Lloyd Spetz and K. Uvdal, J. Phys. D: Appl. Phys., 2007, 40, 6435–6442 CrossRef CAS.
- F. Fabbri, F. Rossi, G. Attolini, G. Salviati, S. Iannotta, L. Aversa, R. Verucchi, M. Nardi, N. Fukata, B. Dierre and T. Sekiguchi, Nanotechnology, 2010, 21, 345702 CrossRef PubMed.
- F. Fabbri, F. Rossi, P. Lagonegro, M. Negri, J. S. Ponraj, M. Bosi, G. Attolini and G. Salviati, J. Phys. D: Appl. Phys., 2014, 47, 394006 CrossRef.
- F. Fabbri, F. Rossi, M. Melucci, I. Manet, G. Attolini, L. Favaretto, M. Zambianchi and G. Salviati, Nanoscale Res. Lett., 2012, 7, 680 CrossRef PubMed.
- F. Rossi, E. Bedogni, F. Bigi, T. Rimoldi, L. Cristofolini, S. Pinelli, R. Alinovi, M. Negri, S. C. Dhanabalan, G. Attolini, F. Fabbri, M. Goldoni, A. Mutti, G. Benecchi, C. Ghetti, S. Iannotta and G. Salviati, Sci. Rep., 2015, 5, 7606 CrossRef CAS PubMed.
- A. Cacchioli, F. Ravanetti, R. Alinovi, S. Pinelli, F. Rossi, M. Negri, E. Bedogni, M. Campanini, M. Galetti, M. Goldoni, P. Lagonegro, R. Alfieri, F. Bigi and G. Salviati, Nano Lett., 2014, 14, 4368–4375 CrossRef CAS PubMed.
- H. Huang, W. Song, J. Rieffel and J. F. Lovell, Front. Phys., 2015, 3, 1–15 CrossRef.
- A. Ambroise, R. W. Wagner, P. D. Rao, J. A. Riggs, P. Hascoat, J. R. Diers, J. Seth, R. K. Lammi, D. F. Bocian, D. Holten and J. S. Lindsey, Chem. Mater., 2001, 13, 1023–1034 CrossRef CAS.
- T. Bessho, S. M. Zakeeruddin, C. Yeh, E. W. Diau and M. Grätzel, Sol. Cells, 2010, 6646–6649 CAS.
- A. Fateeva, P. A. Chater, C. P. Ireland, A. A. Tahir, Y. Z. Khimyak, P. V. Wiper, J. R. Darwent and M. J. Rosseinsky, Angew. Chem., Int. Ed., 2012, 51, 7440–7444 CrossRef CAS PubMed.
- O. Legrini, E. Oliveros and A. M. Braun, Chem. Rev., 1993, 93, 671–698 CrossRef CAS.
- M. C. DeRosa and R. J. Crutchley, Coord. Chem. Rev., 2002, 233–234, 351–371 CrossRef CAS.
- M. J. Davies, Biochem. Biophys. Res. Commun., 2003, 305, 761–770 CrossRef CAS PubMed.
- J. M. Dąbrowski, B. Pucelik, M. M. Pereira, L. G. Arnaut, W. Macyk and G. Stochel, RSC Adv., 2015, 5, 93252–93261 RSC.
- Y. Liu, W. Chen, S. Wang and A. G. Joly, Appl. Phys. Lett., 2008, 92, 43901 CrossRef.
- R. R. Allison, G. H. Downie, R. Cuenca, X. H. Hu, C. J. H. Childs and C. H. Sibata, Photodiagn. Photodyn. Ther., 2004, 1, 27–42 CrossRef CAS PubMed.
- W. Chen and J. Zhang, J. Nanosci. Nanotechnol., 2006, 6, 1159–1166 CrossRef CAS PubMed.
- J. Hu, Y. Tang, A. H. Elmenoufy, H. Xu, Z. Cheng and X. Yang, Small, 2015, 11, 5860–5887 CrossRef CAS PubMed.
- A. Kamkaew, F. Chen, Y. Zhan, R. L. Majewski and W. Cai, ACS Nano, 2016, 10, 3918–3935 CrossRef CAS PubMed.
- W. Fan, P. Huang and X. Chen, Chem. Soc. Rev., 2016, 45, 6488–6519 RSC.
- Y. Que, Y. Liu, W. Tan, C. Feng, P. Shi and Y. Li, ACS Macro Lett., 2016, 5, 168–173 CrossRef CAS.
