An integral study of cyclodextrins as solubility enhancers of α-methylstilbene, a resveratrol analogue†
Adrián
Matencio
,
Samanta
Hernández-García
 ,
Francisco
García-Carmona
and
José Manuel
López-Nicolás
,
Francisco
García-Carmona
and
José Manuel
López-Nicolás
 *
*
Department of Biochemistry and Molecular Biology-A, Faculty of Biology, University of Murcia, Campus de Espinardo, 30071, Murcia, Spain. E-mail: josemln@um.es; Fax: +34 868 364147; Tel: +34 868 8834777
First published on 6th December 2016
Abstract
trans-α-Methylstilbene (tMS), a resveratrol analogue, has recently been studied in a search for new bioactivities. However, such studies do not take into account that the poor solubility of tMS in aqueous solutions could affect its bioactivity. For this reason, we propose, for the first time, using cyclodextrins (CDs) as solubilizers to increase tMS solubility, in aqueous solutions. The HPLC-RP results obtained, point to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for all the natural (α-, β- and γ-CD) and modified (HPβCD and MβCD) CDs tested. The KFapp (apparent formation constant) for the tMS–CD complexes was seen to be closely dependent on several factors, including the temperature and type of CD. Indeed, the highest KFapp value was obtained for MβCD, while the KFapp decreased with increasing temperature. In addition, the results showed negative entropy (−8.86 × 10−3 ± 0.40 kJ mol−1 K−1) and enthalpy (−16.70 ± 0.98 kJ mol−1) changes and a negative Gibbs free energy value at 25 °C (−14.00 ± 0.55 kJ mol−1) for the encapsulation process. A computational study carried out using molecular docking calculations showed a high degree of correlation between the computed scores and experimental values. Finally, the complexation of trans-stilbene and pinosylvin with HPβCD was compared with tMS.
1 stoichiometry for all the natural (α-, β- and γ-CD) and modified (HPβCD and MβCD) CDs tested. The KFapp (apparent formation constant) for the tMS–CD complexes was seen to be closely dependent on several factors, including the temperature and type of CD. Indeed, the highest KFapp value was obtained for MβCD, while the KFapp decreased with increasing temperature. In addition, the results showed negative entropy (−8.86 × 10−3 ± 0.40 kJ mol−1 K−1) and enthalpy (−16.70 ± 0.98 kJ mol−1) changes and a negative Gibbs free energy value at 25 °C (−14.00 ± 0.55 kJ mol−1) for the encapsulation process. A computational study carried out using molecular docking calculations showed a high degree of correlation between the computed scores and experimental values. Finally, the complexation of trans-stilbene and pinosylvin with HPβCD was compared with tMS.
Introduction
One of the less well-known stilbenes is trans-α-methylstilbene (tMS, Fig. 1), an analogue of resveratrol used in chemical synthesis.1,2 Although the bioactivity of stilbenes is well known (including their antioxidant, antibacterial, anticancer activity, etc.3), the biological activities of tMS have been less investigated.Recently the National Cancer Institute (NIH) of USA studied the bioactivity of tMS for use in the fight against cancer, with disappointing results.4,5 However, the hydrophobic nature of tMS and its low solubility were not taken into account. Indeed, this problem could hinder the bioavailability of the compound, preventing the discovery of its bioactivities. It is therefore clear that tMS solubility needs to be improved if it is to be used in aqueous solutions. One possibility would be to use a polymer or a matrix to encapsulate tMS creating a substrate reservoir that would improve release efficiency.6 Of the polymers available, cyclodextrins (CDs) could be considered a good option.
CDs (Fig. 1) are torus-shaped oligosaccharides made up of α-(1,4) linked glucose units. The most common CDs are α-, β- and γ-CD, which contain six, seven and eight glucose units, respectively.7,8 These types of natural CDs have three GRAS statuses and are included in the lists of additives approved for alimentary use with the corresponding E-numbers α-, β- and γ-CD: E-457, E-459 and E-458, respectively. The cavity of CDs is carpeted by hydrogen atoms and is therefore of a rather hydrophobic nature, unlike the outer surface of the molecule, in which the primary and secondary hydroxyl groups are exposed to the solvent, making the whole molecule highly water-soluble.7,8 Poorly water-soluble compounds and hydrophobic moieties of amphiphilic molecules interact non-covalently with the CD cavity to form the so-called inclusion complexes, which are also highly water-soluble. However, the solubility of these complexes depends on several factors such as the type of CD, and, because CDs are able to increase the bioavailability of different compounds and protect different molecules against the action of external agents, their use in both the pharmaceutical and food industries is increasing.7–9 Among the guest molecules encapsulated by cyclodextrins are fatty acids,10 vitamins,11 and phenols.9
In recent years, our research group has published several studies on the ability of CDs to encapsulate different molecules of the stilbene family, such as resveratrol, oxyresveratrol, pterostilbene, pinosylvin or piceatannol.9,12 This knowledge has been put to use in industry, where they are added to some products such as nutraceuticals. As the present work will demonstrate, knowledge of these aspects is essential if tMS is to be used as a building block in aqueous reactions or for future nutraceutical use. Indeed, this is the first work to conduct an exhaustive study of the interaction between tMS and CDs.
