An experimental and theoretical investigation of the C(1D) + D2 reaction†
Kevin M.
Hickson
*ab and
Yury V.
Suleimanov
*cd
aUniversité de Bordeaux, Institut des Sciences Moléculaires, F-33400 Talence, France. E-mail: km.hickson@ism.u-bordeaux1.fr
bCNRS, Institut des Sciences Moléculaires, F-33400 Talence, France
cComputation-based Science and Technology Research Center, Cyprus Institute, 20 Kavafi Str., Nicosia 2121, Cyprus
dDepartment of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Ave., Cambridge, Massachusetts 02139, USA. E-mail: ysuleyma@mit.edu
First published on 25th November 2016
Abstract
In a previous joint experimental and theoretical study of the barrierless chemical reaction C(1D) + H2 at low temperatures (300–50 K) [K. M. Hickson, J.-C. Loison, H. Guo and Y. V. Suleimanov, J. Phys. Chem. Lett., 2015, 6, 4194], excellent agreement was found between experimental thermal rate constants and theoretical estimates based on ring polymer molecular dynamics (RPMD) over the two lowest singlet potential energy surfaces (PESs). Here, we extend this work to one of its isotopologues, C(1D) + D2, over the same temperature range. Experimental and RPMD results are in good agreement when contributions from both PESs to this chemical reaction are included in the RPMD simulations. The deviation between experiment and the RPMD calculations does not exceed 25% and both results exhibit a slight negative temperature dependence. The first excited 1A′′ PES plays a more important role than the ground 1A′ PES as the temperature is decreased, similar to our previous studies of the C(1D) + H2 reaction but with a more pronounced effect. The small differences in temperature dependence between the earlier and present experimental studies of C(1D) + H2/D2 reactions are discussed in terms of the use of non-equilibrium populations of ortho/para-H2/D2. We argue that RPMD provides a very convenient and reliable tool to study low-temperature chemical reactions.
1 Introduction
Gas-phase reactions involving neutral carbon atoms are considered to be important in combustion systems, in the chemistry of planetary atmospheres and in the interstellar medium. Many experimental and theoretical studies1–13 of the reactivity of ground state C(3P) atoms have been performed, addressing their kinetic and dynamic aspects over a wide range of temperatures2–6,8,9 and collision energies9–13 and for a variety of different coreagent molecules. The reactivity of the excited 1D state of atomic carbon is generally less well understood, with the exception of the C(1D) + H2 reaction; a process which is simple enough to allow comparison between precise state-selected experimental measurements,14–16 quasi classical trajectory calculations17–19 and detailed quantum mechanical methods,20–29 even for several isotopic forms of the H2 molecule (H2, HD, D2).18,19,30–32 Although there are several experimental works following the dynamics of other C(1D) atom reactions under a range of conditions,13,33–36 few kinetic investigations are reported in the scientific literature. Of these, most are confined to room temperature.37–42 Indeed, the only temperature dependent measurements of the rate constants for C(1D) reactions to have been performed to date are those between C(1D) + CH3OH,7 C(1D) + H243 and the non-reactive quenching of C(1D) atoms with N2.44 Extending such studies to include other isotopic forms of the same molecule would allow us to look for interesting reactivity differences, thereby providing a rigorous test of the underlying potential energy surfaces (PESs).Previous room temperature kinetic studies of the C(1D) + H2, HD and D2 reactions42 demonstrated a noticeable isotope dependence of the rates of these processes, with the rate constant decreasing from H2 to HD to D2. Lin and Guo24 were able to reproduce the experimental results of Sato et al.42 using a wavepacket based statistical model over the lowest 1A′ PES, attributing the reactivity difference to a kinematic effect related to the increasing reduced mass. The recent joint experimental and theoretical investigation of the C(1D) + H2 reaction by Hickson et al.43 has cast some doubt on the reliability of these earlier experiments, measuring rate constants which are significantly larger than those measured by Sato et al.42 On the theoretical side, novel rate theory based on ring polymer molecular dynamics (RPMD)45 was implemented that demonstrated very reliable and predictable behavior in multifarious prototype cases as discussed in a recent review.46 Using the RPMD method, these authors43 were also able to demonstrate that the new experimental results could be well described if reaction occurred adiabatically over both the lowest 1A′ and first excited 1A′′ PESs, considering these surfaces to be uncoupled. Interestingly, Defazio et al. also studied the quantum dynamics of the C(1D) + H2, HD and D2 reactions over uncoupled 1A′ and 1A′′ PESs (although a slightly different fit was used to generate the 1A′ PES)17,20 using a quantum mechanical time dependent wavepacket method, also considering the permutation-inversion symmetry and the Coriolis interactions.22,32 They found that while the upper 1A′′ plays an important role in all these reactions, the reactive contribution of this surface is particularly large in the case of C(1D) + D2. Clearly, an investigation of the temperature dependent kinetics of the reactions of C(1D) atoms with other deuterated forms of hydrogen would provide an ideal opportunity to validate the earlier hypotheses of both Defazio et al.32 and Hickson et al.43 with regard to the role of the 1A′′ PES in these systems.
