Evaluation of clinical safety and beneficial effects of stachyose-enriched α-galacto-oligosaccharides on gut microbiota and bowel function in humans
Ting
Li
 ab,
Xinshan
Lu
ab,
Xinshan
Lu
 a and
Xingbin
Yang
a and
Xingbin
Yang
 *a
*a
aShaanxi Engineering Laboratory for Food Green Processing and Safety Control, College of Food Engineering and Nutritional Science, Shaanxi Normal University, Xi'an 710062, China. E-mail: xbyang@snnu.edu.cn; Fax: +86 29 85310517; Tel: +86 29 85310580
bCentre for Cancer and Inflammation Research, School of Chinese Medicine, Hong Kong Baptist University, Hong Kong, China
First published on 7th December 2016
Abstract
Deshipu stachyose granules (DSG) is a mixture of α-galacto-oligosaccharides derived from the dietary roots of Lycopus lucidus Turcz. Our previous study showed that DSG could improve the faecal microbial composition, and facilitate intestinal peristalsis and fecal excretion in mice. This study was designed to investigate the effect of DSG on gut microbiota and bowel function in humans. Two human intervention studies were conducted. In the first study, 100 healthy adults were treated without or with 5 g per day of DSG for 14 days. The microbiota composition in fecal samples was quantitatively analyzed before and after DSG supplementation. We found that DSG consumption significantly elevated the fecal bifidobacteria and lactobacilli levels, and also decreased the fecal Clostridium perfringens concentration. In the second study, 103 constipated patients were treated with 5 g per day of placebo or DSG for 30 days, and subsequently subjected to bowel function evaluation. As a result, dietary intake of DSG effectively improved the bowel function of constipated patients, as evidenced by the increased defecation frequency, softer stools and easier defecation. Moreover, clinical safety assessment showed that DSG at 5 g per day did not cause significant adverse effects in both healthy and constipated volunteers. In conclusion, DSG at 5 g d−1 beneficially modulated the gut microbiota in healthy adults and potently improved the bowel function of constipated patients without consequent adverse events. This study suggests that DSG holds promising potential for safe treatment of functional constipation.
1. Introduction
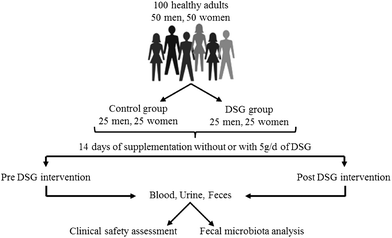
Constipation, which generally refers to less than 2 to 3 bowel movements per week accompanied by small, dry, and/or hard defecation and discharge difficulty, is a common problem with a worldwide prevalence ranging from 2.5% to 79% in adults and 0.7% to 29.6% in children.1 Functional constipation is caused by multiple pathogenic factors involving irregular intestinal wall contractility, visceral sensation dysfunction, unsatisfactory transit and expulsion of endoluminal contents.2 Due to the complex pathophysiological mechanisms, a satisfactory treatment for functional constipation is not yet available.2 Gut microbiota, which can influence a variety of gut functions, plays an important role in the pathology of constipation.3 The gut microbiota was shown to directly or indirectly affect intestinal motor functions.4,5 Imbalance in the microbiota composition has been described in the stools of patients with constipation.2,6 It has been demonstrated that the microbiome in constipated patients exhibits a decrease in obligate bacteria and an increase in potentially pathogenic bacteria.7,8 In this regard, new therapeutic approaches against constipation are based on modulating intestinal microbiota that can influence peristalsis of the colon.9,10Prebiotics are non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of specific members of gut microbiota, thus improving the host health.11 Deshipu stachyose granules (DSG) is a commercial preparation of α-galacto-oligosaccharides derived from the dietary roots of Lycopus lucidus Turcz and is approved by the China Food and Drug Administration as a health food.12 DSG consists of stachyose (55.3%), raffinose (25.8%), verbascose (9.7%) and sucrose (6.9%).12 Galacto-oligosaccharides (GOS), which can be selectively fermented by health-promoting bacteria such as bifidobacteria and lactobacilli, have now been definitely established as prebiotic ingredients.13,14 In our previous study, we have found that DSG can potently promote the growth of beneficial intestinal bacteria and inhibit pathogenic bacteria, and also facilitate intestinal peristalsis and fecal excretion in mice.12
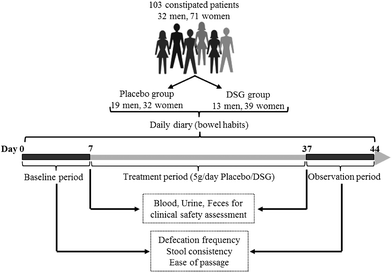
This study, therefore, aims to evaluate the efficacy of an oral supplementation with DSG on the gut microbiota composition in healthy adults and the bowel habit in constipated patients. A non-blind, randomized, controlled trial with healthy adult volunteers (study 1) was performed to investigate the regulative effects of DSG on the number of intestinal bifidobacteria, lactobacilli, Clostridium perfringens, enteric bacilli, enterococci and Bacteroides. To further determine the beneficial effect of DSG on bowel function, a placebo-controlled, randomized, double-blind trial (study 2) was conducted on participants suffering from constipation. The bowel function was assessed by scoring the defecation frequency, stool consistency and ease of passage. Additionally, clinical safety of DSG in both healthy adults and constipated patients was assessed by recording the occurrence of adverse reactions, performing the routine blood, urine and stool tests, and detecting blood biochemical parameters in subjects.