Bearing the above in mind, the main objectives of this work were to:
(1) Analyse the encapsulation mechanism of tMS with different types of natural (α-, β- and γ-CD) and modified (HPβCD and MβCD) CDs.
(2) Evaluate the effect of temperature on the encapsulation mechanism of tMS.
(3) Determine the stoichiometry, KF values and thermodynamic parameters for the tMS–CD complexes.
(4) Study the types of interactions between tMS and CD using molecular docking.
(5) Compare the KF values of tMS, trans-stilbene and pinosylvin.
HPLC was used to characterize the inclusion complexes, a technique increasingly used for observing and characterizing CD–guest inclusion.13,14 Modifications in the retention properties of molecules with different CD concentrations in the mobile phase were seen to be related with the stoichiometry and stability of the inclusion complexes formed.15
Materials and methods
Materials
Quinine (purity ≥90% PubChem CIF 145904), natural CDs (α-, β- and γ-CD, purity ≥97% PubChem CIFs 444913, 444041 and 5287407), modified CDs (HPβCD and MβCD, purity ≥95% PubChem CIF 44134771 and 51051622) were purchased from Sigma-Aldrich (Madrid, Spain). tMS (purity ≥98% PubChem CIF 1549166) was purchased from Alfa Aesar (Karlsruhe, Germany) and used as received. Ethanol (analysis grade, PubChem CIF 702) was purchased from Merck (Madrid, Spain). The samples were stored in the dark. Methanol (HPLC grade, PubChem CIF 887) was purchased from Fisher (Madrid, Spain). MQ water was obtained using a Milli-Q Advantage A10 system by Merck Millipore (Madrid, Spain). Binary mixtures of water/methanol, with methanol percentages of 40–80% were used without further purification.Equipment and experimental procedures
To determine the KF value for the tMS/CD complexes, eqn (1), which relates to the capacity factor, k, and the CD mobile-phase concentration, [CD], was used.16 In this equation two conditions are assumed: (1) the complex has a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and (2) any interaction of the tMS/CD complexes with the stationary phase is negligible15 as explained by Fujimura et al. (1986),15 where its tR for the CD used was nearly the same as that of potassium nitrite used as a marker for measuring the column dead volume.
1 stoichiometry and (2) any interaction of the tMS/CD complexes with the stationary phase is negligible15 as explained by Fujimura et al. (1986),15 where its tR for the CD used was nearly the same as that of potassium nitrite used as a marker for measuring the column dead volume.
 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model.
1 model.
We also studied the possible formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 tMS/CD complex. Eqn (2) is an extension of eqn (1) and includes a second-order term that accounts for the possibility of a 1
2 tMS/CD complex. Eqn (2) is an extension of eqn (1) and includes a second-order term that accounts for the possibility of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 tMS/CD complex formation:17
2 tMS/CD complex formation:17
 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 tMS/CD complex. Values of R2 close to 1 indicate a 1
2 tMS/CD complex. Values of R2 close to 1 indicate a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 model.
2 model.