Here, we report the results of an experimental and theoretical investigation of the C(1D) + D2 reaction at low temperature. A supersonic flow reactor employing pulsed laser photolysis for C(1D) generation coupled with vacuum ultraviolet laser induced fluorescence detection of the deuterium atom products allows us to follow the reaction kinetics over the 50–296 K range. In parallel, RPMD simulations were carried out for the title reaction in the same temperature range. Sections 2 and 3 describe respectively the experimental and theoretical methods used in this work and the results which are further summarized in Section 4.
2 Experimental results
Measurements were performed using a continuous flow reactor, employing axisymmetric Laval nozzles to generate the cold supersonic flows. As this method is well described elsewhere,47,48 only features specific to the current work will be presented here. For the present investigation, it was impossible to use any of our Laval nozzles based on N2 as the carrier gas, as the C(1D) + N2 quenching reaction has been shown to become more efficient as the temperature falls.44 As a result, three nozzles employing the carrier gas argon were used, allowing temperatures of 50 K, 75 K and 127 K to be attained. Details of the flow characteristics of these three nozzles can be found in Table 1 of Grondin et al.49 By removing the Laval nozzle and by reducing the flow velocity, it was also possible to perform room temperature measurements (296 K) with argon as the carrier gas.| T/K | N | [D2]/1014 molecule cm−3 | k C(1D)+D2/cm3 molecule−1 s−1 |
|---|---|---|---|
| a Uncertainties on the calculated temperatures represent the statistical (1σ) errors obtained from Pitot tube measurements of the impact pressure. b Number of individual measurements. c Uncertainties on the measured rate constants represent the combined statistical and systematic errors as explained in the text. | |||
| 296 | 40 | 0.5–12.3 | (1.9 ± 0.2)c × 10−10 |
| 127 ± 2a | 40 | 0.4–9.9 | (2.0 ± 0.2) × 10−10 |
| 75 ± 2 | 41 | 0.4–5.7 | (2.0 ± 0.2) × 10−10 |
| 50 ± 1 | 35 | 0.6–9.0 | (2.1 ± 0.2) × 10−10 |
CBr4 was used as the source of C(1D) atoms in the experiments. These precursor molecules were carried into the reactor by a small argon flow passing over solid CBr4 held in a separate vessel at a known fixed pressure and temperature. This allowed us to estimate the concentration of CBr4 molecules within the supersonic flow to be less than 2 × 1013 molecule cm−3. The pulsed multiphoton dissociation of CBr4 at 266 nm produced both C(1D) and C(3P) atoms along the entire length of the supersonic flow.4–8,44 Although the major photolysis product is C(3P) atoms,7 the C(3P) + D2 → CD + D reaction can be neglected at room temperature and below due to its high endothermicity of 120 kJ mol−1. Due to inefficient gas-phase spin conversion, the D2 used in the present experiments was characterized by a fixed ortho/para ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at all temperatures. This can be contrasted with the expected value at 50 K, which should approach 4
1 at all temperatures. This can be contrasted with the expected value at 50 K, which should approach 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 assuming thermal equilibrium is attained. The D2 concentration was held in large excess with respect to C(1D) atoms in this work, so that pseudo-first-order conditions could be assumed. As C(1D) atoms could not be followed directly in the present experiments, product D(2S) atoms were detected instead through vacuum ultraviolet laser induced fluorescence (VUV LIF) at 121.534 nm. Light at this wavelength was generated by frequency doubling the 729.2 nm output of a pulsed dye laser, yielding a UV beam at 364.6 nm, which was then focused into a cell containing 210 Torr of Kr and 540 Torr of Ar, producing the required tunable VUV light by third harmonic generation. A magnesium fluoride lens positioned at the exit of the tripling cell allowed the VUV beam to be collimated and steered into the reactor where it was allowed to cross the supersonic flow. The on-resonance VUV emission from excited D-atoms within the flow were collected by a lithium fluoride lens and focused onto the photocathode of a solar blind photomultiplier tube.