- H.-G. Jeong and M.-S. Choi, Isr. J. Chem., 2016, 56, 110–118 CrossRef CAS.
- P. Milani and S. Iannotta, Cluster beam synthesis of nanostructured materials, Springer-Verlag, Berlin, 1999 Search PubMed.
- Y. Wu, T. Toccoli, N. Koch, E. Iacob, A. Pallaoro, P. Rudolf and S. Iannotta, Phys. Rev. Lett., 2007, 98, 76601 CrossRef PubMed.
- M. Nardi, R. Verucchi, C. Corradi, M. Pola, M. Casarin, A. Vittadini and S. Iannotta, Phys. Chem. Chem. Phys., 2010, 12, 871–880 RSC.
- M. Nardi, R. Verucchi, R. Tubino and S. Iannotta, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 1–9 CrossRef.
- R. Verucchi, L. Aversa, G. Ciullo, A. Podestà, P. Milani and S. Iannotta, Eur. Phys. J. B, 2002, 26, 509–514 CAS.
- R. Verucchi, L. Aversa, M. V. Nardi, S. Taioli, S. A. Beccara, D. Alfè, L. Nasi, F. Rossi, G. Salviati and S. Iannotta, J. Am. Chem. Soc., 2012, 134, 17400–17403 CrossRef CAS PubMed.
- L. Aversa, R. Verucchi, G. Ciullo, L. Ferrari and P. Moras, Appl. Surf. Sci., 2001, 184, 350–355 CrossRef CAS.
- M. Negri, S. C. Dhanabalan, G. Attolini, P. Lagonegro, M. Campanini, M. Bosi, F. Fabbri and G. Salviati, CrystEngComm, 2015, 17, 1258–1263 RSC.
- V. Maiorano, F. Matino, R. Cingolani, J. Thompson and R. I. R. Blyth, J. Synchrotron Radiat., 2005, 12, 690–695 CrossRef CAS PubMed.
- http://tools.lifetechnologies.com/content/sfs/manuals/mp36002.pdf .
- L. Liu, J. Y. P. Ko, M. J. Ward, Y. M. Yiu, T. K. Sham and Y. Zhang, J. Phys.: Conf. Ser., 2009, 190, 12134 CrossRef.
- L. Liu, Y. M. Yiu, T. K. Sham, L. Zhang and Y. Zhang, J. Phys. Chem. C, 2010, 114, 6966–6975 CAS.
- M. Nardi, R. Verucchi, L. Aversa, M. Casarin, A. Vittadini, N. Mahne, A. Giglia, S. Nannarone and S. Iannotta, New J. Chem., 2013, 37, 1036 RSC.
- F. Fabbri, F. Rossi, G. Attolini, M. Bosi, G. Salviati, S. Iannotta, L. Aversa, R. Verucchi, M. Nardi, N. Fukata, B. Dierre and T. Sekiguchi, Mater. Sci. Forum, 2012, 717–720, 557–560 CrossRef CAS.
- R. Wiglusz, J. Legendziewicz, A. Graczyk, S. Radzki, P. Gawryszewska and J. Sokolnicki, J. Alloys Compd., 2004, 380, 396–404 CrossRef CAS.
- T. Förster, Discuss. Faraday Soc., 1959, 7–17 RSC.
- A. L. Bulin, C. Truillet, R. Chouikrat, F. Lux, C. Frochot, D. Amans, G. Ledoux, O. Tillement, P. Perriat, M. Barberi-Heyob and C. Dujardin, J. Phys. Chem. C, 2013, 117, 21583–21589 CAS.
- Y. Tang, J. Hu, A. H. Elmenoufy and X. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 12261–12269 CAS.
- S. Clement, W. Deng, E. Camilleri, B. C. Wilson and E. M. Goldys, Sci. Rep., 2016, 6, 19954 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed fitting analysis of the CL spectra of bare and functionalized NWs (Fig. S1a). CL emission spectrum of SiC/SiOx core/shell NWs, overlapped with the absorption spectrum of H2TPP(F) (Fig. S1b). Water contact angle measurements on planar (bare and functionalized) SiO2 substrates (Fig. S2). X-ray absorption spectroscopy (XAS) of a monolayer of H2TPP(F) on a planar SiO2 substrate (Fig. S3). Plot of the SOSG integrated fluorescence intensity of 10 repeated experiments (Fig. S4). See DOI: 10.1039/c7me00005g |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |