Impact of multiwall carbon nanotubes on the accumulation and distribution of carbamazepine in collard greens (Brassica oleracea)†
Yingqing
Deng
a,
Brian
Eitzer
b,
Jason C.
White
*b and
Baoshan
Xing
*a
aStockbridge School of Agriculture, University of Massachusetts Amherst, Amherst, Massachusetts 01003, USA. E-mail: bx@umass.edu
bDepartment of Analytical Chemistry, The Connecticut Agricultural Experiment Station, 123 Huntington Street, New Haven, Connecticut 06511, USA. E-mail: Jason.White@ct.gov
First published on 30th November 2016
Abstract
Pre-existing pharmaceutical residues in agricultural soils may encounter engineered nanomaterials, resulting in poorly understood co-contamination interactions. In this study, the bioaccumulation and translocation of the pharmaceutical residue carbamazepine (100 μg L−1) in collard greens (Brassica oleracea) was evaluated upon concurrent exposure to pristine or carboxyl-functionalized multiwall carbon nanotubes (pCNTs or cCNTs) at 50 mg L−1 under hydroponic exposure and at 500 mg kg−1 in soil-grown conditions. B. oleracea toxicity was more evident under hydroponic exposure, with growth inhibtion dependent on carbamazepine concentrations; however, biomass enhancement was noted in cCNTs-treated plants. Without CNTs, B. oleracea accumulated and translocated significant amounts of carbamazepine; up to 2500 μg kg−1 in the leaves and 300 μg kg−1 in the roots, depending on growth condition. The co-exposure of carbon materials notably suppressed carbamazepine accumulation in both hydroponic and soil systems. Specifically, root carbamazepine content in soil-grown plants was suppressed 29%, 53% and 89% by pCNTs, cCNTs and AC, respectively. Generally, the adsorption capacity of the carbon materials correlated well with the suppression of carbamazepine accumulation. The results also suggest that functionalization of CNTs enhanced carbamazepine translocation potential and significantly affected nanomaterial/co-contaminant interactions as compared to its pristine analog. These findings show that the CNTs in the environment may significantly affect the bioavailability and translocation pattern of coexisting organic contaminants.
Environmental significanceThere is limited information on nanomaterial interactions with co-contaminants, including the subsequent impact on biological uptake of these materials. We evaluated the impact of multiwall carbon nanotube (CNT) co-exposure on the fate of the pharmaceutical carbamazepine in a model plant. The work involved two exposure conditions, employed activated carbon as a control, and evaluated the role of functionalization on carbamazepine fate within the plant. The plants accumulated significant amounts of carbamazepine; up to 2500 μg kg−1 in the leaves and 300 μg kg−1 in the roots. CNT co-exposure suppressed carbamazepine accumulation; the adsorption capacity of the carbon correlated well with the suppression of pharmaceutical accumulation. CNT functionalization enhanced carbamazepine translocation and significantly affected nanomaterial/co-contaminant interactions as compared to its pristine analog. |
Introduction
Engineered nanomaterials (ENMs) are incorporated into a wide variety of commercial products in the sectors as diverse as information technology, biological and environmental science, energy sources, material science, medicine and others.1 As one of the top 10 nanomaterials in global production, multiwall carbon nanotubes (CNTs) have attracted great interest because of their extraordinary characteristics, including unique electronic properties, high thermal conductivity and exceptional stiffness, strength and resilience.2,3 However, given the widespread and increasing use, significant concerns have been raised over the release of various ENMs into the environment.4–8 In addition, there is general consensus in the scientific community that nanomaterial fate and effects in the environment is poorly understood. Without addressing these critical knowledge gaps, the sustainable use of ENMs and the ability to accurately characterize associated risk will remain elusive.In recent years, concern over the ecological risk of nanomaterials has expanded from direct effects on biota to the impact on co-existing contaminant fate and effects. Adsorption studies have shown that CNTs affect the fate and transport of sorbed organic contaminants in the environment, which may subsequently alter bioavailability and toxicity.9 For example, single-wall CNTs exhibited high adsorption capacity for phenanthrene and reduced toxicity of the residue to algae (P. subcapitata); however, the presence of four other multiwall CNTs had no effect on phenanthrene toxicity.10 In another study with diuron, the presence of CNTs reduced the adverse effects of the herbicide on green algae Chlorella vulgaris.11 In addition, previous studies have revealed that CNTs may penetrate cell walls and membranes, raising the possibility of these materials serving as molecular transporters and consequently affecting contaminant bioaccumulation.12,13 Although concerns over CNT impacts on co-contaminant fate in terrestrial systems are significant, relatively little work has been done in this area. Petersen et al. reported decreased pyrene accumulation (25–50%) by earthworms (Eisenia foetida) in two soils amended with 3000 mg kg−1 CNTs.14 Wild et al. reported that in wheat roots exposed to multiwall CNTs, root cap cells pierced by the nanomaterial accumulated phenanthrene approximately 50% faster than roots not exposed to CNTs.15 In another study with four crop species, multiwall CNTs were found to decrease the accumulation of the weathered pesticides chlordane and DDx (DDT + metabolites) from soil in a dose-dependent fashion.16 It is clear that the literature on nanomaterial/co-contaminant interactions is both limited and contradictory; without a further understanding of these processes through significant additional investigations, an accurate assessment of nanomaterial risk in terrestrial systems will not be possible.
The current study focused on co-contamination between carbon nanotubes and a pharmaceutical compound in a model agricultural soil. Carbamazepine was chosen as a representative pharmaceutical residue because of its frequent detection and persistence in water bodies.17 Carbamazepine was detected in WWTP effluents up to 0.95 μg L−1 and in biosolids at 281.2 μg kg−1.18–20 It has also been reported that carbamazepine would irreversibly bind to soil and therefore exhibit low leaching potential.21,22 In addition, carbamazepine has a moderate octanol–water partitioning coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow 2.45) and will remain non-ionic under soil conditions (pKa 13.4); as such, the residue has a significant potential to accumulate in plants through biosolids application or reclamation of treated water.23,24 Here, collard greens (B. oleracea) were exposed to carbamazepine in the presence of CNTs under both hydroponic and soil-grown conditions. To assess the effect of carbamazepine–CNTs interactions on B. oleracea, biomass was monitored under all conditions and carbamazepine bioaccumulation was determined through liquid chromatography with high-resolution mass spectrometry (LC-HRMS). The adsorption of carbamazepine onto CNTs was also evaluated under abiotic conditions. The results of this study will further our understanding of the interactions between nanomaterials and organic contaminants in agricultural soils and provide critical information concerning risk from the presence of nanomaterials in the environment.
Kow 2.45) and will remain non-ionic under soil conditions (pKa 13.4); as such, the residue has a significant potential to accumulate in plants through biosolids application or reclamation of treated water.23,24 Here, collard greens (B. oleracea) were exposed to carbamazepine in the presence of CNTs under both hydroponic and soil-grown conditions. To assess the effect of carbamazepine–CNTs interactions on B. oleracea, biomass was monitored under all conditions and carbamazepine bioaccumulation was determined through liquid chromatography with high-resolution mass spectrometry (LC-HRMS). The adsorption of carbamazepine onto CNTs was also evaluated under abiotic conditions. The results of this study will further our understanding of the interactions between nanomaterials and organic contaminants in agricultural soils and provide critical information concerning risk from the presence of nanomaterials in the environment.