The column void volume, t0, was determined using a reagent grade copper sulphate solution (0.01 mg mL−1).18
The HPLC-RP method gives us an apparent KF (KFapp) because of methanol/CD interactions.19
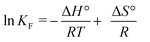 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KFversus 1/T, the slope and intercept are −ΔH°/R and ΔS°/R, respectively. To determine the Gibbs free energy change for the interactions that take place during the inclusion process, we used eqn (4):
KFversus 1/T, the slope and intercept are −ΔH°/R and ΔS°/R, respectively. To determine the Gibbs free energy change for the interactions that take place during the inclusion process, we used eqn (4):| ΔG° = ΔH° − TΔS° | (4) |
Results and discussion
Selection of the optimal conditions to characterize the encapsulation of tMS with different CDs
Several authors have demonstrated that the use of CDs as additives in the mobile phase in reversed-phase high performance liquid chromatography (RP-HPLC) decreases the retention time of the guest as a result of host–guest interactions.13 However, changes in retention are strongly dependent on factors such as the type of organic modifier or flow rate due to the host–guest complex stability. For this reason, the first step in our investigation was to select the most suitable composition of the mobile phase to be used for the analysis.The formation of CD inclusion complexes in the liquid phase proceeds more easily in an aqueous solution. However, an aqueous-organic solvent was used as a mobile phase in the present system (using a non-polar stationary phase) because when water alone was used as the mobile phase, very long retention times, with the associated experimental error and a column deterioration, were observed during the analysis.
In the selection of the most appropriate organic solvent for this work, two parameters were borne in mind (i) the affinity of the organic modifier for the CD cavity and the solubility of CDs in the organic solvent, due to their influence on the retention value, and (ii) the resolution of the sample solute and the binding constant of inclusion complexes of the solute. Although several types of organic solvents such as ethanol, acetonitrile or methanol have been used to identify different stilbenes by RP-HPLC,23 we introduced methanol in the corresponding mobile phases for the following reasons: (i) the very weak association of methanol with β-CD, as represented by the low value of Km, the constant which describes the affinity of the organic modifier for the CD cavity.19 Indeed the Km value described for the interaction between methanol and β-CD (Km = 0.32 M−1) or αCD (Km = 0.93 M−1) makes it a more favourable medium for the tMS–CD encapsulation process than other alcohols such as ethanol (Km for β-CD = 0.93 M−1; Km for α-CD = 5.62 M−1) or 1-propanol (Km for β-CD = 3.71 M−1; Km for α-CD = 23.44 M−1); (ii) the fact that the solubility of β-CD in methanol is greater than in acetonitrile and THF permits the concentration of the β-CD in the mobile phase to be increased, thus facilitating characterization of the tMS/β-CD complexes. For these reasons, binary mixtures of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water were used as the optimum composition of the mobile phase in RP-HPLC to study the encapsulation process of tMS by β-CD.
water were used as the optimum composition of the mobile phase in RP-HPLC to study the encapsulation process of tMS by β-CD.
Effect of MβCD on the retention time of tMS in HPLC-RP
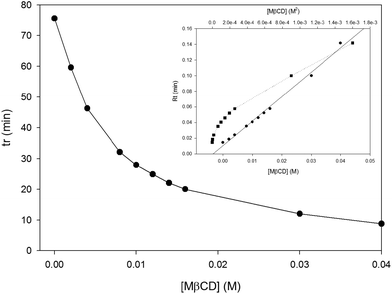
The next step was to test the experimental conditions by injecting tMS without CDs, the result of which was an increase in the retention time (tR) to 75.06 ± 3.02 min. Although tR could have been shortened using higher methanol concentrations, this would have greatly decreased the encapsulation efficiency as mentioned above. When the injection of tMS was assayed with different concentrations of MβCD (Fig. 2), tR decreased with increasing MβCD concentrations (from 75.06 ± 3.02 to 8.78 ± 0.5 at 40 mM of MβCD), perhaps because the encapsulation of tMS affects the solubility of hydrophobic compounds in aqueous solutions.24To validate the use of CDs as additives in the mobile phase, it was first necessary to confirm that the effect of CDs on the tMS tR was not due to the glucidic nature of the CDs, but to their ability to complex hydrophobic compounds.
To do this, the possibility that the reduction of tR of tMS was due to the presence of D-glucose in the reaction medium was studied since glucose is a molecule that forms a part of the CD structure. Thus, different amounts of D-glucose (14 and 280 mM), corresponding to 2 and 40 mM of MβCD (each molecule of MβCD contains seven units of D-glucose in a ring), were added to the 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (v/v) (methanol
40 (v/v) (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water) mobile phase and the tR of tMS was ascertained. The tMS tR in the absence of any additive was 75.06 ± 3.02 min but decreased in the presence of 2 mM (59.62 ± 2.87 min) and 40 mM (8.78 ± 0.5 min) MβCD, whereas the addition of D-glucose did not significantly alter the retention time (74.37 min).
water) mobile phase and the tR of tMS was ascertained. The tMS tR in the absence of any additive was 75.06 ± 3.02 min but decreased in the presence of 2 mM (59.62 ± 2.87 min) and 40 mM (8.78 ± 0.5 min) MβCD, whereas the addition of D-glucose did not significantly alter the retention time (74.37 min).