2. Materials and methods
2.1 Sample and chemicals
Deshipu stachyose granules (DSG), a powder of small granules with 5 g of recommended daily allowance (RDA) per person, was obtained from Xi'an Deshipu Bio-industry Co., Ltd (Xi'an, China). BBL-agar, the selective medium for bifidobacteria, and Bile Esculin Azide-agar for enterococci were prepared in our laboratory. MRS-agar for lactobacilli and TSC-agar for Clostridium perfringens were obtained from OXOID Ltd (United Kingdom) and Merck KGaA (Germany), respectively. EMB-agar for enteric bacilli and GAM broth for Bacteroides were purchased from the Kaifeng City Institute of Medical Biology (China). All the other chemicals were of the highest grade available.2.2 Assessment of gut microbiota regulating effects (study 1)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CFU per gram of wet feces. Data were expressed as mean ± SD of at least three independent experiments. Statistical analyses were conducted with SPSS 11.5 software. A paired t-test was applied to compare the differences between pretest and posttest in the same group, and the two independent samples t-test was employed to compare the differences between the DSG group and the control group. For the two independent samples t-test, the homogeneity test of variances was first conducted. Data with abnormal distribution or unequal variances were subjected to an appropriate variable transformation to satisfy the Gaussian distribution or obtain equal variances. Statistical analysis was then performed using the transformed data. For the data inconformity to the requirements after variable transformation, a rank-sum test was used. The difference was considered significant if p < 0.05.
10 CFU per gram of wet feces. Data were expressed as mean ± SD of at least three independent experiments. Statistical analyses were conducted with SPSS 11.5 software. A paired t-test was applied to compare the differences between pretest and posttest in the same group, and the two independent samples t-test was employed to compare the differences between the DSG group and the control group. For the two independent samples t-test, the homogeneity test of variances was first conducted. Data with abnormal distribution or unequal variances were subjected to an appropriate variable transformation to satisfy the Gaussian distribution or obtain equal variances. Statistical analysis was then performed using the transformed data. For the data inconformity to the requirements after variable transformation, a rank-sum test was used. The difference was considered significant if p < 0.05.