Experimental section
Chemicals and plants
Pristine carbon nanotubes (pCNTs; 95% purity, <8 nm o.d., 2–5 nm i.d.; 10–30 μm length, specific surface area/SSA 500 m2 g−1) and carboxyl-functionalized carbon nanotubes (cCNTs; 95% purity, <8 nm o.d., 2–5 nm i.d.; 10–30 μm length, SSA 500 m2 g−1, 3.8% COOH groups) were purchased from Cheaptubes (Brattleboro, VT). A Zetasizer (90Plus, Brookhaven, Holtsville, NY) was employed to characterize the CNT suspensions for hydrodynamic diameter and zeta potential. The two CNTs were dispersed in 1/2 strength Hoagland solution at 50 mg L−1 and were sonicated with a probe sonicator (Misonix S-4000, Farmingdale, NY) for 30 minutes before analysis. Not surprisingly, significant aggregation occurred in solution. The average particle diameter and surface charge of pCNTs in the solution were 3380 nm and −6.8 mV (pH = 6.5), respectively; cCNTs had a hydrodynamic size of 1600 nm and was more negatively charged with a zeta potential of −25.9 mV. The homogeneous dispersion of cCNTs was facilitated with electrostatic repulsion; conversely, pCNTs in solution were less stable, with an observable settling of undispersed CNTs after 24 h. A commercial activated carbon (AC) from Fisher (Norit, neutral, decolorizing, SSA 1380 m2 g−1 (ref. 25)) was used as a control for carbon nanomaterials. Carbamazepine (purity 99%) was purchased from Acros Organics (NJ, USA). A standard of isotopically labelled carbamazepine (D10, 98%) was purchased from Cambridge Isotope Laboratories (Tewksbury, MA). Collard greens (Brassica oleracea, “Georgia”) seeds were obtained from Burpee Garden Products Co (Warminster, PA). Seeds were germinated in vermiculite for 5 days before transplanting.Selection of fixed exposure concentrations
To limit the experimental matrix in the co-exposure assay, nominal CNTs concentrations of 50 mg L−1 in the hydroponic experiments and 50 mg kg−1 in the soil experiments were selected in accordance with concentrations commonly used in previous exposure studies.26–28 To determine the optimum carbamazepine concentration, B. oleracea were cultivated in nutrient solution containing carbamazepine at 0.1, 0.5, 1.0, 5.0 or 10 mg L−1, with or without 50 mg L−1 pCNTs. With increasing carbamazepine concentrations over 1 mg L−1, B. oleracea exhibited overt phytotoxicity, including sporadic necrosis, mottling and chlorosis at leaf margins; notably, these effects were more severe without CNTs co-exposure. To avoid phytotoxicity, carbamazepine was therefore prepared at 100 μg L−1 in nutrient solution or irrigation water for the exposure assays. This concentration is also environmentally relevant18–20 and within the range of 1.0–232.5 μg L−1 used in other carbamazepine investigations.29–31Co-exposure assay in hydroponics
The hydroponic experiments were conducted in a greenhouse, with controlled temperature regime (24 °C day/20 °C night), and 4 h supplemental lighting (PAR source, 400 μmols m−2 s−1). Glass jars (120 mL) were wrapped with aluminum foil and connected with a commercial air pump (EcoPlus) through equal multi-outlets. The aerator constantly provided oxygen to roots and maintained the CNTs suspension. The plant growth medium was a modified Hoagland solution with the pH adjusted to 6.5. CNTs and activated carbon were added and dispersed with the aid of ultrasonic probe sonication at 75 W for 30 min (Misonix, Farmingdale, NY) shortly prior to exposure. Uniform seedlings were transplanted into the hydroponic jars containing the following solutions: 1) negative control (no carbamazepine, no CNTs); 2) carbamazepine alone; 3) cCNTs alone; 4) pCNTs alone; 5) carbamazepine + cCNT; 6) carbamazepine + pCNTs; 7) carbamazepine + AC. One jar was planted with one seedling and there were 7 replicates in each treatment. The growth media with/without contaminants was completely replaced every 7 d. The B. oleracea were exposed for 28 d before harvest. Separately, the quantification of carbamazepine adsorption on CNTs in Hoagland's solution was determined at conditions approximating the above-described exposure experiments; additional experimental details can be found in the ESI.†Co-exposure assay in soil
A Hadley sandy loam soil (50% sand, 45% silt, 5% clay; 4.8% organic matter; pH 5.9; cation exchange capacity 11.4 cmol kg−1) was collected from the University of Massachusetts Agricultural Experiment Station Farm. The two CNTs or activated carbon were separately added to the soil at 500 mg kg−1 in forms of dry powder and the jars (120 mL) were shaken vigorously overnight to maximize homogeneous mixing. Carbamazepine at 100 μg L−1 was applied through the irrigation water; all replicate jars received equal volumes so as to ensure an identical dose. Throughout the exposure, 600 mL carbamazepine-amended water was used for irrigation, equating to a final concentration of 1.2 mg carbamazepine per kg soil. There was one seedling per jar and 7 replicates per treatment. Similar to the hydroponic experiment, the treatments were 1) negative control; 2) carbamazepine alone; 3) cCNTs alone; 4) pCNTs alone; 5) carbamazepine + cCNT; 6) carbamazepine + pCNTs; 7) carbamazepine + AC. Collards in soil grew more slowly than in the hydroponic solution and required 42 d to reach approximately the same size as plants grown for 28 d under hydroponic conditions.Harvest and vegetation extraction
At harvest, small amounts of leaf tissue were reserved for chlorophyll and anthocyanin content determination. Leaves and roots were rinsed and separated before extraction. The extraction method was modified from previously reported QuECHeRs protocol.32,33 Briefly, approximately 15 g fresh tissue was homogenized and transferred into a 50 mL centrifuge tube, spiked with 100 ng D10 carbamazepine as an internal standard and extracted with 15 mL acetonitrile with the aid of a wrist action shaker (Burrell Scientific) for 30 min. The extract was then separated from aqueous phase and solids through centrifuge (Thermo Centra GP6) at 3000 rpm and further purified with 1.5 g anhydrous magnesium sulfate (1.5 g), 0.5 g primary and secondary amine (PSA) bonded silica and 2.0 mL toluene. After cleanup, the extract was subsequently concentrated to 1 mL through TurboVap® II concentration workstation (Biotage).An additional experiment was conducted in which the regular rinsing was replaced with surfactant washing assisted with sonication (sodium dodecyl benzene sulphonate, SDBS, 0.5%, 30 min). The sonication was performed identically to the previously described method. SDBS was chosen from preliminary tests and due to its reported high ability to suspend CNTs.34 Individual roots were divided in half and were treated separately with regular washing and the surfactant/sonication procedure. The root samples after the two different washing procedures were further extracted by the QuEChERS procedure and were analyzed as described below.