Several conclusions can be deduced from these results. Firstly, the addition of CD to the mobile phase reduces the tMS tR due to its capacity to complex hydrophobic substances since no glucose/tMS complexes are formed. Secondly, the possible elution modifications observed in the presence of CD cannot be attributed to modifications in the solvent strength.
Stoichiometry and apparent complexation constant of tMS by natural and modified CDs
It is important to ascertain the KF to know how CD should be added to the solution, the complexation behaviour, etc. in order to optimize the process. In the previous section, it was seen that MβCD could encapsulates tMS efficiently; but, when the concentration of some molecule needs to be increased, it is necessary to test many types of CDs. Furthermore, knowing the KF is important for optimizing the quantity of each reactant to be added, for which reason the encapsulation process was studied with a high number of natural (α-, β- and γ-CD) and modified (HPβCD and MβCD) CDs. Table 1 shows the different KFapp (apparent KF) values obtained and their stoichiometry (1 CD per tMS or 2 CDs per tMS) obtained using eqn (1) for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 encapsulation process or eqn (2) for a 2
1 encapsulation process or eqn (2) for a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 encapsulation process (ESI Fig. 2†). The highest KFapp value was obtained using MβCD (298.01 ± 15.03 M−1). The results showed that the tMS/MβCD complex has a 1
1 encapsulation process (ESI Fig. 2†). The highest KFapp value was obtained using MβCD (298.01 ± 15.03 M−1). The results showed that the tMS/MβCD complex has a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (R2 > 0.99, Fig. 2 inset) and not a 2
1 stoichiometry (R2 > 0.99, Fig. 2 inset) and not a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (R2 ≈ 95, Fig. 2 inset). Furthermore, the best KFapp for natural CDs was β-CD followed by γ- and α-CD, all with a 1
1 stoichiometry (R2 ≈ 95, Fig. 2 inset). Furthermore, the best KFapp for natural CDs was β-CD followed by γ- and α-CD, all with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry.
1 stoichiometry.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 tMS/CD complexes, respectively) at 25 °C in the C18 column and 60
2 tMS/CD complexes, respectively) at 25 °C in the C18 column and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 MeOH
40 MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water; and Vina simulated binding energy
water; and Vina simulated binding energy
| Type of CD | K Fapp (M−1) | SD (±) |
R
2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
R
2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Binding energy (kJ mol−1) |
|---|---|---|---|---|---|
| MβCD | 298.01 | 15.03 | 0.99 | 0.95 | −35.98 |
| HPβCD | 108.65 | 6.02 | 0.99 | 0.95 | −24.28 |
| β-CD | 82.67 | 4.01 | 0.99 | 0.95 | −23.85 |
| γ-CD | 23.16 | 1.12 | 0.99 | 0.96 | −23.01 |
| α-CD | 12.15 | 0.80 | 0.99 | 0.95 | −21.34 |
Increase of solubility of tMS by CDs, a direct measurement
As mentioned above, the decrease in tMS tR with increasing CD concentration was assumed to be due to the encapsulation of tMS in the cavity of CDs, which improves solubility. However, to confirm this hypothesis, a direct measurement of the tMS concentration in aqueous solution was carried out. Different aqueous solutions at a fixed tMS concentration were incubated with increasing MβCD concentrations at 14 °C for 24 h at 400 rpm. The resulting solutions were filtered and injected into the HPLC. The results (Fig. 3A) demonstrated that (a) the solubility of tMS in water is negligible and (b) CD can increase the solubility of tMS to give a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, which is demonstrated by the linear fitting of the diagram (above 20 mM) (R2 > 0.98). Moreover, the phase-solubility reflected a negative deviation at low concentrations of MβCD (below 20 mM), perhaps because very hydrophobic compounds generate dimers in aqueous solutions until the CD concentration is sufficient to separate the dimers and so encapsulate them.25
1 complex, which is demonstrated by the linear fitting of the diagram (above 20 mM) (R2 > 0.98). Moreover, the phase-solubility reflected a negative deviation at low concentrations of MβCD (below 20 mM), perhaps because very hydrophobic compounds generate dimers in aqueous solutions until the CD concentration is sufficient to separate the dimers and so encapsulate them.25
tMS/MβCD complex demonstrated by quinine fluorescence quenching