1 assuming thermal equilibrium is attained. The D2 concentration was held in large excess with respect to C(1D) atoms in this work, so that pseudo-first-order conditions could be assumed. As C(1D) atoms could not be followed directly in the present experiments, product D(2S) atoms were detected instead through vacuum ultraviolet laser induced fluorescence (VUV LIF) at 121.534 nm. Light at this wavelength was generated by frequency doubling the 729.2 nm output of a pulsed dye laser, yielding a UV beam at 364.6 nm, which was then focused into a cell containing 210 Torr of Kr and 540 Torr of Ar, producing the required tunable VUV light by third harmonic generation. A magnesium fluoride lens positioned at the exit of the tripling cell allowed the VUV beam to be collimated and steered into the reactor where it was allowed to cross the supersonic flow. The on-resonance VUV emission from excited D-atoms within the flow were collected by a lithium fluoride lens and focused onto the photocathode of a solar blind photomultiplier tube.
None of the gases used in the experiments (Ar 99.999%, D2 99.8%, Kr 99.99%) were purified prior to use. Instead, these gases were flowed directly from cylinders into mass-flow controllers which provided precise control over the carrier gas, reagent gas and precursor concentrations within the supersonic flow. All mass-flow controllers were calibrated using the pressure rise at constant volume method for each individual gas used.
Typical product D(2S) VUV LIF intensity profiles, recorded as a function of delay time between photolysis and probe lasers at 50 K are displayed in Fig. 1.
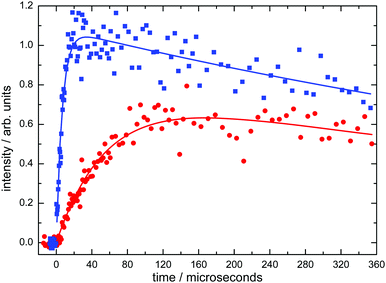 | ||
| Fig. 1 Typical D(2S) atom formation curves from the C(1D) + D2 → CD + D reaction recorded at 50 K. (Blue solid squares) [D2] = 6.8 × 1014 molecule cm−3; (red solid circles) [D2] = 6.0 × 1013 molecule cm−3. Solid lines represent biexponential fits to the data of the form given by eqn (1). | ||
These curves were readily described by a functional fit of the form
| ID = A{exp(−kL(D)t) − exp(−k′t)} | (1) |
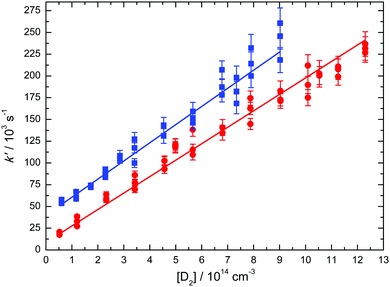
Decay curves were recorded at several D2 concentrations for any particular experiment, to yield a wide range of values for the first-order rate constant k′′. These k′ values were then plotted against the corresponding D2 concentration to yield the second-order rate constant at a given temperature from a weighted linear least squares fit to the data (weighting was performed using the statistical uncertainties generated by the biexponential fitting procedure outlined above). Examples of such plots obtained at 50 K and at 296 K are shown in Fig. 2.