Chemical analysis
A 100 mg L−1 stock of carbamazepine in toluene was diluted to prepare calibration standards of 10–1000 μg L−1. Each standard was amended with 250 μg L−1 D10-labelled carbamazepine as an internal standard. Liquid samples and plant tissues extracts were analyzed through an Agilent (Santa Clara, CA) 1200 Rapid Resolution liquid chromatograph system coupled to a Thermo (West Palm Beach, FL) Orbitrap High-Resolution mass spectrometer (Exactive) with an electrospray interface used in positive ionization. Chromatographic separation was achieved with a Zorbax SB-C18 Rapid Resolution HT column using a 3 μL injection and a flow rate of 0.25 mL min−1. After a 1 min hold at 1% methanol in water, there was a 5 min gradient to 95% methanol in water followed by a 3 minute hold. Carbamazepine was quantified using a 4 ppm window around the m/z = 237.1022 (M + H)+ ion and confirmed with a m/z = 194.0963 fragment ion. The D10 internal standard was monitored using the m/z = 247.1650 (M + H)+ ion. Both the labeled and unlabeled carbamazepine had an elution time of 6.56 min.Statistical analysis
The data are presented as mean ± the standard deviation (SD). Where appropriate, a one-way ANOVA (biomass data) or two-way ANOVA (uptake data) followed by Tukey's multiple comparison test was used to assess statistical significance. Comparisons were considered significantly different at p < 0.05.Results and discussion
Growth response of B. oleracea
The growth of B. oleracea was monitored during hydroponic and soil-based growth under a range of CNT and carbamazepine exposures. Chlorophyll (a, b and total), flavonoid and anthocyanin content were analyzed and were found to not be significantly different across all treatment and growth conditions.Under hydroponic conditions, plant biomass was affected as a function of treatment (Table 1). In the presence of carbamazepine only, B. oleracea biomass decreased by 25% at the highest exposure concentration (10 mg L−1). However, overt visible damage at the leaf margins and modified morphology were evident at concentrations as low as 1 mg L−1. Upon exposure to pCNTs alone, reductions in B. oleracea biomass were evident mainly in the roots; but the total plant biomass was not significantly affected at 42 d (Table 1, row 1). Previous studies addressing carbon nanotube phytotoxicity have produced contradictory findings. With regard to hydroponic conditions, Lin et al. reported that CNTs (pristine, multi-wall) did not affect root growth of five plant species, including Brassica napus (rape), Raphanus sativus (radish), Lactuca sativa (lettuce), Zea mays (corn) and Cucumis sativus (cucumber), at concentrations as high as 2000 mg L−1.35 However, adverse effects from multi-wall CNTs exposure were observed in other studies, including growth inhibition and cell death in Amaranthus tricolor (red spinach) at 125–1000 mg L−1, decreased biomass of Cucurbita pepo (zucchini) at 1000 mg L−1, and suppressed growth with electrolyte leakage in Amaranthus tricolor, Lactuca sativa and Cucumis sativus at 1000 mg L−1.36–38 In general, CNTs phytoxocity was observed at higher and likely unrealistic exposure concentrations. The CNTs level used in the current study was only 50 mg L−1 (hydroponic assay); a lack of apparent phytotoxicity from this level of exposure is not surprising.
| Carbamazepine concentration | Leaf | Root | Total | |||
|---|---|---|---|---|---|---|
| Control | pCNT 50 mg L−1 | Control | pCNT 50 mg L−1 | Control | pCNT 50 mg L−1 | |
| a Each data point is the average of 8 individual measurements. Data analysis was performed through two-way ANOVA with Tukey's test. The interactions between two factors (carbamazepine and pCNTs) were found insignificant. Thus within columns (carbamazepine), values followed by different letters are significantly different at p < 0.05. Within rows (pCNTs), values marked with asterisks (*) are statistically different from values in control column with *p < 0.05 and **p < 0.01. | ||||||
| 0 μg L−1 | 14.8 ± 1.8 A | 13.5 ± 2.6 A | 9.2 ± 3.2 A | 6.8 ± 1.5 A | 24.0 ± 4.3 A | 20.3 ± 3.9 A |
| 100 μg L−1 | 14.0 ± 1.1 A | 12.2 ± 2.4 A | 6.4 ± 1.6 A | 6.3 ± 1.4 A | 20.4 ± 2.5 A | 19.7 ± 1.3 A |
| 500 μg L−1 | 12.9 ± 3.3 A | 12.8 ± 0.6 A | 7.4 ± 1.3 A | 6.5 ± 0.8 A | 20.3 ± 3.9 A | 20.8 ± 0.5 A |
| 1 mg L−1 | 14.2 ± 2.5 A | 14.0 ± 1.4 A | 7.6 ± 2.1 A | 4.6 ± 0.7 A* | 21.8 ± 4.5 A | 18.5 ± 1.4 A* |
| 5 mg L−1 | 13.6 ± 2.1 A | 13.3 ± 2.7 A | 6.3 ± 0.7 B | 3.7 ± 0.7 B** | 19.8 ± 2.4 A | 17.0 ± 3.6 A* |
| 10 mg L−1 | 12.5 ± 1.5 A | 13.2 ± 2.4 A | 5.5 ± 1.5 B | 4.3 ± 1.0 B* | 18.0 ± 2.7 B | 17.5 ± 3.1 A |
We do note that a more limited number of studies have addressed CNT toxicity as a function of surface modification. As shown in this study (Table 2), pCNTs at 50 mg L−1 had no significant adverse effect on biomass production under hydroponic conditions, while cCNTs exposure in the absence of carbamazepine significantly increased leaf biomass by 28% and total plant biomass by 25%. In several previous studies, oxidized or functionalized CNTs were also reported to promote plant growth. Mondal et al. found that oxidized multi-wall CNTs stimulated shoot and root growth; the authors suggested the use of oxidized CNTs as a beneficial growth promoter.39 Notably, there was no parallel exposure to pristine CNTs at identical concentrations. Similarly, root elongation and biomass increases were also reported in Triticum aestivum (wheat) treated with oxidized CNTs.40 While the underlying mechanisms of CNTs surface chemistry effects on plant physiology are unknown, the difference between the two CNTs with regards to biomass production in this study could possibly be linked to water transpiration differences. In a similar hydroponic study, the cumulative transpiration of water in maize exposed to 50 mg L−1 of cCNTs for 18 d was almost twice as much as that in the unexposed and pCNTs-exposed maize.41 Khodakovskaya et al. reported up-regulation of the water-channel LeAqp2 gene in Solanum lycopersicum (tomato) after exposure to COOH-functionalized CNTs.42 Villagarcia et al. also compared the expression of water channel proteins in Solanum lycopersicum exposed to carbon nanotubes functionalized with different groups and observed a strong correlation between protein expression and CNTs surface functionalization.43 In the current study, cCNTs had greater dispersion stability and promoted plant growth, possibly due to enhanced water uptake capacity. In contrast, the pCNTs had no distinct impact on plant growth, likely because agglomerates were more readily formed and the actual CNTs exposure concentration was lowered.