Although complex formation is the most probable explanation for our result, there is a slight possibility that adsorption or another non-specific interaction could have occurred. For that reason, in this section the quenching of quinine, a well-known CD guest,26,27 encapsulated by MβCD at increasing concentrations of tMS was studied. When tMS was not present, the quinine/MβCD complex showed no alteration in fluorescence. However, when tMS was added the equilibrium was displaced and a tMS/MβCD complex was formed, releasing free quinine. The decrease in the fluorescence signal was probably due to a decrease in quantum yield.28 On the other hand, if tMS were absorbed by MβCD there would have been no differences in the signal.29 The results (Fig. 3B) point to a decrease in fluorescence with increasing concentration of tMS. Hence, we can safely say that CDs increased the solubility of tMS by forming a complex.Effect of the CD structure on complexation
In the previous section the KFapp value and stoichiometry of different complexes of tMS with natural and modified CDs of differing structure, size and number of glucose units were determined. Table 1 shows the effect of adding α-, β- and γ-CD concentrations on the KFapp value. As can be observed, the highest KFapp was obtained with β-CD, followed by γ-CD and α-CD.At the molecular level, our data show that the inner diameter of the CD formed by seven units of glucose (β-CD: 6.0–6.4 Å) fitted tMS better than the inner diameter of six (α-CD: 4.7–5.2 Å) or eight (γ-CD: 7.5–8.3 Å) glucose units.
Any modification of β-CD occurs principally at the hydroxyl O2-H of the sugar residues situated on one side of the torus, at the edge of the glucose and inwardly orientated,30 thus increasing the hydrophobicity of the channel. The higher KFapp observed for the tMS/modified CD complexes could be due to an increase in hydrophobicity with no steric hindrances, as a result of the long chain presented by HPβCD. The results were corroborated using ANOVA (for all KFapps) and the Holm31 test (for pairs of KFapp). All the KFapp values differed significantly (P < 0.05) except the difference between α-CD and γ-CD (P ≈ 0.1), which might be explained by a minimal interaction between these CDs and tMS. As the strongest KFapp was obtained with MβCD, this was chosen as the host CD for the following sections of the paper.
Effect of temperature on the complexation constant of the tMS/MβCD complex
One of the most important physicochemical parameters studied in bioavailability is temperature, which is closely related, for example, with the release or integrity. For this reason, it is interesting to study the behaviour of the complex at different temperatures. Indeed, several researchers have studied the changes that occur in the equilibrium between CD and different compounds with temperature. Initially, some authors found that an increase in the temperature of the system leads to an increase in the KFapp values, as is the case with fatty acids–CD complexes.32 However, it has also been found that an increase of the system's temperature causes a dissociation of the complexes.12
Fig. 4 shows the effect of temperature on the complexation constant. An inverse relationship between temperature and KFapp was observed. Applying eqn (1) a stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was observed for all the temperatures tested, leading us to conclude that the encapsulation process is more efficient at low temperatures.
1 was observed for all the temperatures tested, leading us to conclude that the encapsulation process is more efficient at low temperatures.
 | ||
Fig. 4 Effect of temperature on the complexation constant (KF) of tMS/MβCD complexes (C18 column, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 MeOH/water 1.5 mL min−1). Inset: Van't Hoff plot (ln 40 MeOH/water 1.5 mL min−1). Inset: Van't Hoff plot (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KFvs. 1/T) for tMS/MβCD complexes. KFvs. 1/T) for tMS/MβCD complexes. | ||
Thermodynamic parameters for the tMS/MβCD complex
The next step of this study was to obtain the thermodynamic parameters of the encapsulation process (ΔH°, ΔS° and ΔG° at 25 ± 0.2 °C) in order to study the mechanistic aspects of the affinity of tMS for MβCD. A Van't Hoff plot (eqn (3)) pointed to a linear correlation coefficient higher than 0.98 (Fig. 4 inset).The results led to three main conclusions being reached concerning the nature of the encapsulation process of tMS by MβCD: (1) the process is exothermic as seen from the negative values obtained for enthalpy changes (−16.70 ± 0.98 kJ mol−1). This response is typical of hydrophobic interactions, van der Waals interactions, the displacement of water molecules from the inside of MβCD or the formation of hydrogen bonds; (2) the process presents a negative value for entropy changes (−8.86 × 10−3 ± 0.40 kJ mol−1 K−1) due to a decrease in the degrees of freedom of the complexed tMS compared with the free ones; (3) the process is spontaneous, as seen for the negative value obtained for the Gibbs free energy change (−14.00 ± 0.55 kJ mol−1) for the interactions that take place during the inclusion process at 25 ± 0.2 °C.
