Broccoli sprouts in analgesia – preclinical in vivo studies
Nieves
Baenas
ab,
María Eva
González-Trujano
*a,
Omar
Guadarrama-Enríquez
 a,
Francisco
Pellicer
a,
Cristina
García-Viguera
b and
Diego A.
Moreno
*b
a,
Francisco
Pellicer
a,
Cristina
García-Viguera
b and
Diego A.
Moreno
*b
aLaboratory of Neuropharmacology of Natural Products, Neuroscience Research Department, Instituto Nacional de Psiquiatría “Ramón de la Fuente”, Av. México-Xochimilco 101, Col. Sn Lorenzo Huipulco, 14370, México, D. F., Mexico. E-mail: evag@imp.edu.mx; Fax: (+52 55) 5655-9980; Tel: (+52 55) 4160-5085
bPhytochemistry Lab, Department of Food Science and Technology, CEBAS-CSIC, Campus de Espinardo 25, 30100 Murcia, Spain. E-mail: dmoreno@cebas.csic.es; Fax: (+34 968) 396213; Tel: (+34 968) 396369
First published on 25th November 2016
Abstract
Background: Broccoli is a rich source of health-promoting glucosinolates, phenolic compounds, minerals and vitamins, which might have potential to alleviate pain. Aim: To explore the antinociceptive effects of a broccoli sprout aqueous extract (BSE) in experimental models of pain and an opioid mechanism. Materials and methods: the BSE was administered to mice and rats that were subjected to the writhing and formalin tests, respectively. Gastric damage or sedative-like response, as adverse effects observed in anti-inflammatory non-steroidal and opioid analgesic drugs, respectively, were also explored. Results: Antinociception, but not sedative or gastric injury response, was observed in a significant and dose-dependent manner with the BSE (50–500 mg kg−1, i.p. and 500–2000 mg kg−1, p.o.) in comparison to the control group; these effects resembled those observed with the analgesic tramadol (30 mg kg−1, i.p.) in writhing and formalin assessments. Blockage of opioid receptors by naloxone (1 mg kg−1, i.p.) produced partial inhibition of the antinociceptive effect of the BSE in both assays. Conclusion: This study gives evidence of the potential activity of broccoli sprouts in pain therapy.
Introduction
Pain is an enormous problem of health. It is associated with a wide range of injuries and diseases often caused by inflammation and it is sometimes the disease itself. The prevalence, incidence, and vast social and health consequences of pain require attention from the public health system to address this global issue.1 Because of the significant side effects of opioids, steroidal and NSAID medications, currently the most applied analgesics, there is greater interest in natural alternatives, such as dietary supplements and herbal remedies, which have been used for centuries to reduce pain and inflammation, not only for mild to moderate aches but also for chronic pain.2Studies have revealed that dietary consumption of Brassica vegetables may potentially have a chemopreventive and anti-inflammatory effect diminishing the risk of several chronic diseases.3 The in vitro and in vivo studies have shown that Brassica phytochemicals may palliate inflammatory and antioxidant pathways through inhibition of the nuclear factor kappa B (NF-κB) activity, which is consistently related to the control of inflammatory diseases in animal models.4 Broccoli sprouts (Brassica oleracea var. italica) are considered a rich source of health-promoting compounds including glucosinolates (GLS), isothiocyanates (ITC),5 phenolic compounds,6 vitamins and minerals,7 and bioactive compounds also related to the reduction of oxidative stress and inflammation.8
Very few preliminary studies of broccoli have reported significant central nervous system (CNS) actions by administration of extracts from mature plant organs (inflorescences), sprouts or isolated phytochemicals to induce antinociceptive activity, for example, the oral administration of a hydro-alcoholic extract of mature broccoli9 or the anxiolytic-like activity of pure sulforaphane.10 As a plant material used in this work, the Brassica oleracea sprouts were elicited with the phytohormone methyl jasmonate (MeJA) (250 μM), following the protocol studied by Baenas et al.,11 obtaining safe and ready-to-eat vegetables enriched with bioactive compounds. As mentioned, these sprouts have been investigated because they contain higher levels of constituents with anti-inflammatory and antioxidant activities than the mature plant, useful in cancer prevention and development. However, there is very limited information to support the antinociceptive properties of broccoli sprouts and their possible mechanisms of action. Therefore, we evaluated the potential of an aqueous broccoli sprout extract (BSE) for analgesic and anti-inflammatory activities and the involvement of the opioid mechanism in vivo using experimental models of nociception, and also examining gastric damage and sedative effects in rodents as the possible adverse effects observed when using analgesic therapy.
