Effects of daily blueberry consumption on circulating biomarkers of oxidative stress, inflammation, and antioxidant defense in postmenopausal women with pre- and stage 1-hypertension: a randomized controlled trial
Sarah A.
Johnson
*ab,
Rafaela G.
Feresin
 bd,
Negin
Navaei
bc,
Arturo
Figueroa
b,
Marcus L.
Elam
be,
Neda S.
Akhavan
bc,
Shirin
Hooshmand
f,
Shirin
Pourafshar
bc,
Mark E.
Payton
g and
Bahram H.
Arjmandi
bc
bd,
Negin
Navaei
bc,
Arturo
Figueroa
b,
Marcus L.
Elam
be,
Neda S.
Akhavan
bc,
Shirin
Hooshmand
f,
Shirin
Pourafshar
bc,
Mark E.
Payton
g and
Bahram H.
Arjmandi
bc
aDepartment of Food Science and Human Nutrition, Colorado State University, Fort Collins, CO 80523, USA. E-mail: Sarah.Johnson@colostate.edu; Tel: +1-970-491-3807
bDepartment of Nutrition, Food and Exercise Sciences, Florida State University, Tallahassee, FL 32306, USA
cCenter for Advancing Exercise and Nutrition Research on Aging (CAENRA), Florida State University, Tallahassee, FL 32306, USA
dDepartment of Dietetics and Nutrition, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA
eDepartment of Human Nutrition and Food Science, California State Polytechnic University, Pomona, CA 91768, USA
fSchool of Exercise and Nutritional Sciences, San Diego State University, CA, USA
gDepartment of Statistics, Oklahoma State University, Stillwater, OK, USA
First published on 2nd December 2016
Abstract
Oxidative stress and inflammation are central to the development of a number of chronic diseases including cardiovascular disease and previous research suggests that blueberry consumption may attenuate these processes. The present study investigated the effects of blueberries on blood biomarkers of oxidative stress, inflammation, and antioxidant defense in postmenopausal women with pre- and stage 1-hypertension. In a randomized, parallel-arm, double-blind, placebo-controlled clinical trial, 40 pre- and stage 1-hypertensive postmenopausal women aged 45 to 65 years were randomly assigned to receive 22 g freeze-dried highbush blueberry powder per day (Blueberry) or 22 g placebo powder per day (Control) for 8 weeks. A blood biomarker of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine (8-OHdG), as well as blood biomarkers of oxidative stress, inflammation, and antioxidant defense were assessed at baseline, 4 and 8 weeks. 8-OHdG levels were significantly (P = 0.008) lower in Blueberry compared to Control at 4 weeks with a significant time-by-treatment interaction (P = 0.04). Levels were not different between groups at 8 weeks. Other biomarkers measured were not affected by blueberry consumption. Daily consumption of blueberries for 4 weeks, but not 8 weeks, attenuated a biomarker of oxidative DNA damage in pre- and stage 1-hypertensive postmenopausal women. Future clinical studies should directly evaluate the effects of blueberry consumption on oxidative stress, inflammation, and antioxidant defense at the cellular level and in the vasculature in this population.
1. Introduction
Hypertension is a major risk factor for a number of chronic diseases including cardiovascular, cerebrovascular, and renal diseases, type 2 diabetes and cancer, particularly renal cell carcinoma.1 This is a major public health concern given that approximately 80 million adults in the United States (US), or 32.6% of the adult population, have hypertension.2 Oxidative stress and chronic inflammation not only play a role in the pathogenesis of hypertension but are perpetuated by high blood pressure, leading to a vicious cycle and increasing chronic disease risk.3,4Oxidative stress is an imbalance between the production of reactive oxygen species (ROS; e.g. superoxide anions) and the activity of detoxifying antioxidants including enzymatic and non-enzymatic endogenous and exogenous molecules. Examples of endogenous enzymatic antioxidant molecules include superoxide dismutase (SOD), glutathione reductase (GR), and glutathione peroxidase (GPx). Examples of exogenous antioxidant molecules include vitamins A, C, E, and polyphenols. Oxidative stress leads to accumulation of ROS in the body resulting in damage of cellular components and the formation of products of oxidative stress. This includes DNA damage (e.g. 8-hydroxy-2′-deoxyguanosine, 8-OHdG), lipid oxidation and peroxidation (e.g. oxidized low-density lipoprotein, OxLDL; thiobarbituric acid reactive substances, TBARS; and 8-isoprostane), and protein damage (e.g. protein carbonyls).5 Chronic inflammation is characterized by increased production of pro-inflammatory cytokines (e.g. tumor necrosis factor-alpha, TNF-α), and hepatic acute-phase proteins (e.g. C-reactive protein, CRP). Oxidative stress and chronic inflammation have a reciprocal relationship such that each exacerbates the other.6 Importantly, both oxidative stress and inflammation are central to the development and progression of a number of chronic diseases.5–7 As such, interventions aimed not only at improving blood pressure but also mitigating oxidative stress and chronic inflammation in individuals with high blood pressure may be more effective at reducing disease risk (e.g. coronary artery, cerebrovascular, and renal diseases, heart failure, type 2 diabetes, and cancer) than interventions that only improve blood pressure.