Molecular modelling of the complexes established between tMS and CDs
To understand the interactions between tMS and CDs, docking simulations were carried out and the binding affinity was obtained. Scoring functions are fast approximate mathematical methods used to predict the strength of the non-covalent interaction (also referred to as binding affinity) between two molecules after they have docked. Table 1 shows that the binding affinities were directly proportional to the Gibbs free energy of the encapsulation process and, thus, to the KFapp values. The good correlation observed between the binding affinities and experimental values suggests that our modelling methodology captures the essentials of the host–guest energetic interactions. Additionally, the structural information concerning the different binding poses obtained by docking (Fig. 5) might explain the experimental data obtained. With regard to the ring size of the different CDs, the weakest interaction corresponds to the complex with α-CD, as depicted in Fig. 5A. The main reason for this was weak hydrophobic stabilisation due to the poor host–guest fit. The γ-CD is probably more stable (Fig. 5C) due to a better fit between γ-CD and CD. However, in general terms, the stability of the encapsulation process was greater with β-CD and its derivatives HPβCD and MβCD (Fig. 5B, D and E). For CDs with seven sugar rings, the most stable combination corresponded to β-CD followed by its derivatives HPβCD and MβCD. The good fit between tMS and β-CD, possibly due to the optimal diameter, could explain the better KF than γ-CD. HPβCD provided a worse fit than MβCD; perhaps because of steric hindrances between tMS and hydroxypropyl groups (which would make it difficult to separate the hydroxypropyl groups from the phenolic ring, decreasing efficiency of the encapsulation process) or the increase of polar contacts with hydroxypropyl groups; in fact, tMS almost does not enter into the cyclodextrin.Comparative study of tMS, trans-stilbene and pinosylvin encapsulation process with HPβCD
In recent years, our group has encapsulated many stilbenes with CDs. The slight differences observed between stilbenes (hydroxyl groups, methyls, etc.) led us to compare their KF values. For that reason, we compared the complexation of HPβCD with tMS, trans-stilbene33 and pinosylvin,34 whose structures are depicted in Fig. 1.One obvious detail is that tMS (KFapp = 108.65 ± 6.02 M−1) and pinosylvin (KF = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 112 ± 761 M−1) have a 1
112 ± 761 M−1) have a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry whereas trans-stilbene (KF12 = 1.01 × 109 ± 0.67 × 106 M−2) has a 1
1 stoichiometry whereas trans-stilbene (KF12 = 1.01 × 109 ± 0.67 × 106 M−2) has a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry, perhaps because any substituent in the structure affects the fitting with two CDs. A comparison between the experimental KF values of tMS and pinosylvin was not possible due to the apparent value of tMS KF (pinosylvin was studied using a fluorescence technique, which provides a real KF). However, a computational study was used to compare them. The binding energy for tMS/HPβCD (−24.28 kJ mol−1, Fig. 5E) was far lower than pinosylvin/HPβCD (−33.47 kJ mol−1, Fig. 5F), perhaps due to the existence of hydrogen bonds in the pinosylvin/HPβCD complex, as the hydrogen bond is one of the most important types of interactions for the stabilization of inclusion complexes of guest molecules with CDs.35,36 Furthermore, a better fit was obtained for the simulation of pinosylvin, helping the stabilization. To conclude, in this section we have illustrated that: (a) a change in the bond between the two phenols could change the stoichiometry and (b) the presence of hydrogen bonds increases the strength of the complexation enormously.
2 stoichiometry, perhaps because any substituent in the structure affects the fitting with two CDs. A comparison between the experimental KF values of tMS and pinosylvin was not possible due to the apparent value of tMS KF (pinosylvin was studied using a fluorescence technique, which provides a real KF). However, a computational study was used to compare them. The binding energy for tMS/HPβCD (−24.28 kJ mol−1, Fig. 5E) was far lower than pinosylvin/HPβCD (−33.47 kJ mol−1, Fig. 5F), perhaps due to the existence of hydrogen bonds in the pinosylvin/HPβCD complex, as the hydrogen bond is one of the most important types of interactions for the stabilization of inclusion complexes of guest molecules with CDs.35,36 Furthermore, a better fit was obtained for the simulation of pinosylvin, helping the stabilization. To conclude, in this section we have illustrated that: (a) a change in the bond between the two phenols could change the stoichiometry and (b) the presence of hydrogen bonds increases the strength of the complexation enormously.