| Treatment | Hydroponics 28 d | Soil 42 d | ||||
|---|---|---|---|---|---|---|
| Leaf | Root | Total | Leaf | Root | Total | |
| a Each data point is the average of 8 individual measurements. Data analysis was performed through two-way ANOVA with Tukey's test. Values marked with asterisks (*) are statistically different from control with *p < 0.05. Carbamazepine was applied at 100 μg L−1; pCNTs and cCNTs were tested at 50 mg L−1 in hydroponics and 0.5 mg g−1 in soil. | ||||||
| Control | 7.1 ± 1.4 | 2.6 ± 0.5 | 9.7 ± 2.6 | 7.8 ± 2.0 | 2.9 ± 0.8 | 10.7 ± 2.6 |
| Carbamazepine | 7.6 ± 1.4 | 2.7 ± 0.7 | 10.3 ± 3.5 | 6.9 ± 1.0 | 2.9 ± 0.8 | 9.9 ± 1.2 |
| pCNTs | 6.7 ± 2.2 | 2.3 ± 0.5 | 9.0 ± 3.0 | 7.4 ± 0.9 | 3.3 ± 0.6 | 10.7 ± 1.6 |
| pCNTs + carbamazepine | 7.5 ± 1.5 | 2.7 ± 0.6 | 10.2 ± 3.4 | 6.7 ± 1.4 | 2.4 ± 0.8 | 9.1 ± 2.1 |
| cCNTs | 9.1 ± 2.7* | 3.0 ± 0.7 | 12.1 ± 3.6* | 6.8 ± 1.3 | 2.9 ± 0.8 | 9.7 ± 2.6 |
| cCNTs + carbamazepine | 7.4 ± 2.4 | 2.7 ± 0.7 | 10.1 ± 3.5 | 6.5 ± 1.2 | 2.4 ± 0.5 | 8.9 ± 1.5 |
In addition to exposure under single analyte conditions (carbamazepine or CNTs), the results from hydroponic co-exposure scenario indicated that the interaction between carbamazepine and CNTs did not have a significant effect on biomass production. In contrast to the hydroponic scenario, leaf, root and total plant biomass in soil were unaffected by any of the treatments (Table 2). In soil, CNTs aggregation and attachment to soil particles likely significantly reduced the accessibility of the nanomaterial to root tissues. These differential findings based on experimental or exposure conditions highlight the importance of evaluating phytotoxicity under conditions of environmental relevance.
Carbamazepine accumulation through hydroponic exposure
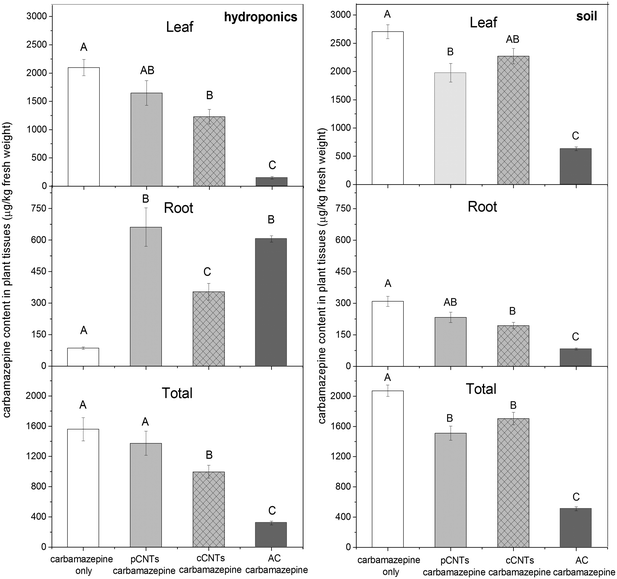
During the 28 d hydroponic exposure, B. oleracea treated with carbamazepine accumulated substantial amount of the pharmaceutical into leaf and root tissues, regardless of CNTs co-exposure (Fig. 1). The plants from the control group contained no detectable carbamazepine and are excluded from the figure. In all treatments except AC co-exposure, carbamazepine accumulation in leaf tissues was significantly higher than that in root tissues. Carbamazepine demonstrated exceptional translocation potential into the edible portion of the plant. Specifically, in the absence of carbon materials, carbamazepine content in the leaf and root tissues were 2099 μg kg−1 and 86 μg kg−1, respectively, with a transfer factor of 24.4 (Table S1,† transfer factor = leaf/root concentration ratio, TF). This high translocation potential was also reported in several other plant species, including Capsicum annuum (pepper), Lactuca sativa L. (lettuce), Raphanus sativus (radish) and Lycopersicon esculentum (tomato).23,44,45 After uptake and translocation, carbamazepine could possibly undergo metabolic transformation within the plant. Possible metabolic pathways have been characterized in the literature. Using these pathways the chemical formulas of the metabolites were determined. From these formulas the exact masses of the (M + H)+ ion of the metabolites were determined. These masses were then extracted from the total ion chromatogram using a 4 ppm window around the mass. Based on these extracted chromatograms, the major metabolite found in the B. oleracea tissues was suspected to be 10,11-dihydro-10,11-epoxycarbamazepine (CBZ-EP); full confirmation and quantitation of this metabolite would have required a standard of the metabolite which was not available. Assuming the same response factor, CBZ-EP had an average relative area ratio of 0.08 (leaf) and 0.01 (root) to that of the parent compound, while other suspected metabolites presented a significantly lower relative area ratio (<0.005). Oxidized from carbamazepine, CBZ-EP is an active primary metabolite and has been previously detected in human urine and Typha spp. tissues.20,46,47 In addition, the presence of CNTs did not affect the relative ratio of metabolized carbamazepine. The results indicated that both carbamazepine and its pharmaceutically active metabolite were present in the edible tissues of plants growing in contaminated hydroponic media.Co-exposure to carbon materials significantly altered carbamazepine accumulation and translocation within B. oleracea. As shown in Fig. 1 (hydroponics), carbamazepine leaf concentrations were decreased in the presence of carbon materials. Compared to carbamazepine only exposure, co-exposure to pCNTs and cCNTs reduced carbamazepine leaf content by 21% and 41%, respectively. The activated carbon treatment dramatically reduced leaf carbamazepine levels (93%) as compared to controls. Not surprisingly, the carbamazepine root concentration was significantly elevated by carbon co-exposure after routine root washing. Specifically, the carbamazepine root concentrations in the presence of pCNTs, cCNTs and AC were 6.6, 3.1 and 6.0 times greater than the control. The elevated root carbamazepine levels were likely due to the substantial deposition of carbon nanomaterials that had previously adsorbed the co-contaminant carbamazepine prior to root surface association. Even though surfactant washing and sonication removed significant amounts of carbamazepine from CNTs-treated roots, the remaining residue content in root was still significantly greater than the controls (Fig. 3). The results showed that more carbamazepine was incorporated into roots with the presence of CNTs. However, in general, carbamazepine accumulation in the whole plant (as well as edible leaves) was suppressed by CNTs and AC co-exposure. In addition to transfer factors, the bioconcentration factors (BCF, concentration in tissues/concentration in growth media) were calculated due to the variability in root concentrations (ESI,† Table S1). The transfer potential of carbamazepine from root to leaf was significantly lowered by carbon co-exposure. In particular, the TF was decreased from 24.4 (control) to 2.5, 3.5 and 0.2 in the pCNTs, cCNTs and AC treatments, respectively. As evident from the root and leaf BCF values, root accumulation of carbamazepine was enhanced by carbon co-exposure while the leaf bioaccumulation potential was lowered. Regardless of the difference among carbon material type, the in planta distribution pattern of the pharmaceutical was dramatically altered in the presence of carbon materials under hydroponic conditions. The application of carbon could decrease the risk of human exposure to selected pharmaceuticals from crops grown in contaminated media. With respect to optimizing crop protection in contaminated environments, AC is a better agent than CNTs under these growth conditions.
