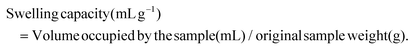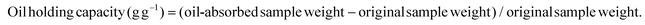Recovery of dietary fiber and polyphenol from grape juice pomace and evaluation of their functional properties and polyphenol compositions
LuLu
Zhang†
a,
MengTing
Zhu†
a,
Ting
Shi
a,
Cong
Guo
a,
YouSheng
Huang
b,
Yi
Chen
*a and
MingYong
Xie
a
aState Key Laboratory of Food Science and Technology, Nanchang University, Nanchang 330047, People's Republic of China. E-mail: chenyi-417@163.com; Tel: +0791-88304449
bJiangXi Institute of Analysis and Test, Nanchang 330029, People's Republic of China
First published on 5th December 2016
Abstract
The present work aimed at the recovery and characterization of dietary fiber and polyphenolic compounds extracted from red grape pomace, a by-product generated after grape fruit processing. High contents of total DF were found in the dietary fiber extracts (57.24%), whereas insoluble fiber was the major fraction (51.70%). And it showed good functional properties, including swelling capacity (4.01–8.32 mL g−1), water holding capacity (1.91–4.23 g g−1) and oil holding capacity (0.59–0.65 g g−1). After separation from the dietary fiber, phenolic extracts with high concentrations of total phenolic compounds and total flavonoids, showed high antioxidant activities, while the separated dietary fiber showed little antioxidant activities. This indicated that the phenolic composition is essential for the antioxidant activity of “antioxidant dietary fiber (ADF)”. The identification of individual polyphenols was performed applying the HPLC-ESI-MS/MS technique and 31 compounds have been identified belonging to 4 groups, including anthocyanins, flavonols, flavan-3-ols and phenolic acids. Based on this study, we believe grape juice pomace could potentially be exploited as an inexpensive source of natural dietary fiber and phenolics and possibly used as a functional food ingredient.
1. Introduction
Grape (Vitis sp.) is the largest fruit crop mostly used for the production of juice, wine and jams. The by-product of grape wine or juice industry weighing approximately 20% (w/w) of the grapes after pressing, is known as grape pomace. Grape pomace mainly consists of skin, and also of seeds, stems and remaining pulp. During the winemaking or juice making process, only part of the bioactive compounds such as flavonoids, polyphenols and dietary fiber in grapes is transferred to the wine or juice, and a high proportion still remains in the residues, especially in the grape pomace.1 Grape pomace was found to contain a large amount of phenolic compounds distributed in the pulp (10%), seeds (60–70%) and skin (28–35%).2 However, such a large amount of grape pomace is always disposed as waste, which causes a serious environmental problem. In order to avoid this, some industries will re-use it, but is still limited to animal feed or fertilizers. Thus it is essential to exploit its potential as a source of bioactive compounds and an alternative to the reuse of waste.Polyphenols include extractable polyphenols and non-extractable polyphenols. Extractable polyphenols refer to the phenolic compounds that can be extracted from the food matrix by aqueous-organic solvents.3 From a physiological point of view, such extractable polyphenols could be absorbed through the gastrointestinal tract. These extractable polyphenols mainly consist of the groups of catechins (46.8 percent), benzoic acids (16 percent), anthocyanidins (16.2 percent) and flavonols (14 percent),4 and have been shown to possess such beneficial effects as antioxidant, anti-inflammatory, and anti-carcinogenic, antimicrobial activities, and preventing cardiovascular diseases as well.5 However, extractable polyphenols amount to only 21% of total polyphenols in grape pomace, since the rest are non-extractable polyphenols which are usually hydrolysable tannins and condensed tannins associated with the dietary fiber (DF) matrix. Although non-extractable polyphenols are not available in the gastrointestinal tract, on reaching the colon it may be fermented by the colonic microflora and release substances with specific health-beneficial properties.6 Overall, this composition confers appreciable nutritional properties to the grape pomace.
Thus, the good nutritional value of grape is also related to their dietary fiber (DF) content. Dietary fiber has been proved to have several important health benefits such as the regulation of intestinal transit and prevention or treatment of diabetes, hypertension, coronary heart disease (CHD), cardiovascular disease and colon cancer.7 Dietary fiber is subdivided into insoluble dietary fiber (IDF) and soluble dietary fiber (SDF) depending on its solubility in water. SDF is associated with a decreased risk of cardiovascular disease owing to its cholesterol and glycemic index lowering properties. IDF consists of insoluble hemicelluloses, cellulose, resistant starch, and lignin. IDF possesses passive water-attracting properties that are beneficial for weight loss and digestive health. The by-products of fruits from industrial applications could be potential sources of DF that can be incorporated into food products. Dried apple pomace is considered as a potential food ingredient having a dietary fiber content of about 36.8%.8 According to Larrauri et al.,9 compared with dietary fiber from cereals, fruit fiber is of better quality due to its higher total and soluble fiber content, water and oil holding capacity and colonic fermentability, as well as its lower phytic acid contents and caloric values. In addition, fruit fiber has significant amounts of secondary compounds associated with it, such as polyphenols and other bioactive compounds.10 This fiber is called “antioxidant dietary fiber (ADF)” which refers to a dietary fiber concentrate containing significant amounts of natural antioxidants associated with non-digestible compounds.11 This concept indicates that ingredients and products might have health benefits from fiber together with the powerful antioxidant activity from secondary metabolites such as polyphenols from grapes.