Materials and methods
Plant material and extract preparation
Broccoli seeds for sprouting (Brassica oleracea var. italica Plenck.) were provided by Intersemillas S.A (Valencia, Spain). Sprouts were germinated under controlled conditions for 8 days according to Baenas et al.11 protocols, and elicited by spraying methyl jasmonate (MeJA, 250 μM) once a day, during 4 days prior to harvest. Briefly, sprouts were collected, flash frozen in liquid nitrogen, and stored at −80 °C until lyophilized and ground into a fine powder.The BSE was obtained by maceration and continuous shaking of 1.6 g of ground and freeze-dried broccoli sprouts in 50 mL MilliQ water overnight at room temperature. The sample was sonicated before and after the maceration, and then the BSE was centrifuged and the supernatant was lyophilized to obtain a fine powder used in the experiments.
Drugs
Tramadol, pharmaceutical grade (98%) (Grünenthal de México, S.A. de C.V.), bismuth subsalicylate (Pepto-Bismol®), and sodium pentobarbital (Sedalpharma®) were used. Naloxone, diazepam and indomethacin were purchased from Sigma (Sigma-Aldrich Co., St Louis MO, USA) and dissolved in saline solution (s.s.) or resuspended in 0.5–1% Tween 80 in s.s. correspondingly. Control animals received distilled water as vehicle. Formalin and acetic acid (J.T. Baker, USA) were prepared at 1% solutions to induce nociception. Absolute ethanol (J.T. Baker, USA) was used for analyzing the gastric damage. Drugs were freshly prepared on the day of the experiments; all treatments were administered intraperitoneally (i.p.) or orally (p.o.) in a volume of 0.1 ml per 10 g or 100 g body weight in mice and rats, respectively.Identification and quantitative analysis of broccoli sprouts and BSE by HPLC-DAD-ESI-MSn and UHPLC-QqQ-MS/MS
The GLS and phenolic compounds in broccoli sprouts and the BSE were identified and quantified by HPLC-DAD-ESI-MSn according to Baenas et al.12 Briefly, lyophilized samples were extracted with methanol (70% v/v) and heated at 70 °C in order to avoid enzyme myrosinase activity which hydrolyzes GLS to ITC, allowing the quantification of individual compounds that were identified based on retention times, UV absorption maxima, and mass spectra or comparison with authentic compounds when possible. The ITC in broccoli sprouts and the BSE were extracted according to the Cramer and Jeffery13 protocol and quantified by UHPLC-QqQ-MS/MS following Domínguez-Perles et al.14Pharmacological evaluation
Experimental groups
Groups of at least six mice or rats, depending on the experimental model, were organized as follows: vehicle group (mice or rats received distilled water); three or four groups receiving acute administration of BSE, p.o. (500, 1000 and 2000 mg kg−1) or i.p. (50, 100, 250 and 500 mg kg−1), respectively, 30 min previous to the experimental test, and one group receiving the positive control (opioid analgesic tramadol, i.p.). Bismuth subsalicylate (antiulcer drug, p.o.) and indomethacin (anti-inflammatory inhibitor of cyclooxygenases, p.o.) were used as reference drugs in gastric protection and gastric ulcer, respectively.Antinociceptive activity
The Writhing test was used as previously described by Collier et al.15 Immediately after 1% acetic acid, i.p., administration, latency to the onset of the writhes and the total number of writhes were recorded in the following periods: 0–5, 5–10, 10–15, 15–20, 20–25 and 25–30 min. The area under the curve (AUC) of the temporal courses was plotted to describe the dose-response in the effects of the BSE in comparison to tramadol and the vehicle group.Formalin test: Immediately after 50 μl injection in the subplantar area of the right hind paw with 1% formalin by using a 30-gauge needle, each rat was placed into a glass cylinder provided with mirrors to enable a total panorama of the nociceptive behavior. The number of shakings and/or the accumulated time spent in licking the injected paw was taken as the nociceptive response.16 Two periods of high shaking activity were considered: the first one was present immediately after injection and lasted 5 min; this was known as the early phase (neurogenic phase). The second period was observed 20–25 min after formalin injection and denoted as the late phase (inflammatory phase). Animals were administered with the aqueous extract 30 min before the intraplantar injection of formalin. Control animals received vehicle by the same route (p.o. or i.p.) and time of administration.
At the end of the nociceptive evaluation, rats were euthanized in a CO2 chamber and their stomachs were dissected and fixed with 4% formaldehyde to be qualitatively examined for tentative gastric damage by comparing the effect of indomethacin as the positive ulcerogenic drug.
Mechanism of action analysis
In order to explore the participation of the endogenous opioid system in the antinociceptive effect of the BSE, independent groups of mice and rats were administered naloxone (1 mg kg−1, s.c., an opioid receptor antagonist) 15 min previous to an antinociceptive dosage of the extract (50 or 250 mg kg−1, i.p.) or the vehicle. Thirty min after treatments, mice or rats were subjected to the corresponding nociceptive test to compare also with the effect of naloxone per se.Experimental absolute ethanol-induced gastric ulcer
Simultaneous to rat fasting, the groups were administered as follows: BSE (100 mg kg−1, p.o.), indometacin (20 mg kg−1, p.o., ulcerogenic drug) or the antiulcer drug bismuth subsalicylate (17.5 mg mL−1, p.o., 1 mL per rat). Control rats received distilled water as vehicle in the same volume and route of administration.Gastric ulcers were induced as follows: after 30 min of treatment or vehicle administration, each rat received a 0.5 mL volume of absolute ethanol via p.o. as modified from the report previously described.17 One hour after ethanol administration, the animals were sacrificed in a CO2 chamber. The stomach of each rat was dissected and examined to measure gastric lesions.