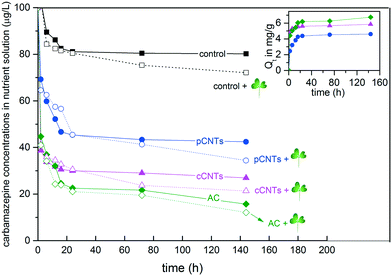
Although all carbon materials similarly suppressed carbamazepine uptake into edible tissues under hydroponic conditions, there are distinct differences based on carbon type. Since the interaction between carbamazepine and carbon materials was predominantly adsorption, the different suppression effect by carbon materials could possibly be explained through this process. The kinetics of this molecular process was evaluated in planted and un-planted systems (Fig. 2). In un-planted reactors, the carbamazepine content in the nutrient solution declined rapidly (within the first 12 h) in the presence of CNTs and AC, and gradually stabilized after 24 h. With B. oleracea seedlings present, carbarmazepine concentrations continued to decline after 24 h. In calculating the adsorbed amount Qt, a pseudo-second-order kinetics model was found to best represent carbamazepine interaction on carbon materials (regression coefficients above 0.999 for three adsorbents). As previously reported for multilayer loading, carbamazepine adsorption on CNTs was likely governed by hydrophobic interactions and π–π electron-donor–acceptor interactions.48 In comparison to CNTs, the conventional activated carbon displayed relatively slower adsorption but higher capacity at equilibrium. The higher capacity could likely be explained through higher specific surface area in AC (1380 m2 g−1) than CNTs (500 m2 g−1). The AC used in this study provided a large number of adsorption sites with a well-developed internal microporosity and therefore significantly reduced the actual exposure concentration of carbamazepine to the plants. As a consequence, the leaf carbamazepine concentration under the AC co-exposure was much lower than with the CNTs and carbon-free exposure.
Notably, cCNTs in this study were different from pCNTs with regard to the adsorption capacities and the subsequent impact on carbamazepine bioaccumulation. The pCNTs and cCNTs had similar properties, including size, length and SSA; the primary exception being the COOH (3.8%) modification. As observed in Fig. 2, cCNTs adsorbed more carbamazepine than pCNTs. The adsorbed carbamazepine on pCNTs and cCNTs reached 4.6 and 5.8 mg g−1 respectively, although we do note that these values may be underestimated due to interferences from nutritional salts in solution or to insufficient agitation from aeration. The surfaces of pCNTs were hydrophobic and had poor wettability in the growth media; in contrast, functionalization of CNTs with COOH groups altered the surface charge and the hydrophobicity. These changes resulted from COOH groups further increased adsorption capacity and affinity, likely by increasing particle stability with a greater negative charge and with hydrogen bonding with the carbamazepine carboxamide groups.49 It is widely recognized that CNT functional groups significantly affect adsorption properties.50 However, few studies have further connected the adsorption properties of CNTs to their impact on the bioaccumulation of co-existing contaminants. In this study, cCNTs had a higher capacity for carbamazepine adsorption and exhibited a significantly greater suppression effect on total carbamazepine bioaccumulation as compared to pCNTs.
With adsorption and uptake data considered collectively, the carbamazepine concentrations in leaf and total plants were decreasing with increasing adsorption capacity of the carbon materials; meaning that the suppression effect in uptake correlated well with the carbon material adsorption capacity. This finding does not align with some published assertions that CNTs could enhance the uptake of adsorbed secondary contaminants.15 As reported in a similar hydroponic co-exposure test, phenanthrene was accumulated in wheat roots more quickly in the presence of CNTs, likely resulting from nanomaterial-induced physical damage to the cell wall.15 It was clear in the current study that CNTs-bound carbamazepine was carried to the root surface upon tube deposition. Consequently, the carbamazepine was more accessible to the root tissue. However, the tertiary interactions between plant root, CNTs and carbamazepine did not lead to a higher in planta accumulation of the residue. In fact, as noted above, the CNTs resulted in reduced leaf and overall carbamazepine content. The lack of effective CNT transport out of the roots is not surprising and has been noted previously; Larue et al. reported a transfer factor of radio labelled-CNTs in wheat and rapeseed as low as 4.7 × 10−6.51 Furthermore, Lin et al. investigated the accumulation NOM-coated CNTs in rice and noted minimal translocation to aerial tissues.26 Although the current study did not include an assessment of in planta CNT content, indirect results with carbamazepine transport suggest minimal potential. Instead of CNTs penetrating and introducing carbamazepine into plants, greater amounts of the pharmaceutical seem to be retained in the growth media or on root surfaces through adsorption on to CNTs. Notably, the increased retention of carbamazepine in the root tissues could have significant food safety implications for crops with edible tubers or other similar tissues.
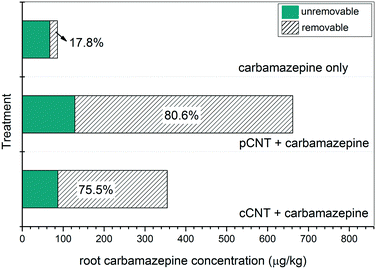
While leaf and total carbamazepine content could be related to the adsorption capacities, the root carbamazepine concentration was more likely to be affected by the dispersion and sedimentation of carbon materials. In comparing the root carbamazepine concentrations, it is clear that the more effectively dispersed cCNTs yielded less carbamazepine onto the plant root surfaces than pCNTs did. In control roots exposed to carbamazepine alone, surfactant washing removed roughly 18% of the root carbamazepine (Fig. 3). In the presence of the nanotubes, the results show that a relatively large proportion of carbamazepine detected in roots could be rinsed off by SDBS with sonication; 80.6% and 75.5% from pCNTs and cCNT, respectively. There was no statistical difference in surfactant removal efficiencies between pCNTs and cCNTs treatment, but any tube-specific effects may have been masked by the different initial root carbamazepine concentrations (initial root carbamazepine from pCNTs was roughly two times of that from cCNTs). However, as mentioned above, the presence of CNTs did increase the un-removable fraction of carbamazepine in the root (P < 0.05), which may suggest that more of the residue was incorporated into root cells in the presence of the nanotubes. Although the mechanism of this increased retention is unknown, it is possible that cell wall pores were pierced or physically damaged by CNTs presence; evaluating these processes is a topic in need of additional study. In summary, it is clear that under hydroponic conditions, co-exposure to carbon materials significantly altered the fate of carbamazepine in B. oleracea.
Carbamazepine bioaccumulation in soil exposure
The impact of carbon amendment (CNT and AC) on total carbamazepine accumulation was generally similar to that observed in the hydroponic experiment, and was evident as an overall suppression effect (Fig. 1). AC suppressed carbamazepine uptake to the greatest extent in both root and shoot tissues, with overall reductions in plant content approaching 75%. Similarly, CNTs-treated plants contained significantly lower total carbamazepine levels, with 27% and 18% reductions observed for pCNTs and cCNTs, respectively. When converted to the absolute amount (μg), the significant difference between CNTs and control were more evident in leaf, root and total plant content (Table S2†). Similar to the hydroponic exposure, root and total plant carbamazepine content (μg) of soil-grown B. oleracea correlated well with the adsorption properties of carbon materials. For example, carbon materials suppressed the root carbamazepine uptake by 29%, 53% and 89% in co-exposure with pCNTs, cCNTs and AC, respectively. In soil, the mobility of CNTs was more restricted than in the hydroponic study, with significant interactions within the heterogeneous soil structure; thus, a large proportion of carbamazepine retained in soil through adsorption. Not surprisingly, the BCF of carbamazepine in soil was significantly lower than the hydroponic exposure (Table S1†).Comparisons within the two types of CNTs showed that the pCNTs co-exposure resulted in 34% higher carbamazepine root uptake (μg) than cCNTs co-exposure. This difference is attributable to the higher adsorption capacity resulting from CNTs functionalization. Notably, Hamdi et al. also reported that the presence of CNTs significantly lowered pesticide (chlordane and p,p′-DDE) availability to Lactuca sativa L. (lettuce) seedlings.52 Specifically, the authors noted that amino-functionalized CNTs had a lower adsorption capability for pesticides than non-functionalized CNTs and consequently resulted in significantly greater levels of pesticide residues in lettuce roots and shoots.52 In this study, evidence from adsorption experiment showed that carbon materials with a higher adsorption capacity would present a greater suppression effect in carbamazepine uptake and result in a lower carbamazepine accumulation in planta.