The measured rate constants for the C(1D) + D2 reaction are summarized in Table 1 and displayed as a function of temperature in Fig. 3 alongside the present RPMD rate constants and the previous results, both theory and experiment for this process. We also include our previous experimental and theoretical results for the C(1D) + H2 reaction43 for comparison. Error bars on the present experimental values were derived by combining the statistical uncertainties obtained from second-order fits such as those shown in Fig. 2 with an estimated systematic error of 10% of the nominal rate constant value. This systematic error was considered to potentially arise from calibration errors in various pieces of equipment such as mass-flow controllers and pressure gauges in addition to possible errors in the flow density and velocity calculations.
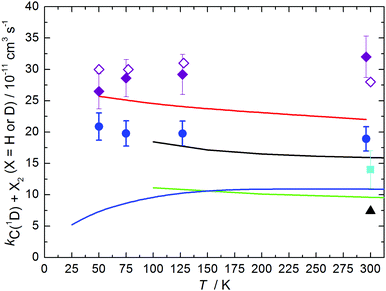 | ||
| Fig. 3 Rate constants for the C(1D) + X2 reactions as a function of temperature (X = H or D). Theoretical values for the C(1D) + D2 reaction: (solid black line) Defazio et al.;32 (solid green line) Joseph et al.;18 (solid blue line) Sun et al.;29 (black triangle) Lin and Guo;24 (solid red line) this work, RPMD method. Experimental values for the C(1D) + D2 reaction: (light blue squares) Sato et al.;42 (dark blue circles) this work, supersonic flow reactor. (Purple diamonds) experimental rate constants for the C(1D) + H2 reaction from Hickson et al.;43 (open purple diamonds) RPMD rate constants for the C(1D) + H2 reaction from Hickson et al.43 Uncertainties on the present experimental results are the combined statistical (1σ) and estimated systematic errors (10%). | ||
While the measured rate constants for the C(1D) + D2 reaction display only a very slight negative temperature dependence (independent of temperature considering the experimental error bars), it is interesting to note that our earlier measurements of the C(1D) + H2 reaction showed a slight positive temperature dependence.43 Although these effects are relatively minor, the differences could be due to the use of non-equilibrated mixtures of ortho/para-H2, -D2 in both studies. If the reactivity of the ortho and para forms of these isotopologues towards C(1D) is significantly different, this should induce the largest impact on the measured rate at the lowest temperatures where deviations from equilibrium are greatest. In the case of the C(1D) + H2 reaction, for such an effect to occur would require the j = 0 level of H2 (para-H2) to react more rapidly than the j = 1 level (ortho-H2) at the lowest temperatures. Consequently, the measured rates would be lower than expected due to an excess of ortho-H2 in these earlier experiments. Although an earlier time-dependent wavepacket study of the C(1D) + H2 reaction over uncoupled 1A′ and 1A′′ PESs22 concluded that the rotational state specific rate constant for the j = 0 level was approximately 15% higher than the corresponding j = 1 level at 300 K, the rate constant for the j = 0 level is seen to increase at 100 K whereas the value for the j = 1 level remains constant. As a result, it may be possible that the relative reactivity trends are inverted at even lower temperatures. In the case of the C(1D) + D2 reaction, a time-dependent wavepacket study indicated that the j = 0 (ortho) and j = 1 (para) levels of D2 displayed the same reactivity at 300 K.32 Unfortunately, no further information was provided with regard to the temperature dependence of the state specific rates for this system, making it impossible to determine whether the present measurements deviate from the expected values at equilibrium. Nevertheless, the temperature dependence of the RPMD results (which assume Boltzmann statistics for the rotational levels of D2) is in good agreement with the experimentally determined one, suggesting that the relative reactivity of j = 0 and j = 1 levels of D2 could be similar below 100 K. We will continue the discussion of the results presented in Fig. 3 in Section 4.