2.3 Assessment of beneficial effects on bowel function (study 2)
2.4 Ethics
The two studies were approved by the ethics committee of China Food and Drug Administration (G20080008), and were performed according to the principles of the Declaration of Helsinki.3. Results
3.1 Clinical safety evaluation of DSG in healthy adults (study 1)
During the whole experimental period, no adverse events were observed in both control and DSG groups, with the exception of anal exsufflation which occurred in a number of participants in the DSG group in response to DSG consumption. No abnormal changes were found in the psychological, dietary and sleeping states, and blood pressure as well as the results of ECG, B-ultrasound and chest X-ray detection in volunteers administered with or without DSG. As shown in Table 1, the results of routine blood, urine, stool tests and blood biochemical parameters of all subjects were maintained at the normal ranges both prior to and after treatment.| Normal ranges | Control (n = 50) | DSG (n = 50) | |||
|---|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | ||
| Routine blood test | |||||
| RBC (×1012 cells per L) | 3.5–5.4 | 4.75 ± 0.85 | 4.60 ± 0.45 | 4.75 ± 0.40 | 4.65 ± 0.40 |
| HGB (g L−1) | 115–160 | 146.37 ± 9.14 | 146.12 ± 9.50 | 146.57 ± 8.51 | 146.52 ± 8.27 |
| WBC (×109 cells per L) | 4–10 | 5.93 ± 1.53 | 6.28 ± 1.42 | 5.95 ± 1.67 | 6.04 ± 1.15 |
| Blood biochemical indices | |||||
| GLU (mg DL−1) | 70–110 | 86.04 ± 12.12 | 81.87 ± 10.37 | 84.54 ± 11.81 | 82.10 ± 9.96 |
| ALT (U L−1) | 5–40 | 25.46 ± 10.71 | 23.55 ± 9.89 | 26.10 ± 12.68 | 22.97 ± 8.55 |
| BUN (mg DL−1) | 6–20 | 13.42 ± 3.24 | 12.17 ± 3.66 | 13.67 ± 3.31 | 11.08 ± 3.74 |
| CHOL (mg DL−1) | 120–220 | 188.47 ± 35.33 | 181.54 ± 27.12 | 192.33 ± 30.64 | 183.25 ± 30.78 |
| TRIG (mg DL−1) | 50–150 | 98.26 ± 43.41 | 95.43 ± 36.22 | 97.12 ± 40.10 | 93.38 ± 38.44 |
| TP (g DL−1) | 6.0–8.7 | 7.64 ± 0.56 | 7.77 ± 0.38 | 7.59 ± 0.45 | 7.48 ± 0.39 |
| ALB (g DL−1) | 3.5–5.5 | 4.82 ± 0.34 | 4.65 ± 0.24 | 4.92 ± 0.29 | 4.70 ± 0.26 |
| CRE (mg DL−1) | 0.57–1.17 | 0.74 ± 0.09 | 0.69 ± 0.12 | 0.71 ± 0.11 | 0.68 ± 0.10 |
| Routine urine test | — | Normal | Normal | Normal | Normal |
| Routine stool test | — | Normal | Normal | Normal | Normal |
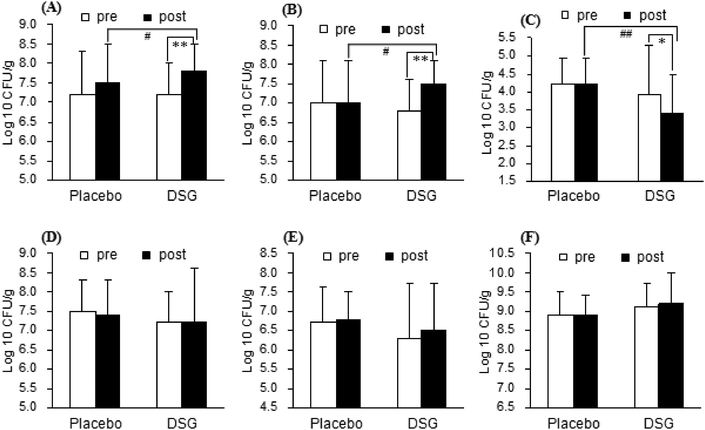
3.2 Effects of DSG on the growth of intestinal bacteria (study 1)
The levels of fecal bifidobacteria, lactobacilli, Clostridium perfringens, enteric bacilli, enterococci and Bacteroides in healthy adults supplemented with or without DSG are shown in Fig. 3. The changes in the intestinal microbiota between pretest and posttest (control group and DSG group) and the differences between groups (pretest and posttest) were assessed. Participants in the control group showed no significant changes in fecal bifidobacteria, lactobacilli, Clostridium perfringens, enteric bacilli, enterococci and Bacteroides concentrations throughout the experimental period. Furthermore, the levels of the six bacterial groups between the control group and the DSG group were similar before treatment. It is noteworthy that administration of DSG evidently elevated the number of fecal bifidobacteria and lactobacilli. The basal levels of fecal bifidobacteria and lactobacilli in the DSG group were 7.2 ± 0.8 and 6.8 ± 0.8 of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CFU g−1, respectively, which were significantly increased to 7.8 ± 0.7 and 7.5 ± 0.6 of log
10 CFU g−1, respectively, which were significantly increased to 7.8 ± 0.7 and 7.5 ± 0.6 of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CFU g−1 after treatment of DSG, respectively (p < 0.01, p < 0.01, Fig. 3A and B). Besides, DSG caused observable reduction in the fecal Clostridium perfringens concentration (Fig. 3C). In comparison with the pretest results, the decrease in the fecal Clostridium perfringens levels in participants receiving 5 g of DSG daily for 14 d was 0.5 of log
10 CFU g−1 after treatment of DSG, respectively (p < 0.01, p < 0.01, Fig. 3A and B). Besides, DSG caused observable reduction in the fecal Clostridium perfringens concentration (Fig. 3C). In comparison with the pretest results, the decrease in the fecal Clostridium perfringens levels in participants receiving 5 g of DSG daily for 14 d was 0.5 of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CFU g−1 (p < 0.05), while no change was observed in the control group. As shown in Fig. 3D, E and F, there are no distinct changes in fecal enteric bacilli, enterococci and Bacteroides populations in the DSG group when compared with the pretest and posttest results (p > 0.05, p > 0.05, respectively). These results suggest that DSG can effectively promote the proliferation of bifidobacteria and lactobacilli, and inhibit the growth of Clostridium perfringens.
