Drop coating deposition of a liposome suspension on surfaces with different wettabilities: “coffee ring” formation and suspension preconcentration
Eva
Kočišová
*a,
Martin
Petr
b,
Hana
Šípová
c,
Ondřej
Kylián
b and
Marek
Procházka
*a
aCharles University, Faculty of Mathematics and Physics, Institute of Physics, Ke Karlovu 5, 121 16 Prague 2, Czech Republic. E-mail: kocisova@karlov.mff.cuni.cz; prochaz@karlov.mff.cuni.cz
bCharles University, Faculty of Mathematics and Physics, Department of Macromolecular Physics, V Holešovičkách 2, 180 00 Prague, Czech Republic
cInstitute of Photonics and Electronics, Academy of Sciences of the Czech Republic, Chaberská 57, 182 51 Prague, Czech Republic
First published on 22nd November 2016
Abstract
Evaporation of a drop of biomolecular solution on a solid surface typically creates a ring-shaped drying pattern, formed by the so-called “coffee ring” effect. The size and shape of the “coffee ring” pattern is strongly dependent on the properties of the surface as well as on the deposited molecular solution or suspension. In this paper, we tested six types of surfaces differing in their physico-chemical surface characteristics (contact angles, wettability and roughness) as well as in the presence or absence of a base metal layer. The tested surfaces include two fluorocarbon coated metallic surfaces (commercial SpectRIM™ from Tienta Sciences, Inc. based on a smoothed stainless steel and non-commercial aluminium surface), three silanized glass surfaces and polished CaF2. The results showed that the formation of a “coffee ring” was influenced by surface wettability as well as by lipid concentration in the drop. Drop coating deposition Raman (DCDR) spectroscopy was used to compare the ability of the tested surfaces to preconcentrate molecules in the ring and therefore improve detection sensitivity. It was shown that surfaces with a contact angle of 90° and higher produce smaller drying patterns than more hydrophilic surfaces. In these drying patterns, the model liposomes were more efficiently preconcentrated, which resulted in a higher Raman signal of the liposomes. The applicability of surfaces with static contact angles less than 90°, high water contact angle hysteresis and no metal layer (silanized glass, CaF2) is limited to samples with high liposome concentrations.
Introduction
The evaporation of solvent from a drop containing dissolved materials such as biomolecules or a suspension of particles is scientifically relevant to many applications. The evaporation of a drop on a solid surface often leads to a nonuniform deposition of dissolved molecules (or particles), the so-called “coffee ring” effect. The complex process of deposition is a result of the interplay between contact line pinning, solvent evaporation and capillary flow.1–3 During the drying process, the edge of the drop is pinned to the substrate, and capillary flow outward from the centre of the drop brings the material to the edge as evaporation proceeds. After evaporation, the suspended material is highly concentrated in a ring-shaped pattern of a diameter similar to the size of the original drop.1 The “coffee ring” effect was manifested in various systems including microspheres,1–3 nanoparticles,4,5 individual molecules6–10 and complex bodily fluids.11–13 The size of the ring-shaped deposit is influenced by both the properties of the surface (mostly wettability and roughness) and of the drop (mainly size, shape, charge and concentration of dissolved entities, solvents used, etc.). Various substrates based on hydrophobic surfaces (e.g. Teflon-coated stainless steel,6–10 polished metals,6,13,14 silanized glass slices,15,16 quartz,17–19 mica,2,3 metals with self-assembled monolayers of alkanethiols,6,20 or calcium fluoride (CaF2)11,12) can facilitate the “coffee ring” formation of the deposited liquid drops.Drop coating deposition Raman (DCDR) spectroscopy is an example of a technique benefiting from the “coffee ring” formation as a means of analyte preconcentration.6 Raman spectroscopy (RS) is based on inelastic scattering of light and enables the identification of molecules on the basis of their specific molecular fingerprints and thus it is applicable in various fields of chemical, physical and biological research.21 The main advantages of RS include simple sample preparation, small sample volumes, lack of principal water interference and simple and rapid measurement. However, RS is intrinsically weak and requires high sample concentrations (∼0.1–0.01 M). Using DCDR spectroscopy, lower