Consumption of fruits and vegetables rich in bioactive compounds including vitamins and polyphenols has been demonstrated to protect against oxidative stress and inflammation and the development of chronic diseases.8,9 Blueberries are one of the most widely consumed fruits in the US and are rich in bioactive compounds including vitamins and minerals, dietary fiber, and polyphenols, particularly flavonoids such as anthocyanins and flavanols, as well as phenolic acids primarily as chlorogenic acid, and stilbenes.10–12 Previous research supports the potential for consumption of blueberries to reduce oxidative stress and damage.13 Blueberries have been demonstrated to exert anti-carcinogenic effects.10,14 Additionally, the beneficial effects of blueberries on blood pressure, vascular function, and cardiometabolic health in humans have been reported12,15–19 and reviewed.10,20,21
We previously demonstrated that blueberries reduced systolic and diastolic blood pressure and arterial stiffness, as assessed by brachial-ankle pulse wave velocity, and increased circulating nitric oxide metabolite levels after 8 weeks in pre- and stage 1-hypertensive postmenopausal women in a randomized controlled trial.22 Although the prevalence of hypertension is associated with aging in both genders, the increase in blood pressure in women after menopause exceeds that of men.2 Further, estrogen deficiency in postmenopausal women results in accelerated age-related increases oxidative stress and chronic inflammation making this population particularly vulnerable to developing a number of chronic diseases.4,23 Preclinical evidence suggests that blueberries attenuate oxidative stress, inflammation, atherogenesis, and hypertension-induced nephropathy through improvements in antioxidant defense.24–26 To our knowledge, the ability of blueberries to reduce systemic oxidative stress and inflammation, and improve antioxidant defense in this population has not been previously investigated.
The objective of this study was to investigate the effects of blueberries on systemic oxidative stress, inflammation, and antioxidant defense in postmenopausal women with pre- and stage 1-hypertension. This is a secondary analysis of data collected as part of our previously published randomized controlled trial in which the primary outcome was blood pressure.22 We hypothesized that daily consumption of blueberries for 8 weeks would reduce biomarkers of oxidative stress and inflammation and improve antioxidant defense compared to control.
2. Methods
2.1 Subjects and study design
A detailed description of the study design and participant characteristics have been previously reported.22 Briefly, postmenopausal women between the ages of 45 and 65 years with pre- and stage 1-hypertension were recruited via newspaper advertisements and flyer distribution from the greater Tallahassee, Florida and the surrounding areas between January 2012 and March 2013. After an initial pre-screening over the telephone, potential participants were invited to the study site for their first visit during which they provided written informed consent. Brachial blood pressure measurements were taken in duplicate after 10 min of seated rest with an automatic device (Omron Healthcare, Inc.). A complete medical and nutrition history was obtained from participants by a registered dietitian nutritionist for screening purposes. Forty-eight women that met all inclusion criteria participated in an 8-week, randomized (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), parallel-arm, double-blind, placebo-controlled clinical trial. The research protocol was approved by the Human Subjects Committee of the Institutional Review Board at Florida State University. This trial was registered at clinicaltrials.gov as NCT01686282.
1), parallel-arm, double-blind, placebo-controlled clinical trial. The research protocol was approved by the Human Subjects Committee of the Institutional Review Board at Florida State University. This trial was registered at clinicaltrials.gov as NCT01686282.
2.2 Intervention and compliance
Eligible participants were randomly assigned by the study coordinator using a computer pre-generated randomization list provided by the statistician (PC SAS, version 9.1, 2006, SAS Institute) to one of the two groups: 22 g of freeze-dried blueberry powder (Blueberry) or 22 g of placebo powder (Control). The dose used is equivalent to approximately 1 cup of fresh blueberries. The blueberry powder consisted of freeze-dried highbush blueberries [50/50 Blend of Tifblue (Vaccinium virgatum [ashei]) and Rubel (Vaccinium corymbosum)] and the placebo powder consisted of maltodextrin, fructose, artificial and natural blueberry flavoring, artificial purple and red color, citric acid, and silica dioxide. The nutritional composition of the freeze-dried blueberry powder and placebo powder was determined by Medallion Laboratories and has been reported elsewhere.22 Briefly, the phenolic and anthocyanin content of the 22 g freeze-dried blueberry powder used in this study was approximately 845 mg and 469 mg, respectively. Participants were asked to consume 1/2 of the daily regimen (11 g) mixed with 1 cup (240 mL) water in the morning and the second 1/2 mixed with 1 cup (240 mL) water in the evening at least 6 to 8 h apart. The freeze-dried blueberry powder and placebo powder regimens used in this study were provided by the US Highbush Blueberry Council and were distributed to subjects on a bi-weekly basis. To monitor compliance, participants were given customized calendars and were asked to record the days they missed consuming the study regimen and return any unused portion for compliance monitoring purposes. Participants were asked to maintain their usual diet and physical activity patterns throughout the duration of the study.2.3 Anthropometric assessments
Height without shoes was measured at baseline using a wall-mounted stadiometer to the nearest 0.5 cm and weight was assessed at baseline, 4 and 8 weeks using a digital scale (Seca Corporation) to the nearest 0.1 kg. BMI was calculated as kg m−2.2.4 Blood collection
After an overnight fast (at least 8 h), blood was collected between 8:00 a.m. and 10:00 a.m. from each participant in vacutainers with appropriate anticoagulants at baseline, 4, and 8 weeks. Serum and plasma were separated by centrifuging at 3500g for 15 min at 4 °C within 2 h of collection using an IEC CL31R multispeed centrifuge (Thermo Electron Corporation). Samples were then aliquoted and stored at −80 °C until analysis.2.5 Blood biomarkers
Serum SOD, TNF-α, TBARS, and 8-isoprostane and plasma CRP, GPx, and GR were measured at baseline, 4, and 8 weeks using commercially available kits (Superoxide Dismutase Assay Kit, TNF-α ELISA Kit, TBARS Assay Kit, 8-Isoprostane ELISA Kit, C-Reactive Protein ELISA Kit, Glutathione Peroxidase Assay Kit, and Glutathione Reductase Assay Kit, respectively; Cayman Chemicals). Serum OxLDL was measured at baseline, 4, and 8 weeks using a commercially available kit (Human OxLDL ELISA Kit; MyBioSource). All samples were assayed in duplicate and according to the manufacturers’ instructions.The concentration of free 8-OHdG is low in plasma compared to that which is incorporated into DNA. Therefore, to most accurately determine the concentration of 8-OHdG, DNA was purified from samples using the EpiSeeker DNA Purification and Modification Kit (Abcam). Next, DNA samples were converted to single-stranded DNA by incubating the samples at 95 °C for 5 min and then rapidly chilling them on ice. Single-stranded DNA samples were then digested to nucleosides by incubating the denatured DNA with 9 units of nuclease P1 from Penicillium citrinum (Sigma-Aldrich) for 2 h at 37 °C in a buffer containing 20 mM sodium acetate (pH 5.2), 5 mM zinc chloride, and 50 mM sodium chloride followed by a 1 h incubation at 37 °C with 5 units of alkaline phosphatase from Escherichia coli (Sigma-Aldrich) in 100 mM Tris (pH 7.5). The samples were then centrifuged for 5 min at 6000g and the supernatant was collected for use in the 8-OHdG ELISA Kit (Abcam) which was measured in duplicate at baseline, 4, and 8 weeks according to the manufacturers’ instructions.