10 CFU g−1 (p < 0.05), while no change was observed in the control group. As shown in Fig. 3D, E and F, there are no distinct changes in fecal enteric bacilli, enterococci and Bacteroides populations in the DSG group when compared with the pretest and posttest results (p > 0.05, p > 0.05, respectively). These results suggest that DSG can effectively promote the proliferation of bifidobacteria and lactobacilli, and inhibit the growth of Clostridium perfringens.
3.3 Clinical safety evaluation of DSG in constipated patients (study 2)
All subjects suffering from constipation in study 2 showed no obvious adverse reactions during the whole study period. The data of haematology and clinical chemistry parameters showed that the levels of RBC, WBC, HGB, ALT, AST, BUN and CRE of all participants were in the normal ranges prior to the study, and no significant changes were observed in these parameters after placebo or DSG administration (Table 2). In addition, no abnormality was found in the results of routine urine and stool tests for all volunteers both pre- and post-treatment. No abnormal performances were found in the psychological, dietary and sleeping states, and ECG, B-ultrasound and chest X-ray examination in both control and DSG groups after treatment.| Normal ranges | Placebo (n = 51) | DSG (n = 52) | |||
|---|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | ||
| Routine blood test | |||||
| RBC (×1012 cells per L) | 3.5–5.4 | 4.56 ± 0.56 | 4.45 ± 0.76 | 4.46 ± 0.53 | 4.43 ± 0.77 |
| HGB (g L−1) | 115–160 | 140.46 ± 16.52 | 142.21 ± 26.11 | 141.02 ± 16.10 | 142.14 ± 26.34 |
| WBC (×109 cells per L) | 4–10 | 5.51 ± 1.46 | 5.38 ± 1.37 | 5.54 ± 0.53 | 5.40 ± 1.39 |
| Blood biochemical indices | |||||
| ALT (U L−1) | 0–40 | 24.47 ± 15.17 | 21.21 ± 12.65 | 24.23 ± 14.94 | 21.11 ± 12.67 |
| AST (U L−1) | 0–40 | 25.09 ± 7.71 | 21.97 ± 9.00 | 24.93 ± 7.29 | 21.75 ± 9.08 |
| BUN (mmol L−1) | 2.8–7.2 | 4.58 ± 1.25 | 4.26 ± 0.90 | 4.54 ± 1.23 | 4.24 ± 0.86 |
| CRE (μmol L−1) | 40–140 | 62.75 ± 13.40 | 63.89 ± 14.04 | 62.73 ± 13.42 | 63.90 ± 14.21 |
| Routine urine test | — | Normal | Normal | Normal | Normal |
| Routine stool test | — | Normal | Normal | Normal | Normal |
3.4 Effects of DSG on the bowel function of constipated patients (study 2)
The bowel function of constipated patients with placebo or DSG supplementation is shown in Table 3. No significant differences were found in the basal levels of defecation frequency, stool consistency and ease of passage between the placebo group and the DSG group. After 30 d of the treatment period, patients supplemented with placebo exhibited no evident change in defecation frequency, stool consistency and ease of passage while the mean frequency of defecation in the DSG group was significantly increased from 1.78 ± 0.51 times per week for the base level to 3.02 ± 1.15 times per week after administration of DSG. DSG supplementation also caused remarkable declines on the scores of stool consistency and ease of passage from 1.66 ± 0.48 and 2.45 ± 0.46 before DSG intervention to 1.23 ± 0.33 and 1.86 ± 0.14, respectively (Table 3). Here, the lower scores of stool consistency and ease of passage means even softer stools and easier defecation, respectively.| Placebo group (n = 51) | DSG group (n = 52) | |||
|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | |
| a Values are expressed as means ± SD. b The differences between pretest and posttest in the DSG group are statistically significant (p < 0.05). c The differences between the DSG group and Placebo group after DSG/Placebo treatment are statistically significant (p < 0.05). | ||||
| Defecation frequency (no. per week) | 1.92 ± 0.60 | 1.86 ± 0.58 | 1.78 ± 0.51 | 3.02 ± 1.15b,c |