concentrations of an analyte can be detected, as the Raman spectrum is acquired from the edge of a “coffee ring” pattern, where the analyte is preconcentrated.6 Nowadays, DCDR spectroscopy is a very efficient analytical technique but it requires a suitable surface providing enough high preconcentration of particular molecules in the edge ring pattern. So far, Teflon-coated stainless steel has been regarded as the most efficient DCDR substrate for analyte preconcentration. For example, the Raman detection sensitivity of proteins7 and lipids8,9 was improved by a factor of 103, which made the detection of the molecules down to 1–10 μM of the initial (deposited) concentration possible. Moreover, it was shown that the proteins and lipids dried on the DCDR surface retain their native structure from solution (suspension).7,8 Recently, even 105 times better sensitivity of DCDR over the classical Raman measurement for model porphyrins was reported.10
The main aim of this paper is to test and compare the performances of six surfaces with different physico-chemical surface characteristics in terms of the preconcentration of a liposome suspension. As the test substance, a liposome suspension composed of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) – phosphatidylcholine – was used which is known to form a “coffee ring” pattern when deposited on a Teflon coated stainless steel surface.8,9 Moreover, liposomes prepared in an aqueous environment form a bilayer system and therefore represent an excellent model of biological membranes. The DPPC molecule is composed of two palmitic acid chains attached to the glycerol moiety at positions sn-1 and sn-2 (Fig. 1). A choline is attached through a phosphate to the head of the lipid molecule. As the choline brings positive charge, the total charge of the DPPC molecule is neutral under physiological conditions. In this work, we studied the formation of drying patterns from the liposome drops on various substrates and at various concentrations of DPPC. We also discussed the influence of the properties of dried patterns on Raman spectral sensitivity.
Experimental
Preparation of liposome suspension
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, Avanti Polar lipids, Inc., see the chemical structure in Fig. 1) in the dissolved form (chloroform) was used for the liposome preparation according to the common procedure. DPPC was placed in a flask. A stream of nitrogen gas was used to eliminate the dissolving agents in order to form a thin lipid film on the glass surface. Pure water was subsequently added and mixed to obtain a lipid suspension with a concentration 1 mg mL−1. As the temperature of the phase transition was 41 °C, the suspension was left to hydrate for one hour at a temperature of about 50 °C. Liposomes of defined particle size (100 nm in diameter) were extruded through a polycarbonate membrane filter (more than 30 passages) using LiposoFast-Basic™ apparatus (Avestin, Inc.) keeping them constantly at a higher temperature of about 50 °C.Tested surfaces
Three types of substrates (six in total) were employed in the study:(1) Two fluorocarbon coated surfaces – commercial SpectRIM™ from Tienta Sciences, Inc. based on smoothed stainless steel (mentioned in the text as Tienta) and a non-commercial aluminium surface coated with magnetron sputtered polytetrafluoroethylene (mentioned in the text as sPTFE). More details about preparation of the latter surface can be found elsewhere.22
(2) Three silanized glass slides fabricated at Institute of Photonics and Electronics, AS, Prague mentioned in the text as Silane1, Silane2 and Silane3. The glass slides were rinsed with ethanol and deionized water and immersed for 30 min in an aqueous solution containing 3.6% NH3 and 4.3% H2O2 at 40 °C. They were then rinsed with water, dried with nitrogen and cleaned in a UV-ozone cleaner for 5 min. The substrates were rinsed with ethanol and water, dried with nitrogen, baked at 110 °C for 1 hour and immersed overnight under argon atmosphere in a 2 mM solution of trichloro(octadecyl)silane (Sigma Aldrich, Silane1) and dichlorodimethylsilane (Sigma Aldrich, Silane2) in heptane (spectroscopy grade) or (CH3)2SiCl(CH2)11-(ethylene glycol)6OCH3 (Prochimia, Poland, Silane3) in chloroform (HPLC grade). The substrates were rinsed and immersed in heptane (Silane1, Silane2) or in chloroform (Silane3) and sonicated for 10 minutes, then rinsed with ethanol, water and dried under a stream of nitrogen.
(3) A CaF2 slice treated only by polishing and mentioned as CaF.