Measurement of gastric injury
Each stomach was dissected out from the esophagus to the pyloric portion and inflated with 10 ml of 4% formalin for 15 min to fix both the inner and outer gastric layers. The stomachs were incised along the greater curvature and examined for ulcers. Photographs of the hemorrhagic lesions were taken, scanned and saved in a processor to be measured by using a millimeter grid. The gastric injury was obtained as the ulcer area in mm.2Sedative-like response
Thirty minutes after a significant antinociceptive dose of BSE (100 mg kg−1, i.p.), sedative-like response in mice was investigated by using:Statistical analysis
Data are expressed as the mean ± standard error of the mean (S.E.M.). The area under the curve (AUC) values were calculated from the respective temporal course curves obtained in the writhing test, and were considered as an expression of the overall antinociceptive activity during a 30 min period in area units; the AUC was calculated using the trapezoidal rule. Statistical differences were analyzed using a one-way analysis of variance (ANOVA) followed by Tukey's test for multiple comparisons. A value of P < 0.05 was considered significant.Results
Potential bioactive compounds in broccoli sprouts and BSE
Broccoli sprouts are rich in glucoraphanin, quantified as 40% of the total GLS in our samples (Table 1). This compound is naturally hydrolysed by the enzyme myrosinase to the ITC sulforaphane allowing its detection in the BSE in a concentration of 0.95 mg g−1 dry weight (Table 1). A dosage of 100 mg kg−1 of BSE contains 0.54 μmol (rat) or 0.054 μmol (mice) of the ITC sulforaphane.| Glucosinolates | Broccoli sprouts | BSE |
|---|---|---|
| Mean values (n = 3) ± SD. D.W. (dry weight). | ||
| Bioactive compounds (mg g −1 D.W.) | ||
| Glucoiberin | 6.08 ± 0.38 | — |
| Glucoraphanin | 15.69 ± 0.09 | — |
| 4-Hydroxyglucobrassicin | 1.65 ± 0.20 | — |
| Glucoerucin | 3.59 ± 0.08 | — |
| Glucobrassicin | 3.02 ± 0.29 | — |
| 4-Methoxyglucobrassicin | 1.40 ± 0.10 | — |
| Neoglucobrassicin | 8.81 ± 0.20 | — |
| Aliphatic GLS | 25.36 ± 0.50 | |
| Indole GLS | 14.88 ± 0.64 | |
| Total | 40.25 ± 0.79 | |
| Phenolic compounds | ||
| Chlorogenic acid derivatives | 0.239 ± 0.039 | 0.075 ± 0.03 |
| Flavonols | 0.237 ± 0.039 | — |
| Sinapic acid derivatives | 26.588 ± 6.192 | 19.30 ± 1.75 |
| Total | 27.064 ± 6.270 | 19.38 ± 1.78 |
| Isothiocyanates | ||
| Sulforaphane | 0.383 ± 0.010 | 0.951 ± 0.001 |
| Iberin | 0.009 ± 0.001 | 0.026 ± 0.001 |
| Indole-3-carbinol | — | 0.013 ± 0.003 |
| Total | 0.392 ± 0.010 | 0.990 ± 0.005 |
The ITC iberin and indole-3-carbinol were also present in the BSE, as products of the aliphatic GLS glucoiberin and the indole glucobrassicin, respectively (Table 1). Individual GLS in the BSE were not identified (Table 1).
The predominant class of phenolic compounds in both broccoli sprouts and the BSE is the sinapic acid derivatives, accounting for 98–99% of the total amount, respectively (Table 1). Also, a little concentration of chlorogenic acid derivatives was found in both samples and less than 1% of total phenolics in broccoli sprouts corresponded to flavonols (Table 1).