Therefore, valuable compounds such as phenolics and fiber obtained from different methods of grape pomace recovery are of interest for the development of food ingredients. However, most of the previous research studies were normally performed on the extraction and characterization of extractable polyphenols separately from the skin, seeds and stems, without considering the recovery of dietary fiber simultaneously. The juice or wine industry can generate mixed waste which is not separated. Thus, the aim of this work was to extract and characterize polyphenols and dietary fiber from red grape juice pomace without the separation of parts such as skin, seeds, remaining pulp and stems, as well as evaluate their in vitro antioxidant activities with respect to exploiting their potential as a source of bioactive compounds and an alternative to the reuse of waste.
2. Materials and methods
2.1. Materials
The red grape (the Amur grape) belonging to the species Vitis amurensis was purchased from a local market. 1,1-Diphenyl-2-picrylhydrazyl (DPPH), butylated hydroxytoluene (BHT), Folin–Ciocalteu reagent, hydrogen peroxide (H2O2), 2,4,6-tripyridyl-s-triazine (TPTZ) and gallic acid were purchased from Sigma-Aldrich (St Louis, MO, USA). Ascorbic acid, ferrous sulfate heptahydrate, ferric chloride hexahydrate, salicylic acid and sodium carbonate (Na2CO3) were purchased from J&K Chemicals Ltd (Beijing, China). Hydrochloric acid, ethanol, glacial acetic acid and anhydrous sodium acetate were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). All other chemicals and solvents used in this study were of analytical grade. All organic solvents used for HPLC was from Dikma Scientific, USA.2.2. Laboratory scale preparation of grape juice pomace
Before juice production, the whole grape fruit was washed to remove surface and chemical contaminants. Then the fresh grapes were transferred into a cold press juicer. After pressing the juice pomace is immediately obtained by screening. The resulting juice pomace was freeze-dried. The dried pomace was ground into a homogeneous powder by using a laboratory mill and sieved through a 1.5 mm mesh. The obtained pomace was stored in sealed plastic bags at −40 °C until analysis.2.3. Phenolic and dietary fiber enrichment from grape pomace
Phenolic and dietary fiber were extracted and separated according to the method described by Mohamed Ali Al-Farsi and Chang Yong Lee.12 Briefly, a 5 g sample was extracted with 300 mL 50% aqueous acetone solvents for 1 h at 45 °C in a thermostatic water bath. Solids and liquid phases were separated by centrifugation and filtration under vacuum by Whatman no. 4 filter paper. The supernatant was saved for further analysis. The residues obtained were extracted with 50% aqueous acetone two more times. After that, the residues were collected and marked as R1. The supernatants from the triplicate extraction were combined and marked as E1.The acetone in E1 collected above was evaporated at 60 °C under vacuum using a rotary evaporator (Yarong Instruments, Shanghai, China). Then phenolic in E1 was purified with butanone by the solvent fractionation process using funnel separation. After that, the water fraction and butanone fraction were collected as W1 and B1, respectively. Finally, R1 and W1 were combined and referred to as dietary fibre concentrates. B1 was referred to as a phenolic concentrate. Phenolics and dietary fibre concentrates produced from this process were freeze-dried and ground into powder. Total phenolics, flavonoids and antioxidant analyses were carried out to determine the extraction and purification efficiency.
2.4. Dietary fiber determination
The dietary fiber content was determined according to the enzymatic gravimetric method.12,13 Triplicate samples were weighed and gelatinized with heat resistant α-amylase. Then the protein and starch present were removed by enzymatic digestion with protease and amyloglucosidase. Total dietary fiber was calculated as the sum of soluble dietary fiber and insoluble dietary fiber after correcting for ash and undigested protein. Dietary fiber was expressed as grams per 100 g of sample on a dry weight basis.2.5. Total phenolic
The total phenolic content of grape pomace was determined according to the Folin–Ciocalteu method14 with some modifications. Briefly, 1 mL of Folin–Ciocalteu's phenol reagent was mixed with 1 mL of phenolic extract in a 25 mL volumetric flask and allowed to react for 5 min. Then, 10 mL of 7% sodium carbonate solution (w/v) were added, and the final volume was made up to 25 ml with deionised water. After incubation in the dark for 1.5 h at room temperature, its absorbance was measured at 750 nm against deionized water using a TU-1901 UV–Vis Double Beam Spectrophotometer (PGENERAL, Beijing, China). A calibration curve was prepared with gallic acid as a standard reference. The total phenolic content was calculated as mg of gallic acid equivalent (GAE) by reference to the gallic acid standard curve and the results were expressed as milligrams of GAE per gram dry weight (g per DW) of residues.2.6. Total flavonoid
The total flavonoid content of grape pomace extracts was determined according to Wu et al.15 1 mL of phenolic extract was added into 5 mL of deionized water in a 10 mL volumetric flask. Then 0.3 mL of 5% Na2NO2 was added, and followed by a reaction for 5 min, 0.3 mL of 10% AlCl3 was added. After another 5 min, 3 mL of 1 M NaOH were added and the final volume was made up to 10 mL with 30% ethanol with mixing. Immediately the absorbance of the solution was measured at 510 nm against a prepared blank using an UV–vis spectrophotometer (PGENERAL, Beijing, China). The total flavonoid was determined by referring to a standard calibration curve prepared with a rutin solution, and the value was expressed as grams of rutin equivalents per gram of sample on a wet weight basis.2.7. Measurement of functional properties of dietary fiber
For the above WHC, SWC and OHC determination, the measurements were performed in triplicate for each sample.