Wettability measurements
Surface wettability was evaluated by a goniometer of custom construction. It consisted of a substrate holder, a manually operated syringe made by Hamilton company and a camera connected to a PC. For the determination of static water contact angles, 4 μL water droplets (deionized water at room temperature (25–26 °C)) were used. The dynamic contact angles, which represent the threshold values within which the triple line does not move when the water droplets are inflated and deflated, were determined by means of the method described in the previous work.23 The volume of the droplet was first gradually increased forcing the drop to advance on the sample until a constant contact angle was achieved. This value represents the advancing contact angle. Subsequently, the drop was slowly shrunk until a decrease in the drop radius was observed. The last contact angle value before the change in the droplet radius was the receding contact angle. The reported values of the static, advancing and receding contact angles of water are the average of three measurements.Morphology of samples
Surface morphology was determined by means of atomic force microscopy (AFM, Quesant Q-scope 350) in the semi-contact mode (scan rate 2 s, resolution 512 × 512 points) using ACLA-10 Si probes (tip radius <10 nm, nominal spring constant 58 N m−1, AppNano). The reported values of surface root-mean-square (RMS) roughness represents an average of more than three 10 μm × 10 μm scans performed on randomly selected positions on the samples.Drop coating deposition experiments
2 μL drops of liposome suspension were deposited by a pipette on each studied substrate and left to dry freely in air for about 40 minutes at room temperature. The concentrations of DPPC in the deposited suspension were 1, 0.2 and 0.1 mg mL−1. The white-light images of dried patterns were obtained by using 5× and 10× objectives of a Raman microscope. DCDR spectra were acquired by using a confocal Raman microspectrometer LabRAM HR800 (Horiba Jobin Yvon) with a nitrogen cooled CCD detector, a 300 grooves per mm grating spectrograph, a pinhole diameter of 400 μm, an entrance slit width of 100 μm, an He–Ne laser of 632.8 nm (3 mW power) as the excitation line and a 50× ULWD objective. Raman spectra were accumulated from the centre of edge ring patterns in the 500–3200 cm−1 spectral region with an acquisition time of 60 × 2 s. All the tested surfaces showed almost no Raman signal except for CaF2 which exhibited one band at 322 cm−1.Results and discussion
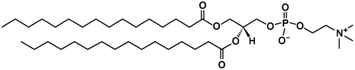
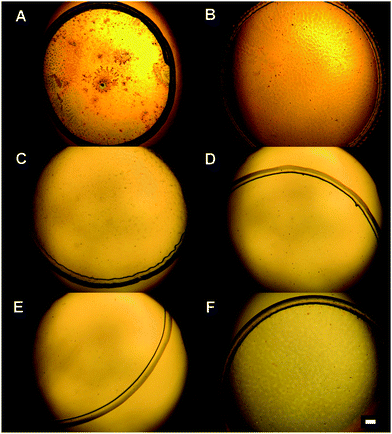
Droplets with 1, 0.1 and 0.2 mg mL−1 concentrations of DPPC were deposited on six different hydrophobic surfaces. After evaporation of the solvent, a pattern with a typical “coffee ring” shape was observed, characterized by a clearly distinguishable ring. 0.1 mg mL−1 was the lowest concentration of DPPC, which created a compact ring. The typical white-light micro-images of the dried patterns of DPPC of 1 mg mL−1 and 0.1 mg mL−1 concentrations are showed in Fig. 2 and 3, respectively. The parameters of rings (diameter of the deposit and width of the ring) at all three DPPC concentrations and all six surfaces are listed in Table 1.| Type of the surface | DPPC concentration | ||
|---|---|---|---|
| c = 1 mg mL−1 | c = 0.2 mg mL−1 | c = 0.1 mg mL−1 | |
| Drop diameter (width of the ring) in μm | |||
| A | |||
| Tienta | 1500 (70) | 760 (20) | 520 (18) |
| sPTFE | 1720 (80) | 980 (20) | 880 (17) |
| Silane1 | 1680 (80) | 880 (20) | 600 (15) |
| Silane2 | 1920 (80) | 920 (20) | 720 (15) |
| B | |||
| Silane3 | 2580 (80) | 2480 (30) | 2350 (30) |
| CaF | 2000 (80) | 2160 (40) | 2200 (30) |
We observed significant differences in the size of the dried patterns as well as the width of the rings, while using different concentrations of DPPC and various substrates. The rough comparison confirms the expected trend that lower concentration of the deposited material forms a dried pattern with a smaller diameter and a smaller width of the rings than higher deposited concentration. Moreover, the parameters of dried patterns strongly depend on the contact angle of the surface used. The results in Table 1 show two different groups of surfaces: (A) the surfaces including two fluorocarbon surfaces (Tienta and sPTFE) and two silanized glasses (Silane1, Silane2); (B) the surfaces including silanized glass Silane3 and CaF. Group B is characterized by dried patterns (2000–2580 μm) with bigger diameters than group A (less than 1920 μm). The largest differences between the two groups were found at the lowest DPPC concentration (0.1 mg mL−1) when the drying patterns on surfaces A reached about 30% of the size of those in group B (in the case of 1 mg mL−1 concentration it is about 70%). Moreover, it can be seen that suspensions of various concentrations of DPPC produce drying patterns of similar size on surfaces in group B. In contrast, in the case of hydrophobic surfaces in group A, the diameters of dried drops significantly decrease with the DPPC concentration: it is 1500–1920 μm at 1 mg mL−1, 760–980 μm at 0.2 mg mL−1 and 520–880 μm at 0.1 mg mL−1 concentration. With regard to the widths of the rings, an interesting trend can be seen from Table 1. No significant dependence on the surface type is observed at the highest concentration when the width of rings varied between 70–80 μm. At lower concentrations of DPPC, a much smaller ring width is obtained (15–40 μm). Following the same trend as the drop diameter, the ring width is smaller on the surfaces in group A (15–20 μm) than that on the surfaces in group B (30–40 μm).