Antinociception
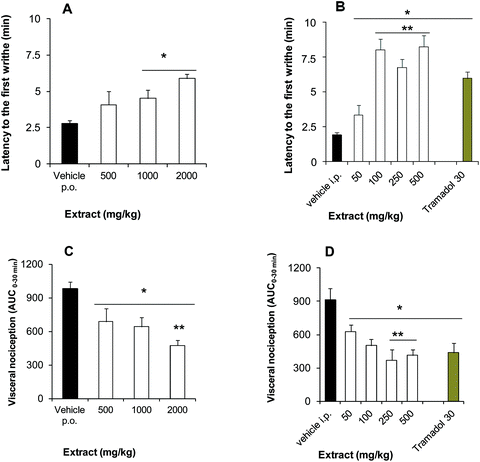
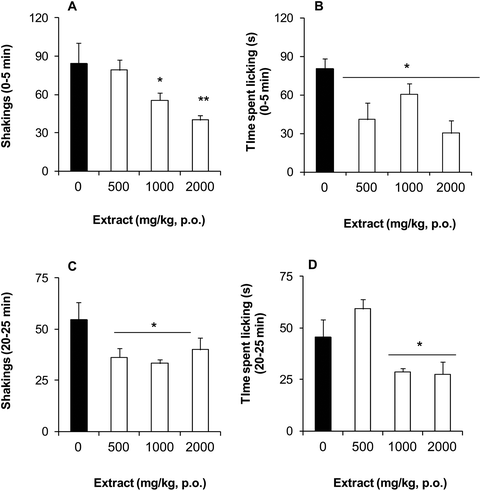
BSE at doses of 1000 to 2000 mg kg−1, p.o. (Fig. 1A) and 50 to 500 mg kg−1, i.p. (Fig. 1B) significantly and in a dose-dependent manner delayed the presence of the first writhe in the acetic acid-induced nociception in comparison to the vehicle group, resembling the delay observed with the analgesic tramadol (30 mg kg−1, i.p.) (Fig. 1B). The intensity of the acetic acid-induced nociception was also significant and in a dose-dependent trend decreased in all the doses of the BSE tested and in both routes of administration, fitting in the range of the observed effects of the analgesic drug taken as the reference (Fig. 1C and D).The BSE, by p.o. administration, significantly reduced the number of shakings (Fig. 2A and C) and the time spent doing licking behavior (Fig. 2B and D) in both phases of the formalin-induced nociception. A dose-dependent effect in shakings of the first phase was observed between the doses of 1000 to 2000 mg kg−1 (Fig. 2A).
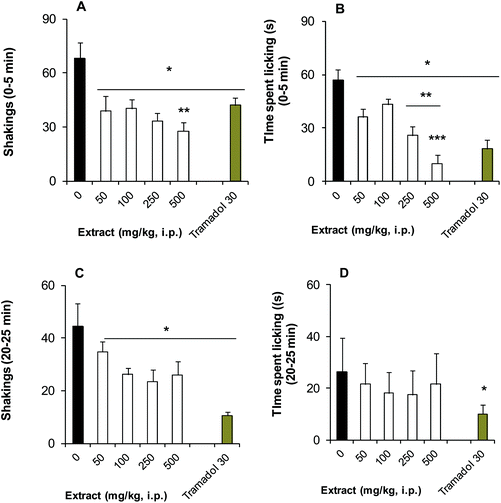
Similarly, a significant and dose-dependent reduction (50 to 500 mg kg−1) was observed in the shakings (Fig. 3A) and time spent doing licking (Fig. 3C) behaviour of the first phase of this test when the BSE was administered by the i.p. route. In the second phase, only shaking behaviour was significantly decreased, but not in a dose-dependent manner (Fig. 3C); while no changes were obtained in the time spent licking (Fig. 3D) in comparison to the vehicle group, resembling the antinociceptive response of the analgesic tramadol (30 mg kg−1, i.p.), mainly in the neurogenic phase.
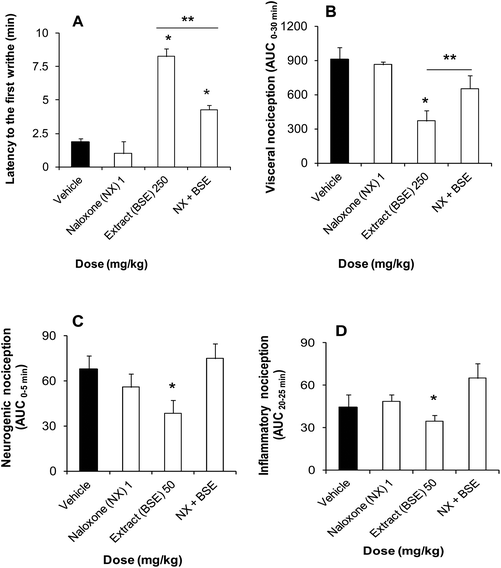
With regard to the mechanism of action, the presence of naloxone (1 mg kg−1, s.c.) per se did not produce different nociceptive responses in comparison to the vehicle group in both tests (Fig. 4). Nevertheless, naloxone partially inhibited the delay to the first writhe (Fig. 4A) and visceral nociception (Fig. 4B) produced by the BSE (250 mg kg−1, i.p.) in the acetic acid-induced nociception in mice. Moreover, this opioid antagonist abolished the reduction produced by this extract (50 mg kg−1, i.p.) in both neurogenic (Fig. 4C) and inflammatory (Fig. 4D) nociceptive responses in the formalin test in rats.