2.8. Antioxidant activity (AA) determinations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v/v). The FRAP reagent was warmed to 37 °C prior to use. To perform this assay, 180 μL Milli-Q water, 1.8 mL of FRAP reagent, and 60 μL sample (W1 and B1 respectively), standard or blank were then mixed in a test tube. After incubation at 37 °C for 4 min, absorbance was recorded at 593 nm with the FRAP working solution as a blank. The relative absorbance value should be within the range 0–2.0; otherwise, the sample should be diluted.19 A series of final concentrations of the extract at 0.063, 0.125, 0.25, 0.5 and 1.0 mg mL−1 were tested respectively. The FRAP values of the sample were calculated from a standard calibration curve plotted with FeSO4·7H2O.
1 (v/v/v). The FRAP reagent was warmed to 37 °C prior to use. To perform this assay, 180 μL Milli-Q water, 1.8 mL of FRAP reagent, and 60 μL sample (W1 and B1 respectively), standard or blank were then mixed in a test tube. After incubation at 37 °C for 4 min, absorbance was recorded at 593 nm with the FRAP working solution as a blank. The relative absorbance value should be within the range 0–2.0; otherwise, the sample should be diluted.19 A series of final concentrations of the extract at 0.063, 0.125, 0.25, 0.5 and 1.0 mg mL−1 were tested respectively. The FRAP values of the sample were calculated from a standard calibration curve plotted with FeSO4·7H2O.
| Hydroxyl radical scavenging ability (%) = [1 − (As − Ab)/Ac]/100 |
2.9. UPLC-ESI/MS/MS conditions
Phenolic compounds in pomace were characterized by the Agilent 1200 HPLC system coupled on-line with a binary pump, a diode array detector (DAD), an autosampler and a column compartment. An Agilent Zorbax Eclipse Plus C18 column (Agilent Technologies, 100 mm × 2.1 mm, 1.8 μm Palo Alto, CA, USA) was used with a LC solvent at a flow rate of 0.3 ml min−1. The mobile phase was composed of 0.1% formic acid in water (eluent A) and acetonitrile (eluent B). The gradient conditions were as follows: 0 min, 5% B; 1 min, 20% B; 6 min, 20% B; 8 min, 80% B; 10 min, 80% B; 10.1 min, 5% B. The phenolic residue of each sample was dissolved in a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v) methanol/H2O and the solutions were filtered through 0.45 μm micropore membranes prior to injection into the HPLC system (injection volume: 5 μL). The detection by DAD was set at 280 nm for the lower molecular weight phenolic compounds, and at 320, 360, and 520 nm for stilbenes, flavonols, and anthocyanins.
30 (v/v) methanol/H2O and the solutions were filtered through 0.45 μm micropore membranes prior to injection into the HPLC system (injection volume: 5 μL). The detection by DAD was set at 280 nm for the lower molecular weight phenolic compounds, and at 320, 360, and 520 nm for stilbenes, flavonols, and anthocyanins.
For identification purposes, mass spectrometry analysis was performed using an Agilent 1260 HPLC system (Nan Chang JiangXi, China) coupled to a Triple Quadrupole Mass Spectrometer equipped with an electrospray ionization (ESI) source. Data acquisition and processing were performed by using the Mass Hunter software. Mass spectra were measured between m/z 50 and 1000 in the negative and positive ionization modes (ESI). The mass spectrometric conditions were as follows: capillary voltage, 4.0 kV; capillary temperature, 350 °C; nebulizer gas pressure of 40 psi; drying and nebulizer gas flow of 10 mL min−1 (N2).
2.10. Statistical analysis
Results were expressed as a mean of triplicate determinations ± standard deviation. Statistical significance (t-test: two-sample equal variance, using two-tailed distribution) was determined using the Microsoft Excel Statistical Data Analysis. Differences at p < 0.05 were considered to be significant.3. Results and discussion
3.1. Dietary fiber
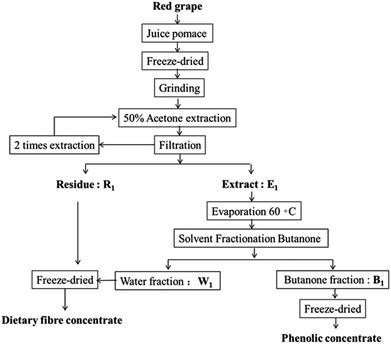
Fig. 1 shows a flow diagram of phenolics and the dietary fiber enriched concentrate from grape pomace. The dietary fiber (soluble, insoluble and total) of grape pomace and the fiber remaining after extraction of phenolics with 50% acetone (ADF) are shown in Table 1. The total dietary fiber content in grape pomace was 57.24 g per 100 g, whereas insoluble fiber was the major fraction (51.70 g per 100 g). A higher content of total dietary fiber (74.5 g per 100 g) in the winery of Manto Negro red grape (Vitis vinifera) pomace has been reported by Llobera & Cañellas et al.21 while Deng et al.22 reported a similar value of 56.31 g per 100 g for total dietary fiber of two white wine grape pomaces. These differences could be related to processing methods, maturation stage, and variety differences. The total dietary fiber of grape pomace after extraction with 50% acetone was increased significantly to 86.06 g per 100 g, as well as their insoluble fiber to 81.4 g per 100 g. This is possibly due to the fact that phenolics as well as other components, such as protein, fat and mono- and di-saccharides could be extracted from grape pomace into 50% acetone solution. This would lead to the reduction in the total weight of grape pomace and thus increase the weight percentage of dietary fiber in the pomace. In particular, soluble fiber including pectins, inulin, gums, arabinoxylan, xylose and raffinose was extracted together with phenolic which lead to their reduction to 4.66 g per 100 g. However, insoluble fiber, mainly consisting of cellulose, lignin and hemicellulose, remained in the pomace.| Soluble (g per 100 g) | Insoluble (g per 100 g) | Total (g per 100 g) | |
|---|---|---|---|
| ADF: dietary fibre remaining after extracting phenolics from pomace by acetone. Values are mean ± SD of three determinations on wet weight basis. Means ± SD followed by the same letter, within a column, are not significantly different (p > 0.05). | |||
| Grape pomace | 5.54 ± 0.14a | 51.70 ± 0.51a | 57.24 ± 0.35a |
| ADF | 4.66 ± 0.20b | 81.4 ± 0.32b | 86.06 ± 0.27b |
This obtained grape pomace after phenolic extraction consists of more than 80% fiber. According to the WHO/FAO report,23 the recommended daily intake of dietary fiber for an adult should be 25 g per day, which equals to only 30 g of such grape pomace. This indicated that such a grape pomace has a great potential for use as a fiber supplementation ingredient.