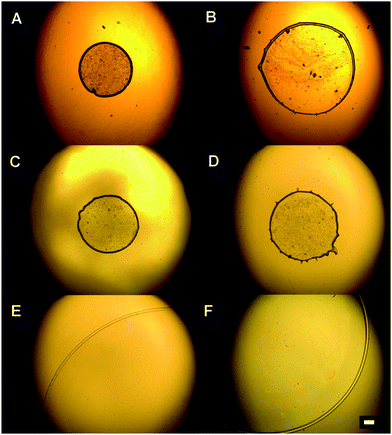
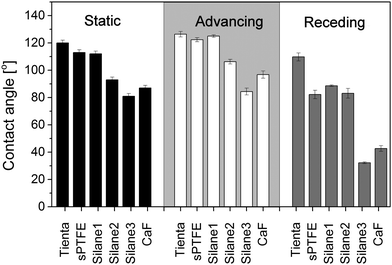
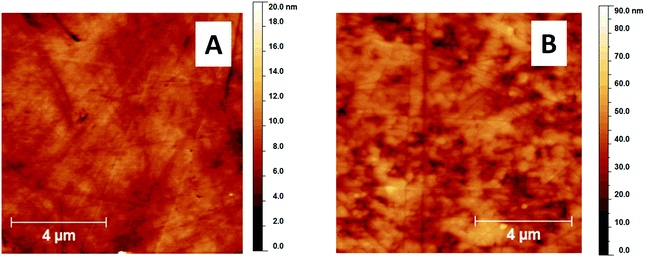
The observed differences in the dried patterns suggest different processes occurring at the interface between the substrates and the liposome suspension that may be connected with surface wettability. Indeed, when the diameters of dried patterns are compared with the values of static water contact angles presented in Fig. 4, all the substrates in group A are characterized by static water contact angles higher than 90°, whereas the substrates in group B exhibit water contact angles lower than 90°. However, the static contact angle alone cannot explain the observed significant difference between samples Silane2 and CaF that have nearly the same static contact angles, but the drop size is about 30–40% and the width of the ring is about 50% smaller for Silane2 than for the CaF surface at low liposome concentrations (Table 1). This difference may be ascribed to the much more heterogeneous surface topography of CaF compared to Silane2. As can be seen in Fig. 5 presenting the AFM images acquired for these two substrates, Silane2 is smooth with an RMS roughness of 1.4 ± 0.2 nm, the whereas CaF surface is characterized by the presence of surface irregularities and an RMS roughness of 7.4 ± 0.2 nm. The nanostructures on the CaF surface in turn influence the process of droplet drying as the liquid may be trapped in the surface crevices: the droplet is pinned on the surface for a longer time that leads to a significant decrease of the receding contact angle as compared to smooth Silane2 (Fig. 4) and to the alteration of the process of accumulation of liposomes on the surface. In other words, not only the surface wettability, but also its hysteresis is a crucial factor governing the shape of the “coffee ring” structure.