| Ease of passage | 2.41 ± 0.44 | 2.38 ± 0.54 | 2.45 ± 0.46 | 1.86 ± 0.14b,c |
| Stool consistency | 1.68 ± 0.49 | 1.76 ± 0.36 | 1.66 ± 0.48 | 1.23 ± 0.33b,c |
4. Discussion
Current medications against constipation, including bulk-forming agents, laxatives, osmotic agents, and prosecretory drugs, often have a short-lived efficacy and/or are accompanied by side effects such as bloating and abdominal cramps.15 Novel therapeutic approaches with high efficacy and few side effects should be explored. Nowadays, increasing interest has grown exponentially in exploring novel prebiotics capable of regulating gut microbiota and relieving constipation with few side effects. Non-digestible oligosaccharides, which are considered as dietary fibers and prebiotics, possess important physicochemical and physiological properties beneficial to human health.16 Currently available prebiotic oligosaccharides on the market mainly include inulin, fructo-oligosaccharides (FOS) and GOS.17 As non-absorbable food ingredients, these oligosaccharides are live microbial food supplements and may beneficially affect the host by selectively stimulating the growth of salutary bacteria in the large intestine.18 Previous studies have suggested that FOS and GOS can effectively increase the proportion of Bifidobacterium and Lactobacillus in the human gastrointestinal tract and hence improve human health.19,20 Indeed, clinical trials have been performed to study the effect of dietary FOS on fecal microbiota and functional constipation in constipated subjects.Regarding the nature of the glycosidic bonds between the sugar molecules, GOS has α- or β-configurations with α(1,2)/α(1,6) or β(1,4) glycosidic linkages, respectively.21,22 Several studies performed in the elderly and adults have demonstrated the prebiotic and bifidogenic effects of β(1,4)-GOS.23,24 Specifically, the GOS in milk powder (mostly β-GOS) can be utilized as selective substrates for the growth of two probiotic strains of bacteria Bifidobacterium lactis DR10 and Lactobacillus rhamnosus DR20.25 DSG is an α-GOS preparation containing stachyose, raffinose, verbascose and sucrose.12 Among them, stachyose, raffinose and verbascose, abundant in seeds of legumes, mallow and mustard, are the most common α-GOS.26,27 We previously demonstrated that DSG exerted prebiotic and constipation alleviative effects in mice, as evidenced by the increase of fecal bifidobacteria and lactobacilli, and the decrease of fecal enteric bacilli as well as the improvement of intestinal peristalsis and bowel function in mice administered with DSG.12 The present study aims to determine whether DSG has beneficial effects on intestinal microbiota and bowel function in humans. We firstly assessed the impact of DSG on the growth of intestinal bifidobacteria, lactobacilli, Clostridium perfringens, enteric bacilli, enterococci and Bacteroides in healthy adults. As a result, DSG potently promoted the growth of beneficial bacteria bifidobacteria and lactobacilli, and effectively inhibited the growth of pathogenic bacteria Clostridium perfringens (Fig. 3). These results suggest that DSG can profitably regulate the intestinal microbiota in humans, which is consistent with previous findings that chickpea (containing significant levels of raffinose and stachyose) and fermented soybean milk (mainly including sucrose, stachyose and raffinose) can positively modulate the intestinal microbial composition to promote intestinal health in healthy adults.28,29 Regarding gut microbial analysis, we used conventional plating methods in this study. The processes including selective culture medium preparation, inoculation of plates and colony counting make these methods time-consuming and labor-intensive.30 In future work, novel powerful DNA-based methods will be utilized.