Conclusions
The bioactivity assays using tMS are almost totally limited by its hydrophobic nature, and any increase in its solubility in aqueous solution could open up new uses and activities. In this work, we propose, for the first time, using cyclodextrins to increase the solubility of tMS in aqueous solution. Our results show that, although the stoichiometry of the complex is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for all the conditions used, the best KF values obtained for the CDs tested were for MβCD, which was chosen for the study. Furthermore, the KFapp values for the tMS–CD complexes are strongly dependent on several factors, such as the temperature, type of CD and structure of the guest molecule. For example, an inverse relationship between temperature and KF was observed. Moreover, the results showed negative entropy (−8.86 × 10−3 ± 0.40 kJ mol−1 K−1) and enthalpy (−16.70 ± 0.98 kJ mol−1) changes and a negative Gibbs free energy value at 25 °C (−14.00 ± 0.55 kJ mol−1) for the encapsulation process. A computational study carried out using molecular docking calculations showed a high degree of correlation between the computed binding affinities and the experimental values. The same numerical order of the complexation was predicted. Finally, a comparison between tMS, trans-stilbene and pinosylvin encapsulation process with HPβCD was carried out finding that any modification of the stilbene structure affected the complexation. The findings as a whole present a new opportunity to improve the assays with this compound in order to find out new bioactivities.
1 for all the conditions used, the best KF values obtained for the CDs tested were for MβCD, which was chosen for the study. Furthermore, the KFapp values for the tMS–CD complexes are strongly dependent on several factors, such as the temperature, type of CD and structure of the guest molecule. For example, an inverse relationship between temperature and KF was observed. Moreover, the results showed negative entropy (−8.86 × 10−3 ± 0.40 kJ mol−1 K−1) and enthalpy (−16.70 ± 0.98 kJ mol−1) changes and a negative Gibbs free energy value at 25 °C (−14.00 ± 0.55 kJ mol−1) for the encapsulation process. A computational study carried out using molecular docking calculations showed a high degree of correlation between the computed binding affinities and the experimental values. The same numerical order of the complexation was predicted. Finally, a comparison between tMS, trans-stilbene and pinosylvin encapsulation process with HPβCD was carried out finding that any modification of the stilbene structure affected the complexation. The findings as a whole present a new opportunity to improve the assays with this compound in order to find out new bioactivities.
Acknowledgements
This work was supported by the “Ministerio de Ciencia e Innovación” (MCINN, FEDER, Spain) (Project AGL2014-57431) and by “Programa de ayudas a Grupos de Excelencia de la Región de Murcia, de la Fundación Séneca, Agencia de Ciencia y Tecnología de la Región de Murcia” No. 19893/GERM/2015. Adrián Matencio holds a “FPU UM” contract from the University of Murcia (R-1042/2015).References
- G. Berti, F. Bottari and B. Macchia, Tetrahedron, 1964, 20, 545–550 CrossRef CAS
.
- X. Quan, V. S. Parihar, M. Bera and P. G. Andersson, Eur. J. Org. Chem., 2014, 140–146 CrossRef CAS
.
- K. M. Kasiotis, H. Pratsinis, D. Kletsas and S. A. Haroutounian, Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc., 2013, 61, 112–120 CrossRef CAS PubMed
.
- PubChem, 2016, https://pubchem.ncbi.nlm.nih.gov/compound/1549166#section=Biological-Test-Results.
- PubChem, 2016, https://pubchem.ncbi.nlm.nih.gov/bioassay/248#section=Data-Table.
- C. M. Bernards, J. Pharm. Sci., 1994, 83, 620–622 CrossRef CAS PubMed
.
- L. Szente and J. Szejtli, Trends Food Sci. Technol., 2004, 15, 137–142 CrossRef CAS
.
- E. M. M. Del Valle, Process Biochem., 2004, 39, 1033–1046 CrossRef CAS
.
- J. M. López-Nicolás, P. Rodríguez-Bonilla and F. García-Carmona, Crit. Rev. Food Sci. Nutr., 2014, 54, 251–276 CrossRef PubMed
.
- A. Matencio, C. J. G. Hernández-Gil, F. García-Carmona and J. M. López-Nicolás, Food Chem., 2017, 216, 289–295 CrossRef CAS PubMed
.