The difference between pCNTs and cCNTs from soil-grown conditions was less significant than observed in the hydroponic experiment. However, the pCNTs and cCNTs modified the in planta distribution of contaminant differently. The presence of cCNT in soil resulted in a statistically greater root to leaf TF (11.7) than the carbon-free control (8.7), pCNTs (8.5) and AC (7.7) (Table S1†). Interestingly, in the hydroponic exposure, the cCNTs co-exposure resulted in the highest carbamazepine TF across all carbon materials. When co-exposed with functionalized cCNTs, carbamazepine had a greater potential to translocate to edible leaf tissues while pCNTs co-exposure tend to retain carbamazepine in the root tissues. Although overall carbamazepine content was suppressed by cCNTs, a potential food safety risks remains in consuming the contaminated leaf tissues. The difference between pristine and functionalized CNTs lead us to hypothesize that surface modification of CNTs may enhance the biocompatibility of CNTs and promote the mobility of the contaminant within the plant. The finding that cCNTs enhanced carbamazepine transfer factors while suppressing overall accumulation demonstrates that the complex nature of interactions of CNTs with coexistent contaminants. Further investigation is required to develop a mechanistic understanding of the interactions and to evaluate the risk to food safety.
Considering data from both hydroponic and soil exposures, carbon amendment into soil generally decreased the availability of carbamazepine and the degree of suppression was directly related to the adsorption properties of carbon materials. There are numerous studies reporting that activated carbon or biochar amendment decreases the mobility and bioavailability of contaminants in soil or sediments, including inorganic heavy metals (Zn, Cd) and hydrophobic organic compounds (PCB, DDT and PAHs).53–55 However, a comparison between conventional AC and carbon nanomaterials is less common, and the available literature on the effect nanomaterial presence on contaminant bioaccumulation is rather limited. With a lower adsorption capacity than AC, CNTs in this study were less effective at crop protection in the presence of the pharmaceutical residue. However, the adsorption capacity of AC is highly dependent on the production/activation process and the specific co-contaminant; CNTs may be more versatile with surface modification and improved adsorption properties, even though AC currently has a significant lower manufacturing cost than CNTs. A number of studies have focused on the concurrent exposure of nanomaterials and contaminants, although there was no use of activated carbon as a control. De La Torre-Roche et al. reported that pristine CNTs co-exposure at 500 mg kg−1 in soil did not affect weathered DDx (DDT + metabolites) and chlordane bioaccumulation in species including C. pepo (zucchini), Zea mays (corn), Glycine max (soybean) and Solanum lycopersicum (tomato), but that exposure to 1000 mg kg−1 or higher did significantly decrease contaminant uptake.16 As mentioned above, Hamdi et al. also reported that both non-functionalized and amino-functionalized CNTs lowered chlordane and p,p′-DDE accumulation but that the amino-functionalized tubes produced a more modest suppression effect.52 Conversely, carbon in the form of C60 has been shown to enhance contaminant bioaccumulation under certain conditions, including chlordane in soil-grown tomato and soybean, DDx in soil-grown zucchini and trichloroethylene in hydroponic poplar.16,56,57 In reviewing the literature, it is clear that the effect of carbon nanomaterials on the uptake of co-existing contaminants is highly variable, and the key factors of exposure concentration, plant species and nanomaterial type/surface chemistry ultimately control bioaccumulation. With interactions specifically involving CNTs, suppression of contaminant uptake in soil exposure with CNTs would be reasonably expected, and mechanistically, functionalization would alleviate or intensify the suppression effect based on the adsorption capacity of the particular contaminant. In environmentally relevant scenarios such as soil, the movement of CNTs was low; the contaminants bound to CNTs were more likely to remain ex planta through adsorption. The bioaccumulation of coexistent contaminant was therefore suppressed due to the actual decreased exposure concentrations. Furthermore, it is worth noting that functionalization of CNTs with COOH significantly improved the in planta translocation of co-existing contaminant in soil, although the mechanism remains unknown.
Overall, this study evaluated B. oleracea growth under a range of co-exposure conditions and quantified the bioaccumulation of carbamazepine in the presence of CNTs and AC, addressing concerns that carbon nanotubes may affect the bioaccumulation of secondary contaminants. With regard to plant growth, the inhibition of plant biomass production was dependent on carbamazepine concentrations in hydroponic conditions, while exposure to 50 mg L−1 cCNTs alone enhanced the plant growth. Suppression of carbamazepine uptake in the presence of carbon materials was observed in both hydroponic and soil conditions, which was likely due to the lowered actual carbamazepine exposure concentration after adsorption to the carbon material and retention in growth media. The extent of suppression was correlated with the adsorption capacities of carbon materials. Interestingly, COOH-functionalized CNTs affected plant carbamazepine uptake and distribution differently from its pristine analog, through enhanced adsorption capacity, better dispersion stability, and possibly increased biocompatibility. With elevated transfer factors, the carboxylated nanotubes demonstrated the potential ability to facilitate xylem-based transport of carbamazepine. Our findings demonstrate that the accumulation and distribution of pharmaceutical residues in crops varies greatly with exposure condition and the type and surface functionalization of carbon materials. In addition, the results presented in this study have significant implications for the use of carbon materials in agriculture and towards efforts to ensure crop protection and food safety.
Acknowledgements
This research was supported by USDA-AFRI (2011-67006-30181) and USDA-AFRI Hatch program (MAS 00475).References
- H. C. Chen, M. C. Roco, J. B. Son, S. Jiang, C. A. Larson and Q. Gao, Global nanotechnology development from 1991 to 2012: patents, scientific publications, and effect of NSF funding, J. Nanopart. Res., 2013, 15(9), 21 Search PubMed.
- E. T. Thostenson, Z. Ren and T.-W. Chou, Advances in the science and technology of carbon nanotubes and their composites: a review, Compos. Sci. Technol., 2001, 61(13), 1899–1912 CrossRef CAS.
- A. A. Keller, S. McFerran, A. Lazareva and S. Suh, Global life cycle releases of engineered nanomaterials, J. Nanopart. Res., 2013, 15(6), 17 CrossRef.
- B. Nowack, J. F. Ranville, S. Diamond, J. A. Gallego-Urrea, C. Metcalfe and J. Rose, et al., Potential scenarios for nanomaterial release and subsequent alteration in the environment, Environ. Toxicol. Chem., 2012, 31(1), 50–59 CrossRef CAS PubMed.