The human intestinal tract is populated by a large, active and complex bacterial community which has an enormous impact on the nutritional and health status of the host, including the digestion of food, the metabolism of endogenous and exogenous compounds, immunomodulation, and the colonization of pathogenic bacteria.31,32 Considering the crucial role of gut microbiota in the pathophysiology of functional constipation,33 together with the beneficial effects of DSG on human gut microbiota, we speculated that DSG should lead to constipation remission. To determine the effects of DSG on bowel function in humans, a placebo-controlled, randomized, double-blind trial was performed on constipated volunteers in this study. Participants were given 5 g of DSG or placebo once daily for 30 d. The bowel function of participants was evaluated by scoring the defecation frequency, stool consistency and ease of passage. As expected, administration of DSG significantly increased the defecation frequency, made stools softer and made defecation easier in constipated participants, indicating the potential of DSG to alleviate constipation. These results are coincident with previous findings that GOS-containing yoghurt reduces the severity of mild constipation in elderly subjects by inducing higher defecation frequency, easier defecation and softer stools.34,35 Numerous human intervention studies have been conducted to explore the mechanisms of action of prebiotics in constipation. Lactulose has been demonstrated to contribute to normal defecation by increasing the stool water content and softening the stools via increasing the osmotic pressure and slightly acidifying the colonic content.36 Chicory inulin contributes to the maintenance of normal defecation by increasing the stool frequency via stimulating bacterial growth in the gut and increasing the bacteria cell mass and faecal bulk.37 These two health claims approved by the European Food Safety Authority reveal the possible mechanisms regarding the role of prebiotics in intestinal motility.36,37 In this study, DSG was shown to regulate the intestinal microbial composition and enhance bowel function in humans. In the future, robust intervention studies and omics techniques (metagenomics, metaproteomics and metabolomics) will be used to validate whether the beneficial effects of DSG on constipation are attributed to its microbe-mediated activity.
Functional constipation may lead to some discomforts, such as abdominal distension, abdominal pain, headache, dizziness and poor appetite, and these symptoms can heavily lower the quality of life in patients.10,38 Unfortunately, many commonly used agents for treating constipation are unable to mitigate and may even aggravate the gastrointestinal symptoms because of their recognized adverse effects.39 To evaluate the safety of DSG consumption, all the participants were required to complete the recording of occurring adverse events, the routine blood, urine and stool tests, and blood biochemical analysis. The low incidence of adverse reactions and the absence of abnormal changes in blood biochemical parameters and indices of blood, urine and stool routine examinations provide evidence for the excellent tolerability and safety of DSG. However, several studies have reported that gastrointestinal symptoms are common side effects of indigestible carbohydrates.35,40,41 Therefore, further investigations should be performed to evaluate the exact effect of DSG on the gastrointestinal symptoms in patients with functional constipation.
In conclusion, DSG at a dosage of 5 g d−1 could instructively regulate the gut microbiota with significant increases in bifidobacteria and lactobacilli and a remarkable decrease in Clostridium perfringens, and also could effectively improve the bowel function of patients suffering from constipation with a marked increase in defecation frequency. Furthermore, DSG at this dosage does not cause obvious adverse effects in both healthy adults and constipated patients. Considering the beneficial effects of DSG at 5 g d−1 on gut microbiota and bowel function, we recommend that DSG should be taken at 5 g d−1 to alleviate functional constipation. This study provides clinical bases for developing DSG as a novel and safe agent for treating functional constipation.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (C31671823), and the Development program for Innovative Research Team of Shaanxi Normal University, China (GK201501006), as well as Science and Technology Innovation as a Whole Plan Projects of Shaanxi Province, China (2015KTCQ02-01).References
- S. M. Mugie, M. A. Benninga and C. Di Lorenzo, Epidemiology of constipation in children and adults: a systematic review, Best Pract. Res., Clin. Gastroenterol., 2011, 25, 3–18 CrossRef PubMed.
- G. Bazzocchi, T. Giovannini, C. Giussani, P. Brigidi and S. Turroni, Effect of a new synbiotic supplement on symptoms, stool consistency, intestinal transit time and gut microbiota in patients with severe functional constipation: a pilot randomized double-blind, controlled trial, Tech. Coloproctol., 2014, 18, 945–953 CrossRef CAS PubMed.
- L. Zhu, W. Liu, R. Alkhouri, R. D. Baker, J. E. Bard and E. M. Quigley, et al., Structural changes in the gut microbiome of constipated patients, Physiol. Genomics, 2014, 46, 679–686 CrossRef CAS PubMed.
- E. M. Quigley, Microflora modulation of motility, J. Neurogastroenterol. Motil., 2011, 17, 140–147 CrossRef PubMed.
- C. H. Choi and S. K. Chang, Alteration of gut microbiota and efficacy of probiotics in functional constipation, J. Neurogastroenterol. Motil., 2015, 21, 4–7 CrossRef PubMed.