2.6 Statistical analysis
As previously described,22 an initial sample size of 48 participants was estimated to allow detection of a significant difference (P < 0.05) in blood pressure at 80% power based on previous research. Statistical analysis was performed using ANOVA methods with PROC MIXED in PC SAS (version 9.1, 2006, SAS Institute). Baseline values of blood biomarker and anthropometric variables, which were normally distributed between the two groups, were compared using two-sample t-tests. The main and interaction effects of the control (placebo) and experimental (blueberry) groups and time (baseline, 4, and 8 weeks) on outcome variables were evaluated. A split plot model of 2 (treatment) × 3 (time) repeated measures ANOVA was used for statistical analysis both within and between treatment groups. The mean changes in outcome variables during the intervention periods were compared by analyzing planned interaction effects of treatment and time, using the SLICE option in a least square means statement. Values are reported as least square mean ± SEM. In all statistical comparisons, differences with P < 0.05 were considered significant.3. Results
3.1 Baseline characteristics and anthropometrics
A total of 48 postmenopausal women who met the inclusion and exclusion criteria were randomly assigned to receive either 22 g of freeze-dried blueberry powder (Blueberry, 25 participants) or 22 g of placebo powder (Control, 23 participants) daily for 8 weeks. Attrition led to a final sample size of 20 participants per group (total of 40 participants). A diagram of participant flow throughout the study, reasons for attrition, and compliance were previously reported.22As previously reported,22 there were no significant differences between groups for baseline characteristics including age, height, weight, and BMI and there were no significant changes in body weight or BMI in either group at any time point.
3.2 Blood biomarkers of oxidative stress, inflammation, and antioxidant defense
Blood biomarkers are presented in Table 1. At 4 weeks, plasma 8-OHdG concentrations were significantly (P = 0.008) lower in Blueberry than in Control with a significant treatment-by-time interaction (P = 0.04). Mean 8-OHdG values increased to that of baseline at 8 weeks in Blueberry and there were no significant differences between the groups at this time point. These findings suggest that daily blueberry consumption was initially protective against oxidative DNA damage but was not able produce a sustained effect over a longer duration.| Blueberry (n = 20) | Control (n = 20) | P | |||
|---|---|---|---|---|---|
| Treatment | Time | Interaction | |||
| Values are mean ± SEM. Labeled means in a column without a common letter differ significantly (P < 0.05, repeated measures ANOVA). *Different from control (P < 0.05, repeated measures ANOVA). Blueberry, 22 g freeze-dried blueberry powder; Control, 22 g placebo powder; CRP, C-reactive protein; GPx, glutathione peroxidase; GR, glutathione reductase; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; 8-iso, 8-isoprostane; OxLDL, oxidized low-density lipoprotein; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substances; TNF-α, tumor necrosis factor-α.a Previously reported.22 | |||||
| CRPa, mg mL−1 | |||||
| Baseline | 2.49 ± 0.87 | 2.69 ± 0.80 | |||
| 4 weeks | 2.44 ± 1.01 | 2.64 ± 0.87 | 0.51 | 0.18 | 0.93 |
| 8 weeks | 2.30 ± 1.08 | 2.29 ± 1.20 | |||
| TNF-α, pg mL−1 | |||||
| Baseline | 6.7 ± 0.68a* | 5.09 ± 0.30a | |||
| 4 weeks | 4.75 ± 0.68b* | 3.03 ± 0.20b | 0.02 | <0.0001 | 0.94 |
| 8 weeks | 5.09 ± 0.63b* | 3.65 ± 0.15b | |||
| SODa, U mL−1 | |||||
| Baseline | 0.21 ± 0.06c | 0.23 ± 0.05c | |||
| 4 weeks | 0.36 ± 0.11b | 0.40 ± 0.06b | 0.64 | <0.0001 | 0.66 |
| 8 weeks | 0.50 ± 0.22a | 0.49 ± 0.15a | |||
| GR, nmol min−1 mL−1 | |||||
| Baseline | 8.91 ± 0.86b | 8.75 ± 0.89b | |||
| 4 weeks | 14.08 ± 1.05a | 14.08 ± 1.19a | 0.62 | <0.0001 | 0.82 |
| 8 weeks | 15.63 ± 0.88a | 16.76 ± 0.90a | |||
| GPx, nmol min−1 mL−1 | |||||
| Baseline | 29.79 ± 4.91c | 29.41 ± 6.64c | |||
| 4 weeks | 91.72 ± 5.18a | 88.19 ± 5.49a | 0.23 | <0.0001 | 0.34 |
| 8 weeks | 64.20 ± 8.20b | 48.65 ± 5.63b | |||
| 8-OHdG, ng mL−1 | |||||
| Baseline | 0.32 ± 0.01 | 0.31 ± 0.01 | |||
| 4 weeks | 0.28 ± 0.01* | 0.35 ± 0.02 | 0.008 | 0.34 | 0.04 |
| 8 weeks | 0.32 ± 0.02 | 0.35 ± 0.01 | |||
| OxLDL, ng mL−1 | |||||
| Baseline | 383.3 ± 5.3a | 424.0 ± 8.2a | |||
| 4 weeks | 337.9 ± 8.6b | 319.4 ± 13.5b | 0.57 | <0.0001 | 0.31 |
| 8 weeks | 400.4 ± 4.6a | 408.8 ± 29.7a | |||
| 8-Iso, pg mL−1 | |||||
| Baseline | 18.59 ± 1.87a | 15.12 ± 3.11a | |||
| 4 weeks | 15.86 ± 1.86ab | 9.92 ± 1.29b | 0.12 | 0.003 | 0.07 |
| 8 weeks | 11.78 ± 1.02b | 11.97 ± 1.68ab | |||
| TBARS, μM | |||||
| Baseline | 1.95 ± 0.11 | 2.01 ± 0.14 | |||
| 4 weeks | 2.65 ± 0.33 | 2.46 ± 0.39 | 0.44 | 0.03 | 0.72 |
| 8 weeks | 2.16 ± 0.19 | 1.85 ± 0.10 | |||
Serum TNF-α concentrations were significantly (P < 0.0001) reduced at 4 and 8 weeks compared to baseline for both Blueberry and Control. Between-group analysis indicated that serum TNF-α concentrations were significantly (P = 0.02) different at each time point. Because the within and between group effects were the same in both groups, this suggests that blueberry consumption did not provide a unique treatment effect on circulating TNF-α concentrations.