3.2. Polyphenol analysis
Polyphenol compounds are known for high antioxidant activities, thus its content can be used as an important indicator of antioxidant capacity as well as a preliminary screen for functional foods as a natural source of antioxidants.24 The total phenol content (TPC) and total flavonoid content (TFC) were 47.6 ± 0.21 mg g−1 GAE DW and 19.54 ± 0.14 mg g−1 LT DW, respectively. Compared with our results, Spigno et al.25 obtained lower polyphenol yield at 42.5 mg g−1 GAE DW in cv. Barbera GP. This data indicated that the technology applied for extraction and purification is a good method for the production of polyphenol extracts from industrial grape juice waste.3.3. Functional properties
| Functional properties | R1 + W1 | R1 |
|---|---|---|
| Values in the same lines with different letters are significantly different (p < 0.05). | ||
| Swelling capacity (mL g−1) | 6.95 ± 0.26a | 13.54 ± 0.37b |
| Water holding capacity (g g−1) | 3.84 ± 0.24a | 8.48 ± 0.30b |
| Oil holding capacity (g g−1) | 0.65 ± 0.02a | 0.59 ± 0.01a |
Regarding applications in food industry, dietary fiber with high WHC can be used as functional ingredients to modify the viscosity and texture of some formulated foods29 and to enhance the health beneficial effects of the food in the aspect of preventing colon cancer by its water binding, faecal bulking ability. However, dietary fiber with low WHC could be used when sugar substitutes are needed to produce low calorie food products such as extruded snacks, corn flakes, cookies and crackers.
As shown in Table 2, the oil holding capacity (OHC) of the total dietary fiber was 0.65 g g−1, which was higher than that of R1 (insoluble fiber concentrate) (0.59 g g−1). It has been reported that the presence of lignin (insoluble dietary fiber) might play some role in oil absorption.31 Indeed, the OHC of the fiber in our study was relatively higher compared to soybean (0.23 g per g DW) and okra (0.20 g per g DW).32 However, it was lower than that of pea (1–0.9 g per g DW), peach (1.02–1.11 g per g DW) and carrot (1.2 g per g DW).33 This was most probably related to various surface properties, fiber composition, particle size, overall charge density and hydrophobic nature of the fiber particles.
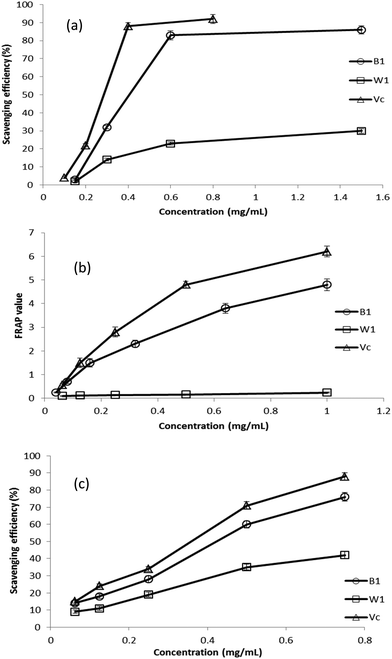
3.4. Antioxidant activities
Previous studies have revealed that the reducing power of bioactive compounds was correlated with the antioxidant activity.34 The reducing power of a compound is related to the electron transfer ability of that compound. The antioxidant compounds can act as electron donors and can react with free radicals to convert them to more stable products and thereby terminate radical chain reactions. This means stronger electron-donating capacity induced higher reducing power, thus higher antioxidant activities.