- B. W. Aichbichler, H. H. Wenzl, C. A. Santa Ana, J. L. Porter, L. R. Schiller and J. S. Fordtran, A comparison of stool characteristics from normal and constipated people, Dig. Dis. Sci., 1998, 43, 2353–2362 CrossRef CAS PubMed.
- I. L. Khalif, E. M. M. Quigley, E. A. Konovitch and I. D. Maximova, Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation, Dig. Liver Dis., 2005, 37, 838–849 CrossRef CAS PubMed.
- G. Zoppi, M. Cinquetti, A. Luciano, A. Benini, A. Muner and E. Bertazzoni Minelli, The intestinal ecosystem in chronic functional constipation, Acta Paediatr., 1998, 87, 836–841 CrossRef CAS PubMed.
- F. Guarner and J. R. Malagelada, Gut flora in health and disease, Lancet, 2003, 361, 512–519 CrossRef.
- F. Valerio, F. Russo, S. de Candia, G. Riezzo, A. Orlando and S. L. Lonigro, et al., Effects of probiotic lactobacillus paracasei-enriched artichokes on constipated patients: a pilot study, J. Clin. Gastroenterol., 2010, 44, S49–S53 CrossRef CAS PubMed.
- T. S. Manning and G. R. Gibson, Prebiotics, Best Pract. Res., Clin. Gastroenterol., 2004, 18, 287–298 CrossRef PubMed.
- T. Li, X. Lu and X. Yang, Stachyose-enriched α-galacto-oligosaccharides regulate gut microbiota and relieve constipation in mice, J. Agric. Food Chem., 2013, 61, 11825–11831 CrossRef CAS PubMed.
- M. C. Marín-Manzano, L. Abecia, O. Hernández-Hernández, M. L. Sanz, A. Montilla and A. Olano, et al., Galacto-oligosaccharides derived from lactulose exert a selective stimulation on the growth of Bifidobacterium animalis in the large intestine of growing rats, J. Agric. Food Chem., 2013, 61, 7560–7567 CrossRef PubMed.
- D. P. M. Torres, M. P. F. Gonçalves, J. A. Teixeira and L. R. Rodrigues, Galacto-oligosaccharides: production, properties, applications, and significance as prebiotics, Compr. Rev. Food Sci. Food Saf., 2010, 9, 438–454 CrossRef CAS.
- D. Pohl, R. Tutuian and M. Fried, Pharmacologic treatment of constipation: what is new?, Curr. Opin. Pharmacol., 2008, 8, 724–728 CrossRef CAS PubMed.
- S. I. Mussatto and I. M. Mancilha, Non-digestible oligosaccharides: a review, Carbohydr. Polym., 2007, 68, 587–597 CrossRef CAS.
- R. A. Rastall, Functional oligosaccharides: application and manufacture, Annu. Rev. Food Sci. Technol., 2010, 1, 305–339 CrossRef CAS PubMed.
- Q. Xu, Y. Chao and Q. Wan, Health benefit application of functional oligosaccharides, Carbohydr. Polym., 2009, 77, 435–441 CrossRef CAS.
- P. K. Gopal, J. Prasad and H. S. Gill, Effects of the consumption of Bifidobacterium lactis HN019 (DR10TM) and galacto-oligosaccharides on the microflora of the gastrointestinal tract in human subjects, Nutr. Res., 2003, 23, 1313–1328 CrossRef CAS.
- C. H. Yen, Y. W. Kuo, Y. H. Tseng, M. C. Lee and H. L. Chen, Beneficial effects of fructo-oligosaccharides supplementation on fecal bifidobacteria and index of peroxidation status in constipated nursinghome residents-a placebo-controlled, diet-controlled trial, Nutrition, 2011, 27, 323–328 CrossRef CAS PubMed.
- W. Van den Ende, Multifunctional fructans and raffinose family oligosaccharides, Front. Plant Sci., 2013, 4, 247 Search PubMed.
- J. M. Bruno-Barcena and M. A. Azcarate-Peril, Galacto-oligosaccharides and Colorectal Cancer: Feeding our Intestinal Probiome, J. Funct. Foods, 2015, 12, 92–108 CrossRef CAS PubMed.
- L. Niittynen, K. Kajander and R. Korpela, Galacto-oligosaccharides and bowel function, Scand. J. Food Nutr., 2007, 51, 62–66 CrossRef.
- Y. Deguchi, K. Matsumoto, A. Ito and M. Watanuki, Effects of β1-4 galacto-oligosaccharides administration on defecation of healthy volunteers with constipation tendency, Jpn. J. Nutr., 1997, 55, 1322 Search PubMed (In Japanese).