- N. Vilanova and C. Solans, Food Chem., 2015, 175, 529–535 CrossRef CAS PubMed
.
- A. Matencio, F. García-Carmona and J. M. López-Nicolás, Food Funct., 2016, 7, 2367–2373 CAS
.
- J. M. López-Nicolás, M. Escorial Camps, H. Pérez-Sánchez and F. García-Carmona, J. Agric. Food Chem., 2013, 61, 11347–11354 CrossRef PubMed
.
- A. Matencio, M. J. Bermejo-Gimeno, F. García-Carmona and J. M. López-Nicolás, Phytochem. Anal., 2016 DOI:10.1002/pca.2654
.
- K. Fujimura, T. Ueda, M. Kitagawa, H. Takayanagi and T. Ando, Anal. Chem., 1986, 58, 2668–2674 CrossRef CAS
.
- J. M. López-Nicolás, E. Núñez-Delicado, A. J. Pérez-López, A. C. Barrachina and P. Cuadra-Crespo, J. Chromatogr., A, 2006, 1135, 158–165 CrossRef PubMed
.
- C. Moeder, T. O'Brien, R. Thompson and G. Bicker, J. Chromatogr., A, 1996, 736, 1–9 CrossRef CAS
.
- I. Clarot, D. Clédat, S. Battu and P. J. Cardot, J. Chromatogr., A, 2000, 903, 67–76 CrossRef CAS PubMed
.
- Y. Matsui and K. Mochida, Bull. Chem. Soc. Jpn., 1979, 52, 2808–2814 CrossRef CAS
.
- M. D. Hanwell, D. E. Curtis, D. C. Lonie, T. Vandermeersch, E. Zurek and G. R. Hutchison, J. Cheminf., 2012, 4, 17 CAS
.
- A. W. Schüttelkopf and D. M. F. van Aalten, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2004, 60, 1355–1363 CrossRef PubMed
.
- O. Trott and A. J. Olson, J. Comput. Chem., 2010, 31, 455–461 CAS
.
- M. E. Juan, R. M. Lamuela-Raventós, M. C. de la Torre-Boronat and J. M. Planas, Anal. Chem., 1999, 71, 747–750 CrossRef CAS PubMed
.
- P. Rodríguez-Bonilla, J. M. López-Nicolás and F. García-Carmona, J. Chromatogr., B: Biomed. Appl., 2010, 878, 1569–1575 CrossRef PubMed
.
- T. Loftsson, D. Hreinsdóttir and M. Másson, Int. J. Pharm., 2005, 302, 18–28 CrossRef CAS PubMed
.
- Z. Fan, C.-H. Diao, H.-B. Song, Z.-L. Jing, M. Yu, X. Chen and M.-J. Guo, J. Org. Chem., 2006, 71, 1244–1246 CrossRef CAS PubMed
.
- X.-M. Wang and H.-Y. Chen, Spectrochim. Acta Part A, 1995, 51, 333–339 CrossRef
.
- M. Hoshino, M. Imamura, K. Ikehara and Y. Hama, J. Phys. Chem., 1981, 85, 1820–1823 CrossRef CAS
.
- T. Kuwabara, A. Nakamura, A. Ueno and F. Toda, J. Phys. Chem., 1994, 98, 6297–6303 CrossRef CAS
.
- D. W. Armstrong, S. M. Han and Y. I. Han, Anal. Biochem., 1987, 167, 261–264 CrossRef CAS PubMed
.
- S. Holm, Scand. J. Stat., 1979, 6, 65–70 Search PubMed
.
- J. M. López-Nicolás, R. Bru, A. Sánchez-Ferrer and F. García-Carmona, Biochem. J., 1995, 308, 151–154 CrossRef
.
- J. M. López-Nicolás, P. Rodríguez-Bonilla, L. Méndez-Cazorla and F. García-Carmona, J. Agric. Food Chem., 2009, 57, 5294–5300 CrossRef PubMed
.
- J. M. López-Nicolás, P. Rodríguez-Bonilla and F. García-Carmona, J. Agric. Food Chem., 2009, 57, 10175–10180 CrossRef PubMed
.
- W. L. Hinze, Sep. Purif. Rev., 1981, 10, 159–237 CrossRef CAS
.
- W. Saenger, Angew. Chem., Int. Ed. Engl., 1980, 19, 344–362 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6fo01677d |
| This journal is © The Royal Society of Chemistry 2017 |