Plasma GR, GPx, and serum SOD (previously reported22) activities were significantly (P < 0.0001) increased at 4 and 8 weeks compared to baseline in both Blueberry and Control. Between-group analysis revealed no significant differences at any time point. Serum OxLDL concentrations were significantly (P < 0.0001) reduced at 4 weeks, but not 8 weeks, compared to baseline in both Blueberry and Control. Between-group analysis revealed no significant differences at any time point. These findings suggest that these parameters may have been impacted due to time rather than the treatment.
8-Isoprostane levels were significantly (P = 0.003) reduced in the Blueberry at 8 weeks with a trend for a treatment-by-time interaction. Between-group analysis revealed that at 4 weeks, plasma 8-isoprostane concentrations were lower in Control than baseline and significantly different than Blueberry. However, levels were not significantly different from each other at 8 weeks. Because both groups demonstrated reduced levels over the 8-week period and were not significantly different from each other at 8 weeks, this suggests that circulating 8-isoprostane may have been impacted by time but not treatment.
No significant results were noted within or between groups for plasma CRP22 and serum TBARS concentrations suggesting no influence of time or treatment on circulating levels of these parameters.
4. Discussion
To our knowledge, this is the first study to investigate the effects of blueberries on circulating biomarkers of oxidative stress, inflammation, and antioxidant defense in postmenopausal women with pre- and stage 1-hypertension. In addition to reducing blood pressure and improving arterial stiffness as previously reported,22 our current findings suggest that 4 weeks of daily freeze-dried blueberry consumption provided modest protection against oxidative DNA damage. However, the hypothesis that daily consumption of blueberries would reduce oxidative stress and inflammation and improve antioxidant defense compared to control cannot be confirmed at this time as improvements in oxidative DNA damage were not sustained at 8 weeks and improvements were not observed in other measured circulating biomarkers.Of note, a treatment effect was observed in the blueberry-treated group after 4 weeks for plasma 8-OHdG concentrations, a biomarker of oxidative DNA damage. However, levels were not different between groups at 8 weeks. Although the reasons or underlying mechanisms responsible for this observation are unknown, it is possible that there is an acclimation to chronic blueberry consumption with respect to their ability to reduce oxidative DNA damage. In fact, it has been reported27 that blackberries provide acute protection against DNA damage with observed effects disappearing after 6 days. Although that study was different from the current study in many regards (e.g. berry type, study duration, and type of DNA damage assessed), their findings support the notion of acclimation to berry consumption over time.
Other investigators have demonstrated the effects of both acute and chronic consumption of blueberries on DNA damage in humans. For instance, in a randomized crossover study, Del Bo’ et al.28 showed that ex vivo hydrogen peroxide (H2O2)-induced DNA damage in peripheral blood mononuclear cells (PBMCs) was significantly reduced 1 h after the consumption of 300 g of fresh highbush blueberries when compared to the control group but returned to baseline levels after 2 h. However, no significant differences in endogenously produced DNA damage or vascular function were noted. Similarly, McAnulty et al.29 found that consumption of 250 g of fresh highbush blueberries daily for 6 weeks plus an acute dose of 375 g of fresh blueberries prior to exercise did not reduce urinary 8-OHdG levels 1 h post-exercise (2.5 h of running at approximately 72% maximal oxygen consumption) in well-trained subjects. In a randomized, repeated-measures crossover study with male subjects at risk for cardiovascular disease, Riso et al.30 reported significant reductions in endogenously oxidized DNA bases and ex vivo H2O2-induced DNA damage in PBMCs after consumption of 25 g of freeze-dried lowbush blueberry powder for 6 weeks but did not observe any significant differences in blood pressure or nitric oxide levels over time or between groups. Because the longest intervention duration in these studies was 6 weeks, and we did not measure DNA damage at this time point, it is difficult to compare our findings to those of the aforementioned studies. Additionally, research design, types of blueberries, and methods used to determine DNA damage, e.g. plasma 8-OHdG vs. endogenously oxidized DNA bases and ex vivo H2O2-induced DNA damage in PBMCs, differ among the studies and thus may be partially responsible for the discrepancies in findings.