Gastric damage or protection
No ulcers were found in the group receiving the vehicle after evaluation in the formalin-induced nociception test, but they showed a slight erosion of the mucous layer; this effect was also observed in the rats receiving the BSE. In contrast, rats administered the analgesic indomethacin showed significant development of gastric ulcers (97.25 ± 43.55 mm2).In the presence of the ulcerogenic agent (0.5 mL of absolute ethanol), the rats receiving vehicle showed a major occurrence of ulcers all along the corpus area in the rat stomach (200 ± 13 mm2); this effect increased because of the combination with indomethacin (235.38 ± 78.39 mm2). The pre-treatment with the BSE prevented the severity of the gastric damage mainly in the surrounding of the corpus of the stomach (108.50 ± 41.50 mm2), but less effectively than the protection obtained with an anti-acid reference drug (26.81 ± 4.20 mm2).
Sedative-like effects
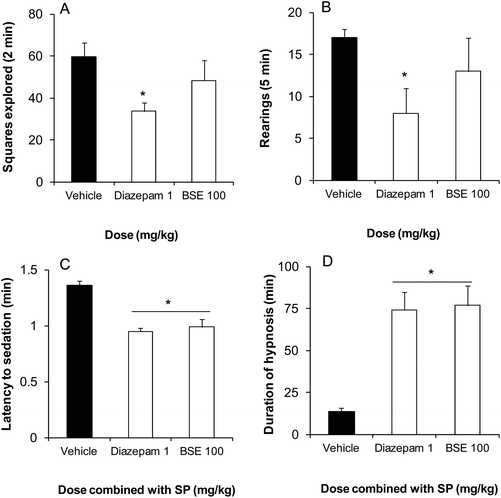
The BSE (100 mg kg−1) did not modify the ambulatory activity of mice in the open-field test (Fig. 5A) or in the exploration behaviour in the cylinder test (Fig. 5B) compared to the vehicle group, and in contrast to diazepam (an anxiolytic and sedative drug) (Fig. 5A and B). However, the extract combined with a sedative-hypnotic like SP was capable to facilitate the beginning of sedative-like behaviour (Fig. 5C) and to enhance significantly the duration of the sleeping time in mice receiving BSE administration (Fig. 5D) in a similar manner to diazepam (Fig. 5C and D).Discussion
Promoting the daily intake of bioactive compounds may be a valuable strategy to improve the management of pain.20 The present work reported a beneficial significant effect of antinociception of the broccoli sprout aqueous extract, administered by enteral (p.o.) and parenteral (i.p.) routes, and using two different models of induced nociception in rodents. The writhing test is used in mice as an acute tonic pain model; whereas the formalin test is applied to evaluate neurogenic and inflammatory nociception in mice and rats, both involve an opioid mechanism.The characteristic bioactive compounds present in broccoli sprouts have been studied before planning their studies in animal models, considering 8-day-old sprouts as optimum for consumption and phytochemical content.12 It has been observed that the profile of bioactive compounds found in sprouts varies with environmental stress, growth conditions and storage.21 In addition, application of the elicitor MeJA (250 μM) has been employed as a tool to increase the GLS content in these broccoli sprouts. Thus, in this study, it was important to evaluate the content of phytochemicals of the samples prior to developing the in vivo antinociceptive assays. Under our conditions, the total GLS content in the sprouts was higher than in other sprouted species22 and mature plants.23 GLS are naturally hydrolyzed to ITC, and these bioactive compounds are considered responsible for the reported bioactivity of cruciferous foods, such as anti-inflammatory and chemopreventive foods.3
The preliminary antinociceptive effects of Brassica vegetables have been described with a dose-dependent activity in the writhing test in mice by administrating a hydro-methanolic extract of Brassica juncea (200–400 mg kg−1, p.o.)24 and in both phases of the formalin test in rats by a hydro-alcoholic mature broccoli extract (2000 mg kg−1, p.o.).9 In our study, the inhibitory response in the abdominal constrictions produced by the BSE via oral administration was improved when the extract was tested by the parenteral route of administration in mice. Antinociceptive effects were also observed when the BSE was administered orally, it reduced painful behavior in both neurogenic and inflammatory phases of the formalin test in rats. These results together suggest that the bioavailability of constituents is modulated by the route of administration, but they do not affect the antinociceptive properties; moreover, central activity is possible in the effects of broccoli sprouts since the neurogenic phase (I) showed major and significant changes in both behaviors (shakings and time spent licking) in comparison to their diminution in the inflammatory phase (II) of the formalin test. The antinociceptive effects of the BSE in the writhing and the two phases of formalin tests suggest an involvement at both central and peripheral levels of pain combining the antinociceptive and anti-inflammatory activities.
Intraperitoneal administration of dilute acetic acid induces nociception in mice leading to writhing behavior, a widely used test commonly employed as a screening method for analgesic drugs.15 Analgesia is demonstrated by the decrease of the number of writhing by blockage of prostaglandin synthesis, a peripheral mechanism of pain inhibition. In addition, during inflammatory pain evoked by injection of pro-inflammatory agents such as formalin into a hind paw, neuronal nitric oxide synthase (nNOS) and iNOS are up-regulated.25 Also in the formalin test, the inhibition of COX-2 plays an important role in the nociceptive behavior suppression associated with acute inflammation. This anti-inflammatory activity agreed with the fact that the intake of broccoli sprouts per se modulates the inflammatory and vascular prostanoids26 suggesting the central and also peripheral involvement in its antinociceptive response.