3 Theoretical results
The present theoretical simulations of the title reaction were performed using the RPMD method. This recently proposed45 semiclassical method takes into account quantum mechanical effects of nuclear motions via the so-called “classical isomorphism” between the quantum mechanical formalism of statistical mechanics and the classical one for the ring polymer with beads being classical copies of the original system connected with neighbors in the necklace via fictitious temperature-dependent harmonic interaction. The classical isomorphism is exact for various static properties but it was also shown to provide a reliable approximation to real-time Kubo-transformed correlation functions responsible for various dynamical properties.50 It has been found that RPMD provides very reliable estimates for real-time correlation functions used to calculate thermal rate constants.46,51–53 Using simple gas-phase chemical reactions,43,54–70 the method was thoroughly compared with rigorous quantum mechanical results (when possible), other dynamics approximations and experiment and its accuracy and reliability has been confirmed as described in a recent review by one of us (YVS).46 In particular, RPMD exhibits very reliable and predictable behavior for barrierless chemical reactions with deviation from the rigorous quantum dynamics results within the convergence error of the computational procedure (typically below 15%).60,61 As mentioned in the introduction, RPMD demonstrated an excellent agreement with experiment in our previous calculations for the C(1D) + H2 reaction in the temperature range 50–300 K.43In the present RPMD calculations we used the same computational strategy as in our previous study of the C(1D) + H2 reaction. All calculations were performed using the RPMD rate code.71 The parameters of the simulations are summarized in Table S1 of the ESI.† They are similar to the previous parameters for the C(1D) + H2 system, except that fewer beads were required to converge the RPMD rate constants for the title D-transfer reaction. As previously,43 we used the multi-reference configuration-interaction PESs of Bañares et al.17 for the ground state (1A′) and of Bussery-Honvault et al.21 for the first excited state (1A′′). Both PESs were obtained from ab initio data and are of insertion type with deep wells (4.32 and 3.46 eV relative to the reactants for 1A′ and 1A′′, respectively) and barrierless minimum energy paths in the entrance channel (perpendicularly constrained approach and bent approach of C towards D2 around 60° for 1A′ and 1A′′, respectively). As for the C(1D) + H2 reaction, we used the 1A′ and 1A′′ saddle points to initiate the RPMD rate calculations, though we note that the RPMD rate is rigorously independent of our choice of the initial transition state structure.52
The results of the RPMD rate simulations are summarized in Fig. 4 and 5 and in Table 2. Fig. 4 shows that for both PESs, ring polymer free energy profiles demonstrate small thermodynamically induced barriers near the entrance to the well which diminish as we decrease the temperature. The 1A′′ surface exhibits a more pronounced decrease. Fig. 5 shows that for the 1A′ PES, recrossing dynamics are significantly enhanced as the temperature is decreased. Though the free energy barrier decreases with the temperature, this does not compensate the decrease due to recrossing dynamics. As a result, the RPMD rate coefficient for the 1A′ PES decreases with decreasing temperature, see Table 2. For the 1A′′ PES, the temperature dependences of the ring polymer free energy barriers and the transmission coefficient are qualitatively the same, though the decrease in the plateau values of the ring polymer transmission coefficients is less pronounced and, as a result, the RPMD rate constant increases with the temperature. As a result, the rate constants for the 1A′ PES are higher than those for the 1A′′ one only at 300 K. At temperatures below 75 K, the rate constants for the 1A′′ PES are twice as large as those for the 1A′ PES.