3.5. Identification of polyphenol constituents by HPLC–DAD–MSn analysis
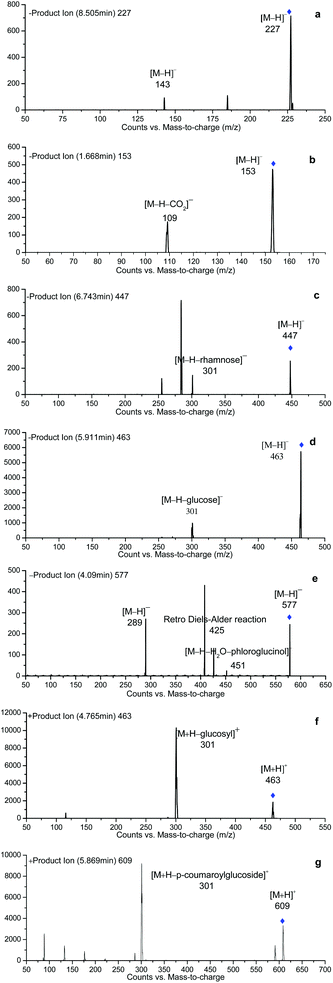
In this study, LC-MS, and the subsequent fragmentations of the predominant ions in MS/MS spectra were used to analyze the polyphenol composition of the samples. In addition, some literature data were used to support the identifications. Peak identification was based on the comparison of their retention times and mass spectral data (Fig. 3). Table 3 provides a summary of all the compounds studied, including retention times, experimental m/z, MS/MS fragments, compound name, as well as ion abundance.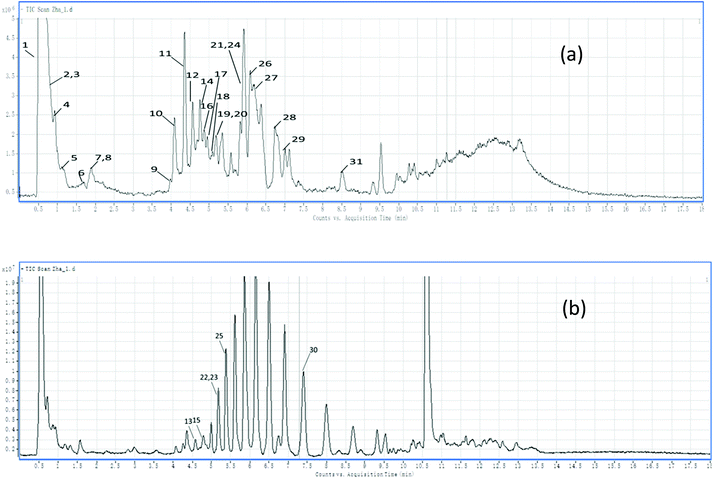 | ||
| Fig. 3 HPLC chromatograms of the phenolic compounds of the grape pomace: (a) negative TIC scan; (b) positive TIC scan. | ||
| Peak | R t (min) | Compound name | [M − H]+/[M − H]− | MS/MS fragment |
|---|---|---|---|---|
| Hydroxybenzoic acids and derivatives | ||||
| 1 | 0.539 | Quinic acid | [−]/191 | 173, 127 |
| 6 | 1.668 | Protocatechuic acid | [−]/153 | 109 |
| 4 | 0.941 | Gallic acid | [−]/169 | 125 |
| 5 | 1.206 | Galloyl glucoside | [−]/331 | 169 |
| Hydroxycinnamic acids and derivatives | ||||
| 9 | 3.967 | Caffeic acid hexose | [−]/341 | 179.05, 135 |
| 16 | 4.771 | Feruloyltartaric acid | [−]/325 | 193 |
| 12 | 4.57 | p-Coumaric acid-O-glycoside | [−]/325 | 163, 145 |
| Flavonols | ||||
| 26 | 6.087 | Eriodictyol hexoside | [−]/449 | 287, 259, 201 |
| 20 | 5.297 | Myricetin-O-glycoside | [−]/479 | 317, 179, 151 |
| 21 | 5.815 | Quercetin 3-O-galactoside | [−]/463 | 301 |
| 24 | 5.911 | Quercetin 3-O-glucoside | [−]/463 | 301 |
| 27 | 6.332 | Quercetin 3-O-glucuronide | [−]/477 | 301, 178, 151 |
| 28 | 6.743 | Quercetin 3-O-rhamnoside | [−]/447 | 301 |
| 29 | 7.015 | Isorhamnetin 3-O-glucoside | [−]/477 | 315, 314 |
| Flavan-3-ols | ||||
| 18 | 5.066 | Procyanidin trimer | [−]/865 | 695, 577, 289 |
| 8 | 1.883 | Gallocatechin or Epigallocatechin | [−]/305 | 179, 221, 261, 165, 125 |
| 11 | 4.335 | Catechin | [−]/289 | 245, 179, 125 |
| 17 | 4.865 | Epicatechin | [−]/289 | 245 |
| 10 | 4.09 | Procyanidin B3 | [−]/577 | 451, 425, 407, 289 |
| 14 | 4.694 | Procyanidin B4 | [−]/577 | 451, 425, 407, 289 |
| 19 | 5.146 | Procyanidin B2 | [−]/577 | 425, 407 |
| Anthocyanins and stilbenes | ||||
| 13 | 4.572 | Cyanidin glucoside or galactoside | [+]/449 | 287 |
| 22 | 5.869 | Peonidin-3-O-(6′′-O-p-coumaroyl)glucoside | [+]/609 | 301 |
| 23 | 5.883 | Petunidin 3-galactoside | [+]/479 | 317.4 |
| 25 | 5.924 | Delphindin 3-glucoside | [+]/465 | 303 |
| 15 | 4.765 | Peonidin 3-glucoside or galactoside | [+]/463 | 301 |
| 30 | 7.341 | Malvidin 3-galactoside | [+]/493 | 331.4 |
| 31 | 8.505 | trans-Resveratrol | [−]/227 | 185, 142.8 |
| Other compounds | ||||
| 2 | 0.608 | Malic acid | [−]/133 | 155 |
| 3 | 0.787 | Critic acid | [−]/191 | 173, 111 |
| 7 | 1.869 | Tryptophan | [−]/203 | |
Meanwhile, gallic acid (peak 4) was detected as a deprotonated [M − H]− ion at m/z 169, producing a fragment ion at m/z 125 which corresponds to the elimination of CO2 from carboxylic acid.