Writhing behavior is also a model of clinical relevancy in intestinal pain in humans responding to the effect of analgesic opioid drugs.27 A tonic activation of the endogenous opioids has also been described in the inhibitory effect on pain behavior after subcutaneous formalin injection.28 In the antinociceptive effect of the BSE, the presence of naloxone produced a significant blockage in both antinociceptive tests and also in both phases of the formalin test reinforcing central and peripheral actions modulated also by an opioid neurotransmission.
The health beneficial effects of broccoli have been suggested because of the action of some bioactive compounds that positively modulate the immune system and the antioxidant defense. In vivo experiments on humans have reported that the hydrolysis of GLS and, therefore, the absorption of ITC is greater following the consumption of raw plant material with the presence of the enzyme myrosinase, than after ingestion of the cooked plant with the enzyme totally or partially denatured.29 The predominant ITC sulforaphane is able to significantly suppress muramyl dipeptide-induced NF-κB activity, a central participant in inflammatory processes at physiologically relevant concentrations (5–10 μM) achievable via the consumption of broccoli within the diet.4 This compound has also shown anti-inflammatory mechanisms based on the inhibition of lipopolysaccharide-mediated induction of forms of nitric-oxide synthase (iNOS), cyclooxygenase (COX-2), the tumor necrosis factor-α (TNF-α), and interleukin-1 beta (IL-1β) via NFκB inactivation, responsible for chronic inflammation and infections involved in the pathogenesis of many chronic diseases.30,31
The phenolic acids present in Brassicaceae extracts, especially sinapic and chlorogenic acids,32 enhance the cellular defense system avoiding oxidation and inflammation;33 this might contribute to the health-promoting properties of these broccoli sprouts and the BSE. Sinapic acid derivatives contained in broccoli sprouts have been studied as efficacious inhibitors of iNOS and COX-2 expression, responsible for the elevated levels of NO and prostaglandins.31 Other compounds present in broccoli sprouts such as anthocyanins34 and vitamin C35 act as natural antioxidants conferring protection against chronic diseases.
Our results showed that the BSE was not only capable of reducing gastric damage, but was also able to produce certain gastric protection against ulcerogenic substances. These effects might be associated with the presence of bioactive ITC sulforaphane, which stimulates Nrf-2 gene-dependent antioxidant enzyme activity, thereby protecting and repairing cells of the gastric mucosa from oxidative injury and inflammation.36 Finally, in this study the aqueous extract per se did not produce sedative effects as it is observed in the adverse effect of the opioid analgesia. Nevertheless, central actions of the constituents of broccoli sprouts are reinforced by the synergism of the combination between a significant antinociceptive dosage of the extract and the sedative-hypnotic effect of pentobarbital.
Conclusion
The aqueous extract obtained from broccoli sprouts produces a significant and dose-dependent antinociceptive activity suggesting its utility for the treatment of visceral and nociceptive pain mediated by not only central, but also peripheral opioid receptors. These results give scientific evidence to support the beneficial health effects for pain diseases without producing adverse effects as observed in analgesic drug therapy.Chemical compounds
Absolute ethanol (PubChem CID: 702); acetic acid (PubChem CID: 176); bismuth subsalicylate (PubChem CID: 16682734); diazepam (PubChem CID: 3016); formaldehyde (PubChem CID: 712); indomethacin (PubChem CID: 3715); naloxone (PubChem CID: 5284596); sodium pentobarbital (PubChem CID: 4737); tramadol (PubChem CID: 33741); tween 80 (443315).Abbreviations
| ANOVA | Analysis of variance |
| AUC | Area under the curve |
| BSE | Broccoli sprout aqueous extract |
| CNS | Central nervous system |
| COX | Cyclooxygenase |
| GLS | Glucosinolates |
| ITC | Isothiocyanates |
| MeJA | Methyl jasmonate |
| NF-κB | Nuclear factor kappa B |
| NOS | Nitric-oxide synthase |
| NSAID | Nonsteroidal antinflammatory drugs |
| SEM | Standard error of the mean |
| SP | Sodium pentobarbital |
| TNF-α | Tumor necrosis factor-α |
Conflict of interest
The authors declare no conflict of interest.Acknowledgements
We are thankful to Dra. Guadalupe Esther Ángeles, Mr Raúl Cardoso and Mr José Luis Calderon, and the students Frida Blancas and Claret Gutiérrez for the technical assistance. We also thank Aide M. González for proof-reading of the English version of the manuscript and Posgrado en Ciencias Biológicas (Universidad Nacional Autónoma de México) for having received academic training for Omar Guadarrama No.781549. This work was partially supported by the projects CONACYT-226454 and 256448 in Mexico City, and MINECO AGL2013-466247-P in Spain, as well as the Grant for Research Group of Excellence – Fundación Seneca, Murcia Regional Agency for Science and Technology, Project 19900/GERM/15. The authors would also like to thank the CYTED Programme, Action 112RT0460 CORNUCOPIA Thematic Network.References
- D. S. Goldberg and S. J. McGee, Pain as a global public health priority, BMC Public Health, 2011, 11, 770 CrossRef PubMed.