Finally, we employed Raman spectroscopy to detect DPPC accumulated on the edge of the dried drop. It is known that deposition of the sample in the ring is not uniform and provides the most intensive Raman signal in the middle of the ring. The intensity then gradually decreases from the center of the ring to its edges on both sides.7,8 The DCDR spectra were therefore measured from the center of the ring where the Raman signal has the highest intensity. The DCDR spectra obtained from the drops of DPPC suspensions of 1 and 0.1 mg mL−1 concentrations are shown in Fig. 6 and 7, respectively. All DCDR spectra are of good quality and the positions of Raman bands correspond well with the previously published spectra of DPPC.5 Importantly, no Raman signal of the substrates and any undesirable backgrounds were observed in all DCDR spectra except for the CaF substrate, where one characteristic vibration at 322 cm−1 appeared. However, this band is outside the region of characteristic bands of DPPC and therefore does not interfere with the spectra interpretation. In the case of the surfaces in group A and the highest DPPC concentration, the DCDR spectra measured from both fluorocarbon surfaces show similar intensities but the Raman intensity drops about 2–2.5 times for Silane1 and Silane2 surfaces. This can be explained by the presence of a highly refractive metal (steel or aluminium) layer beneath the fluorocarbon film which increases the collected Raman signal, i.e. the effect reported also for the case of SERS based on nanostructured silver films.24 It seems that the efficiency of steel and aluminium to reflect Raman light is roughly the same. The Raman signal of DPPC on surfaces in group B is about 4 times lower than that on Teflon surfaces. The differences in Raman intensities between the various surfaces are even more pronounced at the lowest DPPC concentration. The two fluorocarbon surfaces provide again similar Raman intensities, while for glass slides coated with Silane1 and Silane2 the intensities decrease about 2–3 times. The Raman signal from the surfaces in group B is 8–10 times lower at the lowest DPPC concentration, compared to Teflon substrates.
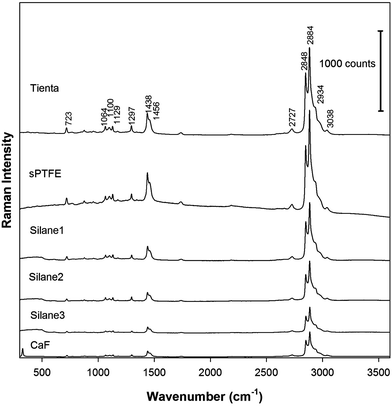 | ||
| Fig. 6 Raman spectra accumulated from the edge ring of the dried patterns. The concentration of DPPC in the drop was 1 mg mL−1. The spectra are shifted in the intensity scale for clarity. | ||
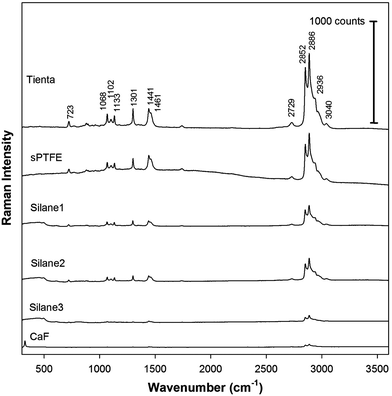 | ||
| Fig. 7 Raman spectra accumulated from the edge ring of the dried drop patterns with the initial DPPC concentration of 0.1 mg mL−1. The spectra are shifted in the intensity scale for clarity. | ||
To determine the ability of the substrates to preconcentrate DPPC in the edge of the ring, we compared the Raman signal from deposits of different DPPC concentrations on the same substrate. In the case of the surfaces in group B, the size of the deposited ring does not change significantly with the DPPC concentration in the initial solution. Therefore, when the concentration of DPPC is decreased 10 times (from 1 to 0.1 mg mL−1) the Raman signal also decreases 10 times (or more). The Raman signal obtained at the lowest concentration from the CaF surface is close to the limit of detection. On the other hand, in the case of the surfaces in group A, when the initial (deposited) concentration decreases 10 times (from 1 to 0.1 mg mL−1), a much smaller dried pattern is obtained and the overall intensity of the Raman signal decreases only by a factor of about 1.5–2 and 2–3 for fluorocarbon and Silane surfaces, respectively. Therefore, it is clearly seen that smaller dried patterns lead to more efficient preconcentration of DPPC in the ring. We can suggest that not all the material will reach the edge ring and stay inside the drop during drying. For bigger dried drops the distance is longer and less material will be found in the edge ring.