- T. M. Benn and P. Westerhoff, Nanoparticle silver released into water from commercially available sock fabrics, Environ. Sci. Technol., 2008, 42(11), 4133–4139 CrossRef CAS PubMed.
- M. A. Kiser, P. Westerhoff, T. Benn, Y. Wang, J. Pérez-Rivera and K. Hristovski, Titanium Nanomaterial Removal and Release from Wastewater Treatment Plants, Environ. Sci. Technol., 2009, 43(17), 6757–6763 CrossRef CAS PubMed.
- A. R. Köhler, C. Som, A. Helland and F. Gottschalk, Studying the potential release of carbon nanotubes throughout the application life cycle, J. Cleaner Prod., 2008, 16(8–9), 927–937 CrossRef.
- Y. D. Deng, J. White and B. S. Xing, Interactions between engineered nanomaterials and agricultural crops: implications for food safety, J. Zhejiang Univ., Sci., A, 2014, 15(8), 552–572 CrossRef CAS.
- K. Yang and B. Xing, Adsorption of Organic Compounds by Carbon Nanomaterials in Aqueous Phase: Polanyi Theory and Its Application, Chem. Rev., 2010, 110(10), 5989–6008 CrossRef CAS PubMed.
- B. Glomstad, D. Altin, L. Sørensen, J. Liu, B. M. Jenssen and A. M. Booth, Carbon Nanotube Properties Influence Adsorption of Phenanthrene and Subsequent Bioavailability and Toxicity to Pseudokirchneriella subcapitata, Environ. Sci. Technol., 2016, 50(5), 2660–2668 CrossRef CAS PubMed.
- F. Schwab, T. D. Bucheli, L. Camenzuli, A. Magrez, K. Knauer and L. Sigg, et al., Diuron Sorbed to Carbon Nanotubes Exhibits Enhanced Toxicity to Chlorella vulgaris, Environ. Sci. Technol., 2013, 47(13), 7012–7019 CAS.
- Q. L. Liu, B. Chen, Q. L. Wang, X. L. Shi, Z. Y. Xiao and J. X. Lin, et al., Carbon Nanotubes as Molecular Transporters for Walled Plant Cells, Nano Lett., 2009, 9(3), 1007–1010 CrossRef CAS PubMed.
- M. F. Serag, N. Kaji, C. Gaillard, Y. Okamoto, K. Terasaka and M. Jabasini, et al., Trafficking and Subcellular Localization of Multiwalled Carbon Nanotubes in Plant Cells, ACS Nano, 2011, 5(1), 493–499 CrossRef CAS PubMed.
- E. J. Petersen, R. A. Pinto, P. F. Landrum and W. J. Weber, Influence of Carbon Nanotubes on Pyrene Bioaccumulation from Contaminated Soils by Earthworms, Environ. Sci. Technol., 2009, 43(11), 4181–4187 CrossRef CAS PubMed.
- E. Wild and K. C. Jones, Novel Method for the Direct Visualization of in Vivo Nanomaterials and Chemical Interactions in Plants, Environ. Sci. Technol., 2009, 43(14), 5290–5294 CrossRef CAS PubMed.
- R. De La Torre-Roche, J. Hawthorne, Y. Q. Deng, B. S. Xing, W. J. Cai and L. A. Newman, et al., Multiwalled Carbon Nanotubes and C-60 Fullerenes Differentially Impact the Accumulation of Weathered Pesticides in Four Agricultural Plants, Environ. Sci. Technol., 2013, 47(21), 12539–12547 CrossRef CAS PubMed.
- M. J. Benotti, R. A. Trenholm, B. J. Vanderford, J. C. Holady, B. D. Stanford and S. A. Snyder, Pharmaceuticals and Endocrine Disrupting Compounds in US Drinking Water, Environ. Sci. Technol., 2009, 43(3), 597–603 CrossRef CAS PubMed.
- C. D. Metcalfe, X. S. Miao, B. G. Koenig and J. Struger, Distribution of acidic and neutral drugs in surface waters near sewage treatment plants in the lower Great Lakes, Canada, Environ. Toxicol. Chem., 2003, 22(12), 2881–2889 CrossRef CAS PubMed.
- J. E. Drewes, T. Heberer and K. Reddersen, Fate of pharmaceuticals during indirect potable reuse, Water Sci. Technol., 2002, 46(3), 73–80 CAS.
- X. S. Miao, J. J. Yang and C. D. Metcalfe, Carbamazepine and its metabolites in wastewater and in biosolids in a municipal wastewater treatment plant, Environ. Sci. Technol., 2005, 39(19), 7469–7475 CrossRef CAS PubMed.
- R. Navon, S. Hernandez-Ruiz, J. Chorover and B. Chefetz, Interactions of Carbamazepine in Soil: Effects of Dissolved Organic Matter, J. Environ. Qual., 2011, 40(3), 942–948 CrossRef CAS PubMed.
- C. F. Williams, C. F. Williams and F. J. Adamsen, Sorption–Desorption of Carbamazepine from Irrigated Soils, J. Environ. Qual., 2006, 35(5), 1779–1783 CrossRef CAS PubMed.
- C. X. Wu, A. L. Spongberg, J. D. Witter and B. B. M. Sridhar, Transfer of wastewater associated pharmaceuticals and personal care products to crop plants from biosolids treated soil, Ecotoxicol. Environ. Saf., 2012, 85, 104–109 CrossRef CAS PubMed.
- L. D. Nghiem, A. I. Schafer and M. Elimelech, Pharmaceutical retention mechanisms by nanofiltration membranes, Environ. Sci. Technol., 2005, 39(19), 7698–7705 CrossRef CAS PubMed.
- J. A. Menéndez, M. J. Illán-Gómez, C. A. L. y León and L. R. Radovic, On the difference between the isoelectric point and the point of zero charge of carbons, Carbon, 1995, 33(11), 1655–1657 CrossRef.
- S. J. Lin, J. Reppert, Q. Hu, J. S. Hudson, M. L. Reid and T. A. Ratnikova, et al., Uptake, Translocation, and Transmission of Carbon Nanomaterials in Rice Plants, Small, 2009, 5(10), 1128–1132 CAS.
- M. V. Khodakovskaya, B. S. Kim, J. N. Kim, M. Alimohammadi, E. Dervishi and T. Mustafa, et al., Carbon Nanotubes as Plant Growth Regulators: Effects on Tomato Growth, Reproductive System, and Soil Microbial Community, Small, 2013, 9(1), 115–123 CrossRef CAS PubMed.
- J. E. Canas, M. Q. Long, S. Nations, R. Vadan, L. Dai and M. X. Luo, et al., Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species, Environ. Toxicol. Chem., 2008, 27(9), 1922–1931 CrossRef CAS PubMed.
- C. X. Wu, A. L. Spongberg, J. D. Witter, M. Fang and K. P. Czajkowski, Uptake of Pharmaceutical and Personal Care Products by Soybean Plants from Soils Applied with Biosolids and Irrigated with Contaminated Water, Environ. Sci. Technol., 2010, 44(16), 6157–6161 CrossRef CAS PubMed.
- P. A. Herklotz, P. Gurung, B. Vanden Heuvel and C. A. Kinney, Uptake of human pharmaceuticals by plants grown under hydroponic conditions, Chemosphere, 2010, 78(11), 1416–1421 CrossRef CAS PubMed.