Galloyl glucoside (peak 5) was detected with a [M − H]− at m/z 331 and a fragmentation pattern at m/z 169 (corresponding to gallic acid) due to the loss of the hexose moiety.
Peak 12 (Rt 4.57 min) showed a deprotonated [M − H]− at m/z 325.09 and a MS/MS fragment ion at m/z 163.04 due to the neutral loss of a sugar moiety ([M − H − 162]−). By comparing the observed fragmentation pattern with that of Monties et al.,38 this compound has been suggested as p-coumaric acid-O-glycoside.
Peak 9 (Rt 3.967 min) with the precursor ion at m/z 341, gave product ions at m/z 179 (deprotonated caffeic acid) and 135, corresponding to the loss of a sugar residue (hexose, glucose or galactose) and a CO2 group from the carboxylic acid group, respectively. In keeping with the previous report, it was assigned to caffeic acid hex.
Peak 26 with a [M − H]− ion at m/z 449 and a MS2 base peak at m/z 287, resulting from the loss of one hexose unit ([M − H − 162]−), was identified as eriodictyol hexoside.39 Besides, the characteristic product ions, namely m/z 259 ([aglycone − CO]−) and 201 ([aglycone − C2H2O − CO2]−)40 were presented. This fragmentation profile follows the one that described before for eriodictyol.41
Peak 20 eluting at 5.297 min with a [M − H]− at m/z 479 was considered as a derivative of myricetin. Its MS2 fragments at m/z 317 ([M − H − 162]− corresponding to the loss of a sugar moiety), 179 and 151, and maximum absorbance at 350 and 255 nm (data not shown) confirm the presence of a glycosylated flavonol. Finally this peak was therefore identified as myricetin-O-glycoside.
Since all the peaks 21, 24, 27 and 28 exhibited a fragment with a MS2 spectrum at m/z 301 and had a characteristic UV spectrum for flavonol, this fragment was suggested as quercetin (Fig. 4c and d). The fragmentation pattern of peaks 21 and 24 yielded a neutral loss of 162 mass units and referred to the presence of galactoside or glucoside. According to their elution order in HPLC as recorded in the literature report, peaks 21 and 24 were assigned to quercetin-galactoside (Fig. 3c) and quercetin-glucoside ([M − H]− 463), respectively. Likewise, peak 27 ([M − H]− 477) showed the loss of 176 mass units in the fragmentation corresponding to a loss of glucuronic acid. Thus peak 27 was assigned to quercetin 3-O-glucuronide (Fig. 3d). Peak 28 with the loss of 146 mass units led to the assignment as quercetin-3-O-rhamnoside ([M + H]+ 449, [M − H]− 447). Peak 29 gave a fragmental m/z signal at 315 (corresponding to isorhamnetin), resulting from a loss of a glucosyl moiety [M − H − 162]−. Thus peak 29 was attributed to isorhamnetin 3-O-glucoside.
By comparing with the previously reported data, the two peaks eluted at 4.335 and 4.865 min, presenting the same [M − H]− ions at m/z 289, were assigned as flavan-3-ol monomers, catechin and epicatechin, respectively.42
Since all the peaks 10, 14 and 19 exhibited a molecular ion at m/z 577, and a typical fragment pattern for flavan-3-ol dimers at m/z: 559, 451, 425, 407 and 289 were also observed (Fig. 4e), they were identified as flavan-3-ol dimers. And in association with the order of elution found in the literature, they were finally identified as procyanidins B3, B4 and B2 respectively.43
Peak 18 eluted at 5.066 min with a deprotonated ion at m/z 865 was assigned as trimeric procyanidins of (epi) catechin. Its MS2 fragmentation yielded the product ions at m/z 221 due to the loss of catechin units from the trimer; and the ion at m/z 289, corresponded to catechin and epicatechin monomers.
The peak 22 (Rt = 5.869 min) with a molecular ion at m/z 609.0 was assigned as 3-p-coumaroylglucoside derivatives of peonidin. Its mass spectra were characterized with the M+ molecular ion and the fragment ion at m/z 301 which was through the loss of a p-coumaroylglucoside residue [M − 308]+ (Fig. 4g).
Peak 31 (Rt = 8.505 min) was identified as trans-resveratrol based on the most sensitive mass transition from m/z 227 to 142.8 (Fig. 4a).
Peak 3 with a molecular ion at m/z 191 was suggested as citric acid, since it showed a characterized fragmentation pattern at m/z 173 and 111 corresponding to [M − H − H2O]− and [M − H − CO2 − 2H2O]−, respectively.45
Peak 7 eluted at 1.869 min, showed a molecular ion at 203, and was temporarily assigned as tryptophan which has been previously found in zucchini.45
4. Conclusions
Fresh grape juice pomace is a good raw material for the preparation of dietary fiber and polyphenol-rich extracts. The application of separation technology in this research makes it possible to easily obtain both dietary fiber and polyphenol extracts. Both the dietary fiber and phenolic extracts obtained here showed antioxidant capacity, although a higher efficiency was found for the phenolic extracts. This might be due to the strong ability of polyphenols to chelate transition metal ions and donate electrons. The identification of the individual polyphenols present in the polyphenol extracts was performed applying HPLC-DAD-MS and MS/MS techniques and 31 compounds have been identified belonging to 4 groups, including anthocyanins, flavonols, flavan-3-ols and phenolic acids. Due to the low cost and easy availability of fruit residues, which otherwise would be discharged as waste in the environment, they should be regarded as potential nutraceutical resources, capable of offering significant low-cost, nutritional dietary supplements. More research is needed to establish bioavailability and real benefits of these extracts obtained from fruit residues in vivo.Acknowledgements
The financial support from the National Natural Science Foundation of China (No. 21302086, 31471647), the Key Technologies R & D Program of Jiangxi Province (No. 20152ACF60012) and Young Scientists of Jiangxi Province (No. 20142BCB23005) is gratefully acknowledged.References
- A. R. Fontana, A. Antoniolli and R. Bottini, Grape pomace as a sustainable source of bioactive compounds: extraction, characterization, and biotechnological applications of phenolics, J. Agric. Food Chem., 2013, 61, 8987–9003 CrossRef CAS PubMed.