While the mechanisms responsible for the observed protection against oxidative DNA damage at 4 weeks cannot be determined at this time, there are several possible explanations. First, bioactive compounds present in blueberries and/or their metabolites may have either activated endogenous antioxidant signaling pathways resulting in increased expression of antioxidant genes and proteins or served as antioxidants themselves. Previous preclinical research has demonstrated the ability of blueberries to do both.24,31 However, we are not able to determine this from the current study as changes in blood biomarkers observed in this study may not be reflective of cellular antioxidant activity and therefore may not provide an accurate assessment of antioxidant defense. Additionally, other antioxidants such as vitamin C, enzymes such as catalase, and factors regulating antioxidant systems such as nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element transcriptional pathway, as well as endogenous oxidant producing enzymes such as NADPH oxidases were not assessed in this study. In fact, our previously reported findings22 that circulating nitric oxide metabolites were significantly increased after 8 weeks of blueberry treatment with a trend (P = 0.09) for a treatment effect is suggestive of improved nitric oxide bioavailability and endothelial function. Reduced nitric oxide bioavailability is the hallmark of endothelial dysfunction and is largely caused by superoxide-driven oxidative stress due to excessive superoxide anion production by NADPH oxidase, mitochondrial dysfunction, and/or endothelial nitric oxide synthase uncoupling.32 If NADPH oxidase activity was attenuated by blueberry polyphenol metabolites as has been previously demonstrated,18 it is plausible that there would be less superoxide anions to react with nitric oxide and therefore less peroxynitrite which is known to induce oxidative DNA damage.33 Lastly, certain bioactive compounds such as flavonoids have been shown to increase the expression of DNA repair genes.34
The results of the remaining blood biomarkers of oxidative stress and inflammation measured in our study do not demonstrate a distinct response to blueberry treatment. Although certain parameters were significantly improved over the course of the study, namely TNF-α, SOD, GR, GPx, OxLDL, and 8-isoprostane, most of these improvements were observed in both groups and treatment effects were not observed. However, with the exception of TNF-α in which groups were different from each other at all time points, the changes that occurred were strictly time effects. It is currently unknown as to why certain parameters changed in both groups over time; however, it is suggested that generating conclusions based on time effects may be highly misleading as changes in parameters from baseline provides evidence that there is a change over time but not that this is a result of the treatment.35 Nonetheless, we cannot rule out the possibility that a placebo effect, a nutritional component present in both blueberry and placebo powders, and/or lifestyle alterations such as diet, contributed to these changes. In addition, it is possible that some of these parameters were not influenced by the treatment as they were not abnormal to begin with. To our knowledge, established reference values or published findings demonstrating possible reference values of these specific measures in this population do not currently exist for most of the biomarkers measured. An exception to this is CRP, which has been demonstrated to be predictive of future cardiovascular events in postmenopausal women.36
Existing clinical studies demonstrating the impact of blueberry consumption on biomarkers of oxidative stress, inflammation, and antioxidant defense are limited but have reported findings similar to ours. In the study by Riso et al.30 in which men consumed 25 g of freeze-dried wild blueberry powder for 6 weeks, no effects on glutathione, SOD, TNF-α, or CRP were noted although they did find significant reductions in DNA damage. McAnulty et al.29 did not find any significant changes in plasma 8-isoprostane levels after three weeks of 250 g blueberry consuming by adult smokers. Stull et al.17 reported no effect on TNF-α or CRP in obese and insulin resistant men and women who consumed 45 g of freeze-dried highbush blueberry powder per day for 6 weeks.
The present study has several possible limitations. First, only one dose of blueberry powder was assessed thereby precluding the assessment of a possible dose–response. Second, because the sample size and power were calculated based on changes in blood pressure, it is possible that the study was not adequately powered to detect differences in other parameters of interest. Third, compliance was self-reported and were not confirmed via other means (e.g. metabolomics analysis). Additionally, although study participants agreed not to change their diets or physical activity patterns for the duration of the study, diet and physical activity were not assessed. Hence, any changes in diet and physical activity or the ingestion of foods and dietary supplements that may have influenced the results of the study (e.g. consumption of polyphenol-rich foods) are unknown. Fourth, blood biomarkers may not be reflective of what is occurring at the cellular and tissue levels and therefore complicates the interpretation of our findings. For instance, improvements in blood pressure and vascular function (i.e. arterial stiffness and endothelial function) are likely primarily mediated by changes at the cellular level in the vascular endothelium and circulating biomarkers may not reflect this as they are systemic, non-specific measures and can be generated by a number of cells.37 In addition, the blood biomarkers measured in the current study are static biomarkers which may not always respond to a dietary intervention in the same way as functional biomarkers, e.g. ex vivo cytokine release assays in PBMCs, as has been previously shown with strawberries.38,39 Future clinical studies should be designed to assess these parameters at the cellular and tissue levels whenever possible and should aim to include functional measurements.
Additionally, whether plasma 8-OHdG is the best biomarker of oxidative DNA damage is still up for debate. It remains unknown as to which tissues or body fluids should be used to measure oxidative DNA damage, and which methods of detection should be employed. It has been suggested that comprehensive assessment of oxidative DNA damage through measurement of steady state (e.g. in PBMCs, plasma 8-OHdG) coupled with total damage (e.g. urinary 8-OHdG) is the ideal approach. However, when only one type of measurement can be made, it was recommended that steady state 8-OHdG be measured.40 Additionally, recent research suggests that plasma 8-OHdG is more sensitive than urinary 8-OHdG; however, measurement using liquid chromatography tandem mass spectrometry (LC-MS/MS) is more sensitive than ELISA and other methods.41 More work in this important area is needed. Fifth, although the freeze-dried blueberry and placebo powders were almost identical in terms of calories, fat, carbohydrates, and protein, there were other differences in their composition (e.g. dietary fiber, vitamins, and minerals). Hence, it is unknown whether it was the bioactive compounds present in blueberries, the dietary fiber or other nutrients, or the combination that contributed to the beneficial health effects observed. For instance, it has been reported that a higher intake of dietary fiber is associated with a lower blood pressure independent of other nutrients found in fiber-rich foods.42 Future studies should employ metabolomics analyses in order to gain insight into which bioactive compounds in blueberries are responsible for these effects. Finally, the study population was a specific population of postmenopausal women with pre- and stage 1-hypertension and therefore the current findings are not generalizable to other populations.