- J. C. Maroon, J. W. Bost and A. Maroon, Natural anti-inflammatory agents for pain relief, Surg. Neurol. Int., 2010, 1, 80 CrossRef PubMed.
- A. E. Wagner, A. M. Terschluesen and G. Rimbach, Health promoting effects of brassica-derived phytochemicals: from chemopreventive and anti-inflammatory activities to epigenetic regulation, Oxid. Med. Cell. Longevity, 2013, 964539, 23 Search PubMed.
- D. Folkard, G. Marlow, R. Mithen and L. Ferguson, Effect of Sulforaphane on NOD2 via NF-kappaB: implications for Crohn's disease, J. Inflammation, 2015, 12, 6 CrossRef PubMed.
- N. Juge, R. F. Mithen and M. Traka, 2007. Molecular basis for chemoprevention by sulforaphane: a comprehensive review, Cell. Mol. Life Sci., 2007, 64, 1105–1127 CrossRef CAS PubMed.
- M. E. Cartea, M. Francisco, P. Soengas and P. Velasco, Phenolic compounds in brassica vegetables, Molecules, 2011, 6, 251–280 Search PubMed.
- D. A. Moreno, M. Carvajal, C. López-Berenguer and C. García-Viguera, Chemical and biological characterisation of nutraceutical compounds of broccoli, J. Pharm. Biomed. Anal., 2006, 41, 1508–1522 CrossRef CAS PubMed.
- J. W. Fahey, S. L. Wehage, W. D. Holtzclaw, T. W. Kensler, P. A. Egner, T. A. Shapiro and P. Talalay, Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora, Cancer Prev. Res., 2012, 5, 603–611 CrossRef CAS PubMed.
- E. Danesh, S. Khatamsaz, M. Shojaeifard and Z. Khabbaz, Effects of hydro-alcoholic extract of broccoli (Brassica oleracea) on sensory threshold of pain using the for-malin test in adult male rats, J. Biol. Today's World, 2014, 3, 147–151 Search PubMed.
- S. Wu, Q. Gao, P. Zhao, Y. Gao, Y. Xi, X. Wang, Y. Liang, H. Shi and Y. Ma, Sulforaphane produces antidepressant- and anxiolytic-like effects in adult mice, Behav. Brain Res., 2016, 301, 55–62 CrossRef CAS PubMed.
- N. Baenas, C. García-Viguera and D. A. Moreno, Biotic Elicitors Effectively Increase the Glucosinolates Content in Brassicaceae Sprouts, J. Agric. Food Chem., 2014, 31, 31 Search PubMed.
- N. Baenas, D. A. Moreno and C. García-Viguera, Selecting sprouts of Brassicaceae for optimum phytochemical composition, J. Agric. Food Chem., 2012, 60, 11409–11420 CrossRef CAS PubMed.
- J. M. Cramer and E. H. Jeffery, Sulforaphane absorption and excretion following ingestion of a semi-purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men, Nutr. Cancer, 2011, 63, 196–201 CrossRef CAS PubMed.
- R. Domínguez-Perles, S. Medina, D. A. Moreno, C. García-Viguera, F. Ferreres and A. Gil-Izquierdo, A new ultra-rapid UHPLC/MS/MS method for assessing glucoraphanin and sulforaphane bioavailability in human urine, Food Chem., 2014, 143, 132–138 CrossRef PubMed.
- H. O. Collier, L. C. Dinneen, C. A. Johnson and C. Schneider, The abdominal constriction response and its suppression by analgesic drugs in the mouse, Br. J. Pharmacol. Chemother., 1968, 32, 295–310 CrossRef CAS PubMed.
- H. Wheeler-Aceto, F. Porreca and A. Cowan, The rat paw formalin test: comparison of noxious agents, Pain, 1990, 40, 229–238 CrossRef CAS PubMed.
- A. Robert, J. E. Nezamis, C. Lancaster and A. J. Hanchar, Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury, Gastroenterology, 1979, 77, 433–443 CAS.
- M. Ugalde, V. Reza, M. E. González-Trujano, B. Avula, I. A. Khan and A. Navarrete, Isobolographic analysis of the sedative interaction between six central nervous system depressant drugs and Valeriana edulis hydroalcoholic extract in mice, J. Pharm. Pharmacol., 2005, 57, 631–639 CrossRef CAS PubMed.
- M. E. González-Trujano, A. Navarrete, B. Reyes and E. Hong, Some pharmacological effects of the ethanol extract of leaves of Annona diversifolia on the central nervous system in mice, Phytother. Res., 1988, 12, 600–602 CrossRef.