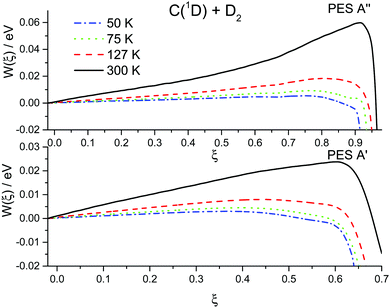 | ||
| Fig. 4 Ring polymer potential of mean force (free energy) for the C(1D) + D2 reaction at 50, 75, 127 and 300 K over two potential energy surfaces 1A′ (lower panel) and 1A′′ (upper panel). | ||
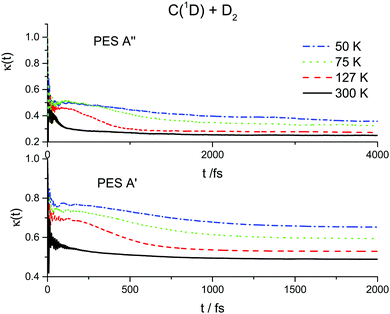 | ||
| Fig. 5 Ring polymer transmission coefficients for the C(1D) + D2 reaction at 50, 75, 127 and 300 K over two potential energy surfaces 1A′ (lower panel) and 1A′′ (upper panel). | ||
| T (K) | k QTST | κ plateau | k RPMD | k RPMD (corrected by Qel) |
|---|---|---|---|---|
| PES 1A′ | ||||
| 300 | 1.20(−09) | 0.489 | 5.84(−10) | 1.17(−10) |
| 127 | 9.43(−10) | 0.529 | 4.99(−10) | 9.98(−11) |
| 75 | 7.48(−10) | 0.594 | 4.44(−10) | 8.88(−11) |
| 50 | 6.16(−10) | 0.652 | 4.02(−10) | 8.04(−11) |
| PES 1A′′ | ||||
| 300 | 2.08(−09) | 0.247 | 5.14(−10) | 1.03(−10) |
| 127 | 2.51(−09) | 0.277 | 6.95(−10) | 1.39(−10) |
| 75 | 2.51(−09) | 0.323 | 8.11(−10) | 1.62(−10) |
| 50 | 2.47(−09) | 0.358 | 8.85(−10) | 1.77(−10) |
When compared to the previous theoretical results for the C(1D) + H2 chemical reaction,43 we note that the above temperature tendencies are similar to the previously observed ones. The final RPMD rate constants are smaller for C(1D) + D2 and, as expected this difference decreases with decreasing temperature (see also Fig. 3). However, the kinetic isotope effect depends on the PES: the rate constants are smaller for the C(1D) + D2 reaction compared to the C(1D) + H2 reaction by 20–40% for the 1A′ PES and by only 13–16% for the 1A′′ PES. The present results show that the 1A′′ PES contribution is more important for the C(1D) + D2 reaction, in line with the previous quantum mechanical time dependent wavepacket results.22,32 Clearly, the chemical dynamics of the title reaction exhibit high sensitivity to the underlying PESs which therefore require very accurate treatment.
4 Discussion and conclusions
Following our previous work on the C(1D) + H2 chemical reaction,43 in this paper we performed a joint experimental and theoretical study of its deuterated counterpart, C(1D) + D2, using the same experimental and theoretical techniques. Measurements were made using a continuous supersonic flow apparatus over the 50–296 K range. Due to the lack of an appropriate method for the direct detection of C(1D) atoms, rate constants were derived instead from product D(2S) atom formation curves. Theoretical results were obtained using novel rate theory based on ring polymer molecular dynamics (RPMD) which has deserved high confidence in the previous intensive examination of the method using prototype chemical reactions,43 including those with profiles of potential energy surfaces similar to the title reaction.Fig. 3 shows that the present RPMD and experimental results are in good agreement, with the former being higher than the latter by only 15–25% but correctly reproducing the very slight temperature dependence observed experimentally. Such deviations are slightly higher than the convergence error of the computational procedure43,71 or the typical error of RPMD observed for prototype insertion chemical reactions.43,60,61 We note that inaccuracy in the present RPMD results can be attributed to the sharp probability resonances found for the C(1D) + D2 reaction32 and which are not taken into account by the RPMD formalism.43 It can also be attributed to inaccuracies of the underlying potential energy surfaces (PESs) and the adiabatic limit used in the present description of the electronic structure (i.e., the two PESs were treated as being uncoupled), as was discussed in our previous study of C(1D) + H2.43 However, we note that the previous attempts to include non-adiabatic effects in calculations of the C(1D) + H2 reaction led to a strong reduction in the overall rate constant and an inversion of the temperature dependence compared to the adiabatic limit.43 Nonadiabatic RPMD studies for the C(1D) + X2 reactions with X = H or D are clearly desirable in the future, when nonadiabatic RPMD rate theory72,73 will become as robust and reliable as its original counterpart for single PESs.