- P. K. Gopala, P. A. Sullivanb and J. B. Smart, Utilisation of galacto-oligosaccharides as selective substrates for growth by lactic acid bacteria including Bifidobacterium lactis DR10 and Lactobacillus rhamnosus DR20, Int. Dairy J., 2001, 11, 19–25 CrossRef.
- H. N. Johansen, V. Glitsø and K. E. B. Knudsen, Influence of extraction solvent and temperature on the quantitative determination of oligosaccharides from plant materials by high-performance liquid chromatography, J. Agric. Food Chem., 1996, 44, 1470–1474 CrossRef CAS.
- D. Qiu, T. Vuong, B. Valliyodan, H. Shi, B. Guo and J. G. Shannon, et al., Identification and characterization of a stachyose synthase gene controlling reduced stachyose content in soybean, Theor. Appl. Genet., 2015, 128, 2167–2176 CrossRef CAS PubMed.
- W. M. Fernando, J. E. Hill, G. A. Zello, R. T. Tyler, W. J. Dahl and A. G. Van Kesse, Diets supplemented with chickpea or its main oligosaccharide component raffinose modify faecal microbial composition in healthy adults, Benef. Microbes, 2010, 1, 197–207 CrossRef CAS PubMed.
- S. Inoguchi, Y. Ohashi, A. Narai-Kanayama, K. Aso, T. Nakagaki and T. Fujisawa, Effects of non-fermented and fermented soybean milk intake on faecal microbiota and faecal metabolites in humans, Int. J. Food Sci. Nutr., 2012, 63, 402–410 CrossRef CAS PubMed.
- P. K. Mandal, A. K. Biswas, K. Choi and U. K. Pal, Methods for rapid detection of foodborne pathogens: an overview, Am. J. Food Technol., 2011, 6, 87–102 CrossRef.
- Y. Tanaka, K. Takami, T. Nishijima, R. Aoki, T. Mawatari and T. Ikeda, Short- and long-term dynamics in the intestinal microbiota following ingestion of Bifidobacterium animalis subsp. lactis GCL2505, Biosci. Microbiota, Food Health, 2015, 34, 77–85 CrossRef PubMed.
- J. M. Laparra and Y. Sanz, Interactions of gut microbiota with functional food components and nutraceuticals, Pharmacol. Res., 2010, 61, 219–225 CrossRef CAS PubMed.
- E. M. Quigley, The enteric microbiota in the pathogenesis and management of constipation, Best Pract. Res., Clin. Gastroenterol., 2011, 25, 119–126 CrossRef CAS PubMed.
- U. Teuria and R. Korpela, Galacto-Oligosaccharides Relieve Constipation in Elderly People, Ann. Nutr. Metab., 1998, 42, 319–327 CrossRef.
- U. Sairanen, L. Piirainen, R. Nevala and R. Korpela, Yoghurt containing galacto-oligosaccharides, prunes and linseed reduces the severity of mild constipation in elderly subjects, Eur. J. Clin. Nutr., 2007, 61, 1423–1428 CrossRef CAS PubMed.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), Guidance on the scientific requirements for health claims related to the immune system, the gastrointestinal tract and defence against pathogenic microorganisms, EFSA J., 2016, 14, 4369 CrossRef.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), Scientific Opinion on the substantiation of a health claim related to “native chicory inulin” and maintenance of normal defecation by increasing stool frequency pursuant to Article 13.5 of Regulation (EC) No 1924/2006, EFSA J., 2015, 13, 3951 CrossRef.
- D. Chatoor and A. Emmnauel, Constipation and evacuation disorders, Best Pract. Res., Clin. Gastroenterol., 2009, 23, 517–530 CrossRef PubMed.
- M. Portalatin and N. Winstead, Medical management of constipation, Clin. Colon Rectal. Surg., 2012, 25, 12–19 CrossRef PubMed.
- J. H. Cummings, G. T. Macfarlane and H. N. Englyst, Prebiotic digestion and fermentation, Am. J. Clin. Nutr., 2001, 73, 415s–420s CAS.
- F. L. Suarez, J. Springfield, J. K. Furne, T. T. Lohrmann, P. S. Kerr and M. D. Levitt, Gas production in human ingesting a soybean flour derived from beans naturally low in oligosaccharides, Am. J. Clin. Nutr., 1999, 69, 135–139 CAS.
| This journal is © The Royal Society of Chemistry 2017 |