Conclusion
Various types of substrates differing in their wettability and the presence or absence of a metal base layer were studied as prospective substrates for DCDR spectroscopy: two types of fluorocarbon substrates, glass substrates modified with various silanes and polished CaF2. The evaporation of droplets of model phospholipid (DPPC) liposomes on all the tested surfaces created nonuniform patterns with a ring, known as the “coffee ring” effect. Fluorocarbon surfaces with a metal base layer were shown to have the highest contact angles with water. They provided the most efficient preconcentration of DPPC and therefore can be used for sensitive detection of low concentrations of DPPC by DCDR. Silanized glass slides with static contact angles of 110° and 90° provided a high DCDR signal as well. The advantage of these substrates is their simple and low-cost preparation without the need of expensive vacuum equipment. Surfaces with static contact angles less than 90° (silanized glass, CaF2) provided much worse preconcentration of DPPC and their applicability is therefore limited only to highly concentrated samples. Our work shows that there are numerous options available for DCDR substrates that can meet diverse demands and specific requirements of sensitivity, price, and ease of preparation for various applications.Acknowledgements
Financial support from the Czech Science Foundation (P205/12/G118) is gratefully acknowledged.References
- R. D. Deegan, O. Bakajin, T. F. Dupont, G. Huber, S. R. Nagel and T. A. Witten, Nature, 1997, 389, 827 CrossRef CAS.
- R. D. Deegan, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 61, 475 CrossRef CAS.
- R. D. Deegan, O. Bakajin, T. F. Dupont, G. Huber, S. R. Nagel and T. A. Witten, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 62, 756 CrossRef CAS.
- T. P. Bigioni, X. M. Lin, T. Nguyen, E. I. Corwin, T. A. Witten and H. M. Jaeger, Nat. Mater., 2006, 5, 265 CrossRef CAS PubMed.
- P. Šimáková, M. Procházka and E. Kočišová, Spectrosc. Int. J., 2012, 27, 449 CrossRef.
- D. Zhang, Y. Xie, M. F. Mrozek, C. Ortiz, V. J. Davisson and D. Ben-Amotz, Anal. Chem., 2003, 75, 5703 CrossRef CAS PubMed.
- V. Kopecký Jr. and V. Baumruk, Vib. Spectrosc., 2006, 42, 184 CrossRef.
- E. Kočišová and M. Procházka, J. Raman Spectrosc., 2011, 42, 1606 CrossRef.
- P. Šimáková, E. Kočišová and M. Procházka, J. Raman Spectrosc., 2013, 44, 1479 CrossRef.
- E. Kočišová and M. Procházka, J. Raman Spectrosc., 2015, 46, 280 CrossRef.
- J. Filik and N. Stone, Anal. Chim. Acta, 2008, 616, 177 CrossRef CAS PubMed.
- J. Filik and N. Stone, J. Raman Spectrosc., 2009, 40, 218 CrossRef CAS.
- P. Hu, X. S. Zheng, C. Zong, M. H. Li, L. Y. Zhang, W. Li and B. Ren, J. Raman Spectrosc., 2014, 45, 565 CrossRef CAS.
- M. F. Mrozek, D. Zhang and D. Ben-Amotz, Carbohydr. Res., 2004, 339, 141 CrossRef CAS PubMed.
- S. Abdolahzadeh, N. M. Boyle, A. Draksharapu, A. C. Dennis, R. Hage, J. W. de Boer and W. R. Browne, Analyst, 2013, 138, 3163 RSC.
- N. Shahidzadeh, M. F. L. Schut, J. Desarnaud, M. Prat and D. Bonn, Sci. Rep., 2015, 5, 10335 CrossRef PubMed.
- R. A. Halvorson, W. N. Leng and P. J. Vikesland, Anal. Chem., 2011, 83, 9273 CrossRef CAS PubMed.
- I. Barman, N. C. Dingari, J. W. Kang, G. L. Horowitz, R. R. Dasari and M. S. Feld, Anal. Chem., 2012, 84, 2474 CrossRef CAS PubMed.
- J. Filik and N. Stone, Analyst, 2007, 132, 544 RSC.
- E. Kočišová, M. Procházka and H. Šípová, J. Raman Spectrosc., 2016, 47, 1394 CrossRef.
- P. R. Carey, Biochemical Applications of Raman and Resonance Spectroscopies, Academic Press, New York, 1982 Search PubMed.
- O. Kylián, M. Petr, A. Serov, P. Solař, O. Polonskyi, J. Hanuš, A. Choukourov and H. Biederman, Vacuum, 2014, 100, 57 CrossRef.
- M. Petr, O. Kylián, J. Hanuš, A. Kuzminova, M. Vaidulych, I. Khalakhan, A. Choukourov, D. Slavínská and H. Biederman, Plasma Processes Polym., 2016, 13, 663 CrossRef CAS.
- M. Šubr, M. Petr, O. Kylián, J. Kratochvíl and M. Procházka, J. Mater. Chem. C, 2015, 3, 11478 RSC.
| This journal is © the Owner Societies 2017 |