Despite the small discrepancy, the present results demonstrate high consistency between theory and experiment. They confirm that both of the two lowest single PESs actively participate in the title reaction. In fact, the contribution from the first excited surface 1A′′ prevails at temperatures below 300 K, being twice as large as the contribution from the 1A′′ surface at 75–50 K. We note that the previous quantum mechanical time dependent wavepacket calculations, also obtained using two uncoupled PESs,32 exhibit similar accuracy though underestimating experimental rates, as can be seen from Fig. 3. Overall, the present RPMD results confirm the validity of this method for low-temperature studies of complex-forming reactions.
Acknowledgements
KMH acknowledges support from the French INSU/CNRS Programs ‘Physique et Chimie du Milieu Interstellaire’ (PCMI) and ‘Programme National de Planétologie’ (PNP). YVS thanks the European Regional Development Fund and the Republic of Cyprus for support through the Research Promotion Foundation (Project Cy-Tera NEA YΠOΔOMH/ΣTPATH/0308/31).References
- D. C. Clary, N. Haider, D. Husain and M. Kabir, Astrophys. J., 1994, 422, 416 CrossRef CAS.
- D. Chastaing, P. L. James, I. R. Sims and I. W. M. Smith, Phys. Chem. Chem. Phys., 1999, 1, 2247–2256 RSC.
- D. Chastaing, S. D. Le Picard and I. R. Sims, J. Chem. Phys., 2000, 112, 8466 CrossRef CAS.
- K. M. Hickson, J.-C. Loison, D. Nuñez-Reyes and R. Méreau, J. Phys. Chem. Lett., 2016, 7, 3641–3646 CrossRef CAS PubMed.
- K. M. Hickson, J.-C. Loison and V. Wakelam, Chem. Phys. Lett., 2016, 659, 70–75 CrossRef CAS.
- K. M. Hickson, J.-C. Loison, J. Bourgalais, M. Capron, S. D. L. Picard, F. Goulay and V. Wakelam, Astrophys. J., 2015, 812, 107 CrossRef.
- R. J. Shannon, C. Cossou, J.-C. Loison, P. Caubet, N. Balucani, P. W. Seakins, V. Wakelam and K. M. Hickson, RSC Adv., 2014, 4, 26342 RSC.
- J. Bourgalais, M. Capron, R. K. A. Kailasanathan, D. L. Osborn, K. M. Hickson, J.-C. Loison, V. Wakelam, F. Goulay and S. D. Le Picard, Astrophys. J., 2015, 812, 106 CrossRef.
- D. C. Clary, E. Buonomo, I. R. Sims, I. W. M. Smith, W. D. Geppert, C. Naulin, M. Costes, L. Cartechini and P. Casavecchia, J. Phys. Chem. A, 2002, 106, 5541–5552 CrossRef CAS.
- R. I. Kaiser, C. Ochsenfeld, M. Head-Gordon, Y. T. Lee and A. G. Suits, J. Chem. Phys., 1997, 106, 1729–1741 CrossRef CAS.
- M. Costes, P. Halvick, K. M. Hickson, N. Daugey and C. Naulin, Astrophys. J., 2009, 703, 1179–1187 CrossRef CAS.
- C. Naulin, N. Daugey, K. M. Hickson and M. Costes, J. Phys. Chem. A, 2009, 113, 14447–14457 CrossRef CAS PubMed.
- F. Leonori, R. Petrucci, E. Segoloni, A. Bergeat, K. M. Hickson, N. Balucani and P. Casavecchia, J. Phys. Chem. A, 2008, 112, 1363–1379 CrossRef CAS PubMed.
- N. Balucani, G. Capozza, L. Cartechini, A. Bergeat, R. Bobbenkamp, P. Casavecchia, F. J. Aoiz, L. Bañares, P. Honvault, B. Bussery-Honvault and J.-M. Launay, Phys. Chem. Chem. Phys., 2004, 6, 4957 RSC.
- N. Balucani, P. Casavecchia, F. J. Aoiz, L. Bañares, J.-M. Launay, B. Bussery-Honvault and P. Honvault, Mol. Phys., 2010, 108, 373–380 CrossRef CAS.