The results of this study demonstrate that consuming 22 g of freeze-dried blueberry powder, equivalent to approximately 1 cup of fresh blueberries, daily for 4 weeks reduced plasma 8-OHdG levels, a marker of oxidative DNA damage, while our previous findings22 showed that 8 weeks of blueberry consumption led to meaningful reductions in blood pressure and arterial stiffness in pre- and stage 1-hypertensive postmenopausal women. However, the current study also demonstrated that blueberries did not exert any significant effects on oxidative DNA damage at 8 weeks or on the other measured blood biomarkers of oxidative stress, inflammation, and antioxidant defense at any time point. The reasons for the time point discrepancies in the beneficial effects of blueberries on DNA damage and blood pressure is unknown at this time. Importantly, the body of literature suggests that a blueberry intervention of at least 8 weeks is needed to produce significant reductions in blood pressure. Our previously reported findings are suggestive of improvements in vascular function, including arterial stiffness, which may have been a major factor contributing to the observed reductions in blood pressure. Indeed, reductions in arterial stiffness are largely driven by improvements in the structural properties of the arterial wall which requires a longer duration of treatment to observe improvements than many biochemical markers.43 As previously mentioned, evidence suggests a possible acclimation to treatment with berries in terms of DNA damage and may be a contributing factor. Although the duration of protection against oxidative DNA damage was limited (i.e. 4 weeks), due to its mutagenic nature as well as the fact that these genetic mutations accumulate over time,44,45 it is likely that any reduction in oxidative DNA damage levels and exposure would provide some protection. This is important given that oxidative DNA damage and resulting genetic mutations contribute to carcinogenic processes in the kidneys46 and the initiation and the development of a number of cancer types47 as well as the development of other chronic diseases such as atherosclerosis.48
5. Conclusion
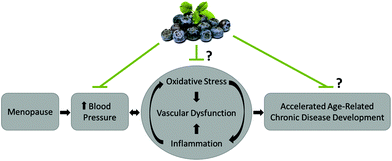
This study demonstrates that daily consumption of blueberries for 4 weeks, but not 8 weeks, provided modest protection against oxidative DNA damage in a high-risk population, while all other measured parameters were not affected by blueberry consumption. Future research with blueberries should investigate ways to increase the duration of protection against oxidative DNA damage, e.g. dose–response, escalating dosing, and intermittent dosing intervention studies. Additionally, future research is needed to confirm the current findings with respect to oxidative DNA damage using additional methodologies for assessing oxidative DNA damage and repair, as well as direct cellular and functional measures of systemic oxidative stress, inflammation, and antioxidant defense in humans. Finally, our research in this population demonstrates that blueberries improve blood pressure and arterial stiffness. Future clinical studies are needed to directly evaluate the effects of blueberries on vascular endothelial function to confirm that blueberries improve vascular function, vascular oxidative stress and inflammation, and their role in preventing the development of age-related chronic disease in this population (Fig. 1).Acknowledgements
This study was supported, in part, by the US Highbush Blueberry Council. Partial results of this study were presented at the Scientific Sessions and Annual Meeting of the American Society for Nutrition at Experimental Biology, April 26–27, 2013, San Diego, CA and March 31, 2015, Boston, MA. The authors thank Lauren T. Ormsbee, Roy Kalfon, Alexei Wong, and Yitong Zhao for their contributions to data collection, as well as Cathy W. Levenson for providing her expertise in measuring DNA damage. We also thank the study participants for their involvement.References
- E. Grossman, F. H. Messerli, V. Boyko and U. Goldbourt, Is there an association between hypertension and cancer mortality?, Am. J. Med., 2002, 112, 479–486 CrossRef PubMed.
- D. Mozaffarian, E. J. Benjamin, A. S. Go, D. K. Arnett, M. J. Blaha, M. Cushman, S. de Ferranti, J. P. Despres, H. J. Fullerton, V. J. Howard, M. D. Huffman, S. E. Judd, B. M. Kissela, D. T. Lackland, J. H. Lichtman, L. D. Lisabeth, S. Liu, R. H. Mackey, D. B. Matchar, D. K. McGuire, E. R. Mohler 3rd, C. S. Moy, P. Muntner, M. E. Mussolino, K. Nasir, R. W. Neumar, G. Nichol, L. Palaniappan, D. K. Pandey, M. J. Reeves, C. J. Rodriguez, P. D. Sorlie, J. Stein, A. Towfighi, T. N. Turan, S. S. Virani, J. Z. Willey, D. Woo, R. W. Yeh and M. B. Turner, C. American Heart Association Statistics and S. Stroke Statistics, Heart disease and stroke statistics–2015 update: a report from the American Heart Association, Circulation, 2015, 131, e29–322 CrossRef PubMed.
- N. D. Vaziri and B. Rodriguez-Iturbe, Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension, Nat. Clin. Pract. Nephrol., 2006, 2, 582–593 CAS.
- L. L. Yanes and J. F. Reckelhoff, Postmenopausal hypertension, Am. J. Hypertens., 2011, 24, 740–749 CrossRef PubMed.
- T. Finkel and N. J. Holbrook, Oxidants, oxidative stress and the biology of ageing, Nature, 2000, 408, 239–247 CrossRef CAS PubMed.