- M. Shenker, D. Harush, J. Ben-Ari and B. Chefetz, Uptake of carbamazepine by cucumber plants - A case study related to irrigation with reclaimed wastewater, Chemosphere, 2011, 82(6), 905–910 CrossRef CAS PubMed.
- M. Anastassiades, S. J. Lehotay, D. Stajnbaher and F. J. Schenck, Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce, J. AOAC Int., 2003, 86(2), 412–431 CAS.
- W. J. Krol, B. D. Eitzer, T. Arsenault, M. J. I. Mattina and J. C. White, Significant Improvements in Pesticide Residue Analysis in Food Using the QuEChERS Method, LCGC North Am., 2014, 32(2), 116–125 CAS.
- V. C. Moore, M. S. Strano, E. H. Haroz, R. H. Hauge, R. E. Smalley and J. Schmidt, et al., Individually Suspended Single-Walled Carbon Nanotubes in Various Surfactants, Nano Lett., 2003, 3(10), 1379–1382 CrossRef CAS.
- D. Lin and B. Xing, Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth, Environ. Pollut., 2007, 150(2), 243–250 CrossRef CAS PubMed.
- P. Begum and B. Fugetsu, Phytotoxicity of multi-walled carbon nanotubes on red spinach (Amaranthus tricolor L) and the role of ascorbic acid as an antioxidant, J. Hazard. Mater., 2012, 243, 212–222 CrossRef CAS PubMed.
- D. Stampoulis, S. K. Sinha and J. C. White, Assay-Dependent Phytotoxicity of Nanoparticles to Plants, Environ. Sci. Technol., 2009, 43(24), 9473–9479 CrossRef CAS PubMed.
- P. Begum, R. Ikhtiari, B. Fugetsu, M. Matsuoka, T. Akasaka and F. Watari, Phytotoxicity of multi-walled carbon nanotubes assessed by selected plant species in the seedling stage, Appl. Surf. Sci., 2012, 262, 120–124 CrossRef CAS.
- A. Mondal, R. Basu, S. Das and P. Nandy, Beneficial role of carbon nanotubes on mustard plant growth: an agricultural prospect, J. Nanopart. Res., 2011, 13(10), 4519–4528 CrossRef CAS.
- X. P. Wang, H. Y. Han, X. Q. Liu, X. X. Gu, K. Chen and D. L. Lu, Multi-walled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum) plants, J. Nanopart. Res., 2012, 14(6), 10 CrossRef.
- G. Zhai, S. M. Gutowski, K. S. Walters, B. Yan and J. L. Schnoor, Charge, Size, and Cellular Selectivity for Multiwall Carbon Nanotubes by Maize and Soybean, Environ. Sci. Technol., 2015, 49(12), 7380–7390 CrossRef CAS PubMed.
- M. V. Khodakovskaya, K. de Silva, D. A. Nedosekin, E. Dervishi, A. S. Biris and E. V. Shashkov, et al., Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(3), 1028–1033 CrossRef CAS PubMed.
- H. Villagarcia, E. Dervishi, K. de Silva, A. S. Biris and M. V. Khodakovskaya, Surface Chemistry of Carbon Nanotubes Impacts the Growth and Expression of Water Channel Protein in Tomato Plants, Small, 2012, 8(15), 2328–2334 CrossRef CAS PubMed.
- C. S. Holling, J. L. Bailey, B. Vanden Heuvel and C. A. Kinney, Uptake of human pharmaceuticals and personal care products by cabbage (Brassica campestris) from fortified and biosolids-amended soils, J. Environ. Monit., 2012, 14(11), 3029–3036 RSC.
- P. A. Herklotz, P. Gurung, B. V. Heuvel and C. A. Kinney, Uptake of human pharmaceuticals by plants grown under hydroponic conditions, Chemosphere, 2010, 78(11), 1416–1421 CrossRef CAS PubMed.
- X.-S. Miao and C. D. Metcalfe, Determination of Carbamazepine and Its Metabolites in Aqueous Samples Using Liquid Chromatography−Electrospray Tandem Mass Spectrometry, Anal. Chem., 2003, 75(15), 3731–3738 CrossRef CAS PubMed.
- A. V. Dordio, M. Belo, D. M. Teixeira, A. J. P. Carvalho, C. M. B. Dias and Y. Pico, et al., Evaluation of carbamazepine uptake and metabolization by Typha spp., a plant with potential use in phytotreatment, Bioresour. Technol., 2011, 102(17), 7827–7834 CrossRef CAS PubMed.
- P. Oleszczuk, B. Pan and B. Xing, Adsorption and Desorption of Oxytetracycline and Carbamazepine by Multiwalled Carbon Nanotubes, Environ. Sci. Technol., 2009, 43(24), 9167–9173 CrossRef CAS PubMed.
- P. S. Bäuerlein, J. E. Mansell, T. L. ter Laak and P. de Voogt, Sorption Behavior of Charged and Neutral Polar Organic Compounds on Solid Phase Extraction Materials: Which Functional Group Governs Sorption?, Environ. Sci. Technol., 2012, 46(2), 954–961 CrossRef PubMed.
- B. Pan and B. Xing, Adsorption Mechanisms of Organic Chemicals on Carbon Nanotubes, Environ. Sci. Technol., 2008, 42(24), 9005–9013 CrossRef CAS PubMed.
- C. Larue, M. Pinault, B. Czarny, D. Georgin, D. Jaillard and N. Bendiab, et al., Quantitative evaluation of multi-walled carbon nanotube uptake in wheat and rapeseed, J. Hazard. Mater., 2012, 227, 155–163 CrossRef PubMed.
- H. Hamdi, R. De La Torre-Roche, J. Hawthorne and J. C. White, Impact of non-functionalized and amino-functionalized multiwall carbon nanotubes on pesticide uptake by lettuce (Lactuca sativa L.), Nanotoxicology, 2015, 9(2), 172–180 CrossRef CAS PubMed.
- L. Beesley, E. Moreno-Jiménez and J. L. Gomez-Eyles, Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil, Environ. Pollut., 2010, 158(6), 2282–2287 CrossRef CAS PubMed.
- L. Beesley, E. Moreno-Jiménez, J. L. Gomez-Eyles, E. Harris, B. Robinson and T. Sizmur, A review of biochars' potential role in the remediation, revegetation and restoration of contaminated soils, Environ. Pollut., 2011, 159(12), 3269–3282 CrossRef CAS PubMed.
- M. Uchimiya, I. M. Lima, K. T. Klasson and L. H. Wartelle, Contaminant immobilization and nutrient release by biochar soil amendment: Roles of natural organic matter, Chemosphere, 2010, 80(8), 935–940 CrossRef CAS PubMed.
- X. M. Ma and C. Wang, Fullerene Nanoparticles Affect the Fate and Uptake of Trichloroethylene in Phytoremediation Systems, Environ. Eng. Sci., 2010, 27(11), 989–992 CrossRef CAS.
- R. De La Torre-Roche, J. Hawthorne, Y. Deng, B. Xing, W. Cai and L. A. Newman, et al., Fullerene-Enhanced Accumulation of p,p'-DDE in Agricultural Crop Species, Environ. Sci. Technol., 2012, 46(17), 9315–9323 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6en00419a |
| This journal is © The Royal Society of Chemistry 2017 |