- A. Bergeat, L. Cartechini, N. Balucani, G. Capozza, L. F. Phillips, P. Casavecchia, G. G. Volpi, L. Bonnet and J. C. Rayez, Chem. Phys. Lett., 2000, 327, 197–202 CrossRef CAS.
- L. Bañares, F. J. Aoiz, S. A. Vázquez, T.-S. Ho and H. Rabitz, Chem. Phys. Lett., 2003, 374, 243–251 CrossRef.
- S. Joseph, P. J. S. B. Caridade and A. J. C. Varandas, J. Phys. Chem. A, 2011, 115, 7882–7890 CrossRef CAS PubMed.
- L. Kang and M. Zhu, J. Mol. Struct.: THEOCHEM, 2010, 945, 116–119 CrossRef CAS.
- B. Bussery-Honvault, P. Honvault and J. M. Launay, J. Chem. Phys., 2001, 115, 10701 CrossRef CAS.
- B. Bussery-Honvault, J. Julien, P. Honvault and J. M. Launay, Phys. Chem. Chem. Phys., 2005, 7, 1476 RSC.
- P. Defazio, C. Petrongolo, B. Bussery-Honvault and P. Honvault, J. Chem. Phys., 2009, 131, 114303 CrossRef PubMed.
- P. Defazio, B. Bussery-Honvault, P. Honvault and C. Petrongolo, J. Chem. Phys., 2011, 135, 114308 CrossRef PubMed.
- S. Y. Lin and H. Guo, J. Chem. Phys., 2004, 121, 1285–1292 CrossRef CAS PubMed.
- S. Y. Lin and H. Guo, J. Chem. Phys., 2003, 119, 11602 CrossRef CAS.
- S. Y. Lin and H. Guo, J. Phys. Chem. A, 2004, 108, 2141–2148 CrossRef CAS.
- J. Liu, B. Fu and D. Zhang, Chem. Phys. Lett., 2009, 480, 46–48 CrossRef CAS.
- Z. Shen, J. Cao and W. Bian, J. Chem. Phys., 2015, 142, 164309 CrossRef PubMed.
- Z. Sun, C. Zhang, S. Lin, Y. Zheng, Q. Meng and W. Bian, J. Chem. Phys., 2013, 139, 014306 CrossRef PubMed.
- N. Balucani, G. Capozza, E. Segoloni, A. Russo, R. Bobbenkamp, P. Casavecchia, T. Gonzalez-Lezana, E. J. Rackham, L. Banares and F. J. Aoiz, J. Chem. Phys., 2005, 122, 234309 CrossRef PubMed.
- F. Gogtas, N. Bulut and S. Akpinar, Int. J. Quantum Chem., 2005, 105, 478–484 CrossRef CAS.
- P. Defazio, P. Gamallo, M. González, S. Akpinar, B. Bussery-Honvault, P. Honvault and C. Petrongolo, J. Chem. Phys., 2010, 132, 104306 CrossRef PubMed.
- F. Leonori, D. Skouteris, R. Petrucci, P. Casavecchia, M. Rosi and N. Balucani, J. Chem. Phys., 2013, 138, 024311 CrossRef PubMed.
- D. C. Scott, J. De Juan, D. C. Robie, D. Schwartz-Lavi and H. Reisler, J. Phys. Chem., 1992, 96, 2509–2518 CrossRef CAS.
- G. M. Jursich and J. R. Wiesenfeld, Chem. Phys. Lett., 1984, 110, 14–19 CrossRef CAS.
- L. Cartechini, A. Bergeat, G. Capozza, P. Casavecchia, G. G. Volpi, W. D. Geppert, C. Naulin and M. Costes, J. Chem. Phys., 2002, 116, 5603 CrossRef CAS.
- R. J. Donovan and D. Husain, Chem. Rev., 1970, 70, 489–516 CrossRef CAS.
- D. Husain and L. J. Kirsch, Trans. Faraday Soc., 1971, 67, 2886–2895 RSC.
- D. Husain and L. J. Kirsch, Trans