- Y. Sui, J. Yang, Q. Ye, H. Li and H. Wang, Infrared, Convective, and Sequential Infrared and Convective Drying of Wine Grape Pomace, Drying Technol., 2014, 32, 686–694 CrossRef CAS.
- J. Perez-Jimenez, M. E. Diaz-Rubio and F. Saura-Calixto, Non-extractable polyphenols, a major dietary antioxidant: occurrence, metabolic fate and health effects, Nutr. Res. Rev., 2013, 26, 118–129 CrossRef CAS PubMed.
- S. G. Sáyago-Ayerdi, A. Brenes and I. Goñi, Effect of grape antioxidant dietary fiber on the lipid oxidation of raw and cooked chicken hamburgers, LWT – Food Sci. Technol., 2009, 42, 971–976 CrossRef.
- S. Rana, S. Gupta, A. Rana and S. Bhushan, Functional properties, phenolic constituents and antioxidant potential of industrial apple pomace for utilization as active food ingredient, Food Sci. Human Wellness, 2015, 4, 180–187 CrossRef.
- J. P. Jimenez, J. Serrano, M. Tabernero, S. Arranz, M. E. Diaz-Rubio, L. Garcia-Diz, I. Goni and F. Saura-Calixto, Effects of grape antioxidant dietary fiber in cardiovascular disease risk factors, Nutrition, 2008, 24, 646–653 CrossRef CAS PubMed.
- M. M. Kaczmarczyk, M. J. Miller and G. G. Freund, The health benefits of dietary fiber: beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer, Metabolism, 2012, 61, 1058–1066 CrossRef CAS PubMed.
- K. J. Carson, J. L. Collins and M. P. Penfield, Unrefined, dried apple pomace as a potential food ingredient, J. Food Sci., 1995, 59, 1213–1215 CrossRef.
- N. Martín-Carrón, I. Goñi, J. A. Larrauri, A. García-Alonso and F. Saura-Calixto, Reduction in serum total and LDL cholesterol concentrations by a dietary fiber and polyphenol-rich grape product in hypercholesterolemic rats, Nutr. Res., 1999, 19, 1371–1381 CrossRef.
- F. Zhu, B. Du, L. Zheng and J. Li, Advance on the bioactivity and potential applications of dietary fibre from grape pomace, Food Chem., 2015, 186, 207–212 CrossRef CAS PubMed.
- A. E. Quiros-Sauceda, H. Palafox-Carlos, S. G. Sayago-Ayerdi, J. F. Ayala-Zavala, L. A. Bello-Perez, E. Alvarez-Parrilla, L. A. de la Rosa, A. F. Gonzalez-Cordova and G. A. Gonzalez-Aguilar, Dietary fiber and phenolic compounds as functional ingredients: interaction and possible effect after ingestion, Food Funct., 2014, 5, 1063–1072 CAS.
- M. A. Al-Farsi and C. Y. Lee, Optimization of phenolics and dietary fibre extraction from date seeds, Food Chem., 2008, 108, 977–985 CrossRef CAS PubMed.
- W. Horwitz, P. Chichilo and H. Reynolds, Official methods of analysis of the Association of Official Analytical Chemists, Association of Official Analytical Chemists, Washington, DC, 1970 Search PubMed.
- S. N. Lou, Y. S. Hsu and C. T. Ho, Flavonoid compositions and antioxidant activity of calamondin extracts prepared using different solvents, J. Food Drug Anal., 2014, 22, 290–295 CrossRef CAS.
- Z. Jia, M. Tang and J. Wu, The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals, Food Chem., 1999, 64, 555–559 CrossRef.
- J. A. Robertson, F. D. de Monredon, P. Dysseler, F. Guillon, R. Amado and J. F. Thibault, Hydration Properties of Dietary Fibre and Resistant Starch: a European Collaborative Study, LWT – Food Sci. Technol., 2000, 33, 72–79 CrossRef CAS.
- A. Elkhalifa, B. Schiffler and R. Bernhardt, Effect of fermentation on the functional properties of sorghum flour, Food Chem., 2005, 92, 1–5 CrossRef CAS.
- S. Kandasamy and S. M. Aradhya, Polyphenolic profile and antioxidant properties of rhizome of commercial banana cultivars grown in India, Food Biosci., 2014, 8, 22–32 CrossRef CAS.
- P. Wanyo, S. Siriamornpun and N. Meeso, Improvement of quality and antioxidant properties of dried mulberry leaves with combined far-infrared radiation and air convection in Thai tea process, Food Bioprod. Process., 2011, 89, 22–30 CrossRef CAS.
- S. Gonçalves, D. Gomes, P. Costa and A. Romano, The phenolic content and antioxidant activity of infusions from Mediterranean medicinal plants, Ind. Crops Prod., 2013, 43, 465–471 CrossRef.
- A. Llobera and J. Cañellas, Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): pomace and stem, Food Chem., 2007, 101, 659–666 CrossRef CAS.