- R. F. Bell, J. Borzan, E. Kalso and G. Simonnet, Food, pain, and drugs: does it matter what pain patients eat?, Pain, 2012, 153, 1993–1996 CrossRef PubMed.
- M. Bjorkman, I. Klingen, A. N. Birch, A. M. Bones, T. J. Bruce, T. J. Johansen, R. Meadow, J. Molmann, R. Seljasen, L. E. Smart and D. Stewart, Phytochemicals of Brassicaceae in plant protection and human health-influences of climate, environment and agronomic practice, Phytochemistry, 2011, 72, 538–556 CrossRef PubMed.
- A. P. Vale, J. Santos, N. V. Brito, D. Fernandes, E. Rosa and M. B. P. P. Oliveira, Evaluating the impact of sprouting conditions on the glucosinolate content of Brassica oleracea sprouts, Phytochemistry, 2015, 115, 252–260 CrossRef CAS PubMed.
- A. Aires, R. Carvalho and E. Rosa, Glucosinolate composition of brassica is affected by postharvest, food processing and myrosinase activity, J. Food Process. Preserv., 2012, 36, 214–224 CrossRef CAS.
- M. Rahmatullah, T. F. Shefa, L. Hasan, T. Hossain, S. Ahmed, A. Al Mamun, R. Islam, S. Rahman and M. H. Chowdhury, A study on antinociceptive and anti-hyperglycemic activity of methanol extract of Brassica Juncea (L.) Czern. leaves in mice, Adv. Nat. Appl. Sci., 2010, 4, 221 Search PubMed.
- A. Schmidtko, I. Tegeder and G. Geisslinger, No NO, no pain? The role of nitric oxide and cGMP in spinal pain processing, Trends Neurosci., 2009, 32, 339–346 CrossRef CAS PubMed.
- S. Medina, R. Domínguez-Perles, D. A. Moreno, C. Garcia-Viguera, F. Ferreres, J. I. Gil and A. Gil-Izquierdo, The intake of broccoli sprouts modulates the inflammatory and vascular prostanoids but not the oxidative stress-related isoprostanes in healthy humans, Food Chem., 2015, 173, 1187–1194 CrossRef CAS PubMed.
- H. F. Miranda, V. Noriega, P. Zanetta, J. C. Prieto, J. C. Prieto-Rayo, N. Aranda and F. Sierralta, Isobolographic analysis of the opioid-opioid interactions in a tonic and a phasic mouse model of induced nociceptive pain, J. Biomed. Sci., 2014, 21, 62 CrossRef PubMed.
- C. H. S. Zhao, Y. X. Tao, J. M. Tall, D. M. Donovan, R. A. Meyer and S. N. Raja, Role of μ-opioid receptors in formalin-induced pain behavior in mice, Exp. Neurol., 2003, 184, 839–845 CrossRef CAS PubMed.
- V. Rungapamestry, A. J. Duncan, Z. Fuller and B. Ratcliffe, Effect of cooking brassica vegetables on the subsequent hydrolysis and metabolic fate of glucosinolates, Proc. Nutr. Soc., 2007, 66, 69–81 CrossRef CAS PubMed.
- E. Heiss, C. Herhaus, K. Klimo, H. Bartsch and C. Gerhäuser, Nuclear Factor κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms, J. Biol. Chem., 2001, 276, 32008–32015 CrossRef CAS PubMed.
- K. J. Yun, D. J. Koh, S. H. Kim, S. J. Park, J. H. Ryu, D. G. Kim, J. Y. Lee and K. T. Lee, Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-kappaB inactivation, J. Agric. Food Chem., 2008, 56, 10265–10272 CrossRef PubMed.
- J. Shan, J. Fu, Z. Zhao, X. Kong, H. Huang, L. Luo and Z. Yin, Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in raw264.7 cells through suppressing NF-κB and JNK/AP-1 activation, Int. Immunopharmacol., 2009, 9, 1042–1048 CrossRef CAS PubMed.
- C. Chen, Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging, Oxid. Med. Cell. Longevity, 2016, 2016, 10 Search PubMed.
- P. Mecocci, C. Tinarelli, R. J. Schulz and M. C. Polidori, Nutraceuticals in cognitive impairment and Alzheimer's disease, Front. Pharmacol., 2014, 5, 147 CAS.
- N. Mikirova, J. Casciari, A. Rogers and P. Taylor, Effect of high-dose intravenous vitamin C on inflammation in cancer patients, J. Transl. Med., 2012, 10, 1479–5876 CrossRef PubMed.
- A. Yanaka, S. Zhang, M. Tauchi, H. Suzuki, T. Shibahara, H. Matsui, A. Nakahara, N. Tanaka and M. Yamamoto, Role of the Nrf-2 gene in protection and repair of gastric mucosa against oxidative stress, Inflammopharmacology, 2015, 13, 83–90 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2017 |