- H. Y. Chung, M. Cesari, S. Anton, E. Marzetti, S. Giovannini, A. Y. Seo, C. Carter, B. P. Yu and C. Leeuwenburgh, Molecular inflammation: underpinnings of aging and age-related diseases, Ageing Res. Rev., 2009, 8, 18–30 CrossRef CAS PubMed.
- P. Libby, Inflammatory mechanisms: the molecular basis of inflammation and disease, Nutr. Rev., 2007, 65, S140–S146 CrossRef PubMed.
- R. H. Liu, Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals, Am. J. Clin. Nutr., 2003, 78, 517S–520S CAS.
- P. M. Kris-Etherton, K. D. Hecker, A. Bonanome, S. M. Coval, A. E. Binkoski, K. F. Hilpert, A. E. Griel and T. D. Etherton, Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer, Am. J. Med., 2002, 113(Suppl 9B), 71S–88S CrossRef CAS PubMed.
- C. C. Neto, Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases, Mol. Nutr. Food Res., 2007, 51, 652–664 CAS.
- A. Rodriguez-Mateos, T. Cifuentes-Gomez, S. Tabatabaee, C. Lecras and J. P. Spencer, Procyanidin, anthocyanin, and chlorogenic acid contents of highbush and lowbush blueberries, J. Agric. Food Chem., 2012, 60, 5772–5778 CrossRef CAS PubMed.
- A. Rodriguez-Mateos, R. Del Pino-Garcia, T. W. George, A. Vidal-Diez, C. Heiss and J. P. Spencer, Impact of processing on the bioavailability and vascular effects of blueberry (poly)phenols, Mol. Nutr. Food Res., 2014, 58, 1952–1961 CAS.
- C. Del Bo, D. Martini, M. Porrini, D. Klimis-Zacas and P. Riso, Berries and oxidative stress markers: an overview of human intervention studies, Food Funct., 2015, 6, 2890–2917 CAS.
- S. A. Johnson and B. H. Arjmandi, Evidence for anti-cancer properties of blueberries: a mini-review, Anticancer Agents Med. Chem., 2013, 13, 1142–1148 CrossRef CAS PubMed.
- A. Basu, M. Du, M. J. Leyva, K. Sanchez, N. M. Betts, M. Wu, C. E. Aston and T. J. Lyons, Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome, J. Nutr., 2010, 140, 1582–1587 CrossRef CAS PubMed.
- A. J. Stull, K. C. Cash, C. M. Champagne, A. K. Gupta, R. Boston, R. A. Beyl, W. D. Johnson and W. T. Cefalu, Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial, Nutrients, 2015, 7, 4107–4123 CrossRef CAS PubMed.
- A. J. Stull, K. C. Cash, W. D. Johnson, C. M. Champagne and W. T. Cefalu, Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women, J. Nutr., 2010, 140, 1764–1768 CrossRef CAS PubMed.
- A. Rodriguez-Mateos, C. Rendeiro, T. Bergillos-Meca, S. Tabatabaee, T. W. George, C. Heiss and J. P. Spencer, Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity, Am. J. Clin. Nutr., 2013, 98, 1179–1191 CrossRef CAS PubMed.
- L. S. McAnulty, S. R. Collier, M. J. Landram, D. S. Whittaker, S. E. Isaacs, J. M. Klemka, S. L. Cheek, J. C. Arms and S. R. McAnulty, Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females, Nutr. Res., 2014, 34, 577–584 CrossRef CAS PubMed.
- A. Rodriguez-Mateos, C. Heiss, G. Borges and A. Crozier, Berry (poly)phenols and cardiovascular health, J. Agric. Food Chem., 2014, 62, 3842–3851 CrossRef CAS PubMed.
- A. Basu, M. Rhone and T. J. Lyons, Berries: emerging impact on cardiovascular health, Nutr. Rev., 2010, 68, 168–177 CrossRef PubMed.
- S. A. Johnson, A. Figueroa, N. Navaei, A. Wong, R. Kalfon, L. T. Ormsbee, R. G. Feresin, M. L. Elam, S. Hooshmand, M. E. Payton and B. H. Arjmandi, Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: a randomized, double-blind, placebo-controlled clinical trial, J. Acad. Nutr. Diet., 2015, 115, 369–377 CrossRef PubMed.
- M. A. Sanchez-Rodriguez, M. Zacarias-Flores, A. Arronte-Rosales, E. Correa-Munoz and V. M. Mendoza-Nunez, Menopause as risk factor for oxidative stress, Menopause, 2012, 19, 361–367 CrossRef PubMed.
- X. Wu, J. Kang, C. Xie, R. Burris, M. E. Ferguson, T. M. Badger and S. Nagarajan, Dietary blueberries attenuate atherosclerosis in apolipoprotein E-deficient mice by upregulating antioxidant enzyme expression, J. Nutr., 2010, 140, 1628–1632 CrossRef CAS PubMed.
- C. M. Elks, S. D. Reed, N. Mariappan, B. Shukitt-Hale, J. A. Joseph, D. K. Ingram and J. Francis, A blueberry-enriched diet attenuates nephropathy in a rat model of hypertension via reduction in oxidative stress, PLoS One, 2011, 6, e24028 CAS.
- A. R. Nair, C. M. Elks, J. Vila, F. Del Piero, D. B. Paulsen and J. Francis, A blueberry-enriched diet improves renal function and reduces oxidative stress in metabolic syndrome animals: potential mechanism of TLR4-MAPK signaling pathway, PLoS One, 2014, 9, e111976 Search PubMed.
- H. L. Nicastro, B. A. Clevidence, C. S. Charron, S. K. Gebauer, H. D. Dawson, M. S. Cooke, V. Mistry, R. Singh, J. A. Milner and J. A. Novotny, Blackberries decrease DNA damage after 3 h, but not after 6 d, in healthy adult volunteers, FASEB J., 2013, 27, 864.4 Search PubMed.