- Q. Deng, M. H. Penner and Y. Zhao, Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins, Food Res. Int., 2011, 44, 2712–2720 CrossRef CAS.
- A. Ness, Diet, nutrition and the prevention of chronic diseases: report of a Joint WHO/FAO Expert Consultation, Int. J. Epidemiol, 2004, 33, 914–915 CrossRef.
- M. Viuda-Martos, M. A. Mohamady, J. Fernández-López, K. A. Abd ElRazik, E. A. Omer, J. A. Pérez-Alvarez and E. Sendra, In vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants, Food Control, 2011, 22, 1715–1722 CrossRef CAS.
- G. Spigno and D. M. De Faveri, Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts, J. Food Eng., 2007, 78, 793–801 CrossRef CAS.
- C. F. Chau and Y. L. Huang, Characterization of passion fruit seed fibres-a potential fibre source, Food Chem., 2004, 85, 189–194 CrossRef CAS.
- M. M. Ma and T. H. Mu, Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin, Food Chem., 2016, 194, 237–246 CrossRef CAS PubMed.
- L. L. Yalegama, D. Nedra Karunaratne, R. Sivakanesan and C. Jayasekara, Chemical and functional properties of fibre concentrates obtained from by-products of coconut kernel, Food Chem., 2013, 141, 124–130 CrossRef CAS PubMed.
- N. Grigelmo-Miguel and O. Martín-Belloso, Characterization of dietary fiber from orange juice extraction, Food Res. Int., 1998, 31, 355–361 CrossRef.
- I. Navarro-González, V. García-Valverde, J. García-Alonso and M. J. Periago, Chemical profile, functional and antioxidant properties of tomato peel fiber, Food Res. Int., 2011, 44, 1528–1535 CrossRef.
- F. W. Sosulski and A. M. Cadden, Composition and physiological properties of several sources of dietary fiber, J. Food Sci., 2006, 47, 1472–1477 CrossRef.
- I. Espinosa-Martos and P. Rupérez, Indigestible fraction of okara from soybean: composition, physicochemical properties and in vitro fermentability by pure cultures of Lactobacillus acidophilus and Bifidobacterium bifidum, Eur. Food Res. Technol., 2008, 228, 685–693 CrossRef.
- M. Elleuch, D. Bedigian, O. Roiseux, S. Besbes, C. Blecker and H. Attia, Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review, Food Chem., 2011, 124, 411–421 CrossRef CAS.
- B. K. Siddhuraju P, Antioxidant Properties of Various Solvent Extracts of Total Phenolic Constituents from Three Different Agroclimatic Origins of Drumstick Tree (Moringa oleifera Lam.) Leaves, J. Agric. Food Chem., 2003, 51, 2144–2155 CrossRef PubMed.
- J. Macdonald, Oxidative stress and gene expression in sepsis, Br. J. Anaesth., 2003, 90, 221–232 CrossRef CAS PubMed.
- S. Gouveia and P. C. Castilho, Antioxidant potential of Artemisia argentea L'Hér alcoholic extract and its relation with the phenolic composition, Food Res. Int., 2011, 44, 1620–1631 CrossRef CAS.
- D. Peričin, V. Krimer, S. Trivić and L. Radulović, The distribution of phenolic acids in pumpkin's hull-less seed, skin, oil cake meal, dehulled kernel and hull, Food Chem., 2009, 113, 450–456 CrossRef.
- B. Monties, M.-L. Bouillant and J. Chopin, C-diholosylflavones dans les feuilles du melon (Cucumis melo), Phytochemicals, 1976, 15, 1053–1056 CrossRef CAS.
- M. Monagas, I. Garrido, R. Lebrónaguilar, B. Bartolome and C. Gómezcordovés, Almond (Prunus dulcis (Mill.) DA Webb) skins as a potential source of bioactive polyphenols, J. Agric. Food Chem., 2007, 55, 8498–8507 CrossRef CAS PubMed.
- N. Fabre, I. Rustan, E. D. Hoffmann and J. Quetin-Leclercq, Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry, J. Am. Soc. Mass Spectrom., 2001, 12, 707–715 CrossRef CAS PubMed.
- F. Cuyckens and M. Claeys, Mass spectrometry in the structural analysis of flavonoids, J. Mass Spectrom., 2004, 39, 1–15 CrossRef CAS PubMed.
- V. Ivanova, M. Stefova, B. Vojnoski, Á. Dörnyei, L. Márk, V. Dimovska, T. Stafilov and F. Kilár, Identification of polyphenolic compounds in red and white grape varieties grown in R. Macedonia and changes of their content during ripening, Food Res. Int., 2011, 44, 2851–2860 CrossRef CAS.
- B. Sun, C. Leandro, J. M. R. D. Silva and I. Spranger, Separation of grape and wine proanthocyanidins according to their degree of polymerization, J. Agric. Food Chem., 1998, 46, 1390–1396 CrossRef CAS.
- W. B. Dunn, S. Overy and W. P. Quick, Metabolomics Evaluation of automated electrospray-TOF mass spectrometry for metabolic fingerprinting of the plant metabolome, Metabolomics, 2005, 1, 137–148 CrossRef CAS.
- M. Gomez-Romero, A. Segura-Carretero and A. Fernandez-Gutierrez, Metabolite profiling and quantification of phenolic compounds in methanol extracts of tomato fruit, Phytochemicals, 2010, 71, 1848–1864 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work and should be considered co-first authors. |
| This journal is © The Royal Society of Chemistry 2017 |