- C. Del Bo, P. Riso, J. Campolo, P. Moller, S. Loft, D. Klimis-Zacas, A. Brambilla, A. Rizzolo and M. Porrini, A single portion of blueberry (Vaccinium corymbosum L) improves protection against DNA damage but not vascular function in healthy male volunteers, Nutr. Res., 2013, 33, 220–227 CrossRef CAS PubMed.
- L. S. McAnulty, D. C. Nieman, C. L. Dumke, L. A. Shooter, D. A. Henson, A. C. Utter, G. Milne and S. R. McAnulty, Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running, Appl. Physiol., Nutr., Metab., 2011, 36, 976–984 CrossRef CAS PubMed.
- P. Riso, D. Klimis-Zacas, C. Del Bo, D. Martini, J. Campolo, S. Vendrame, P. Moller, S. Loft, R. De Maria and M. Porrini, Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors, Eur. J. Nutr., 2013, 52, 949–961 CrossRef CAS PubMed.
- S. G. Lee, B. Kim, Y. Yang, T. X. Pham, Y. K. Park, J. Manatou, S. I. Koo, O. K. Chun and J. Y. Lee, Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-kappaB independent of NRF2-mediated mechanism, J. Nutr. Biochem., 2014, 25, 404–411 CrossRef CAS PubMed.
- D. R. Seals, R. E. Kaplon, R. A. Gioscia-Ryan and T. J. LaRocca, You're only as old as your arteries: translational strategies for preserving vascular endothelial function with aging, Physiology, 2014, 29, 250–264 CrossRef CAS PubMed.
- J. C. Niles, J. S. Wishnok and S. R. Tannenbaum, Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: structures and mechanisms of product formation, Nitric Oxide, 2006, 14, 109–121 CrossRef CAS PubMed.
- S. Guarrera, C. Sacerdote, L. Fiorini, R. Marsala, S. Polidoro, S. Gamberini, F. Saletta, C. Malaveille, G. Talaska, P. Vineis and G. Matullo, Expression of DNA repair and metabolic genes in response to a flavonoid-rich diet, Br. J. Nutr., 2007, 98, 525–533 CrossRef CAS PubMed.
- J. M. Bland and D. G. Altman, Best (but oft forgotten) practices: testing for treatment effects in randomized trials by separate analyses of changes from baseline in each group is a misleading approach, Am. J. Clin. Nutr., 2015, 102, 991–994 CrossRef CAS PubMed.
- P. M. Ridker, C. H. Hennekens, J. E. Buring and N. Rifai, C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women, N. Engl. J. Med., 2000, 342, 836–843 CrossRef CAS PubMed.
- D. Burger and R. M. Touyz, Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cells, J. Am. Soc. Hypertens., 2012, 6, 85–99 CrossRef CAS PubMed.
- S. J. Zunino, M. A. Parelman, T. L. Freytag, C. B. Stephensen, D. S. Kelley, B. E. Mackey, L. R. Woodhouse and E. L. Bonnel, Effects of dietary strawberry powder on blood lipids and inflammatory markers in obese human subjects, Br. J. Nutr., 2012, 108, 900–909 CrossRef CAS PubMed.
- S. J. Zunino, D. H. Storms, T. L. Freytag, B. E. Mackey, L. Zhao, J. S. Gouffon and D. H. Hwang, Dietary strawberries increase the proliferative response of CD3/CD28-activated CD8(+) T cells and the production of TNF-alpha in lipopolysaccharide-stimulated monocytes from obese human subjects, Br. J. Nutr., 2013, 110, 2011–2019 CrossRef CAS PubMed.
- B. Halliwell, Why and how should we measure oxidative DNA damage in nutritional studies? How far have we come?, Am. J. Clin. Nutr., 2000, 72, 1082–1087 CAS.
- C. C. Wang, W. L. Chen, C. M. Lin, C. H. Lai, C. H. Loh, H. I. Chen and S. H. Liou, The relationship between plasma and urinary 8-hydroxy-2-deoxyguanosine biomarkers measured by liquid chromatography tandem mass spectrometry, Environ. Sci. Pollut. Res. Int., 2016, 23, 17496–17502 CrossRef CAS PubMed.
- G. S. Aljuraiban, L. M. Griep, Q. Chan, M. L. Daviglus, J. Stamler, L. Van Horn, P. Elliott and G. S. Frost, Total, insoluble and soluble dietary fibre intake in relation to blood pressure: the INTERMAP Study, Br. J. Nutr., 2015, 114, 1480–1486 CrossRef CAS PubMed.
- H. Tanaka and M. E. Safar, Influence of lifestyle modification on arterial stiffness and wave reflections, Am. J. Hypertens., 2005, 18, 137–144 CrossRef PubMed.
- B. N. Ames, M. K. Shigenaga and L. S. Gold, DNA lesions, inducible DNA repair, and cell division: three key factors in mutagenesis and carcinogenesis, Environ. Health Perspect., 1993, 101(Suppl 5), 35–44 CrossRef CAS PubMed.
- D. Ziech, R. Franco, A. Pappa and M. I. Panayiotidis, Reactive oxygen species (ROS)–induced genetic and epigenetic alterations in human carcinogenesis, Mutat. Res., 2011, 711, 167–173 CrossRef CAS PubMed.
- U. Schmid, H. Stopper, F. Schweda, N. Queisser and N. Schupp, Angiotensin II induces DNA damage in the kidney, Cancer Res., 2008, 68, 9239–9246 CrossRef CAS PubMed.
- L. C. Wilms, P. C. Hollman, A. W. Boots and J. C. Kleinjans, Protection by quercetin and quercetin-rich fruit juice against induction of oxidative DNA damage and formation of BPDE-DNA adducts in human lymphocytes, Mutat. Res., 2005, 582, 155–162 CAS.
- J. C. Wang and M. Bennett, Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence, Circ. Res., 2012, 111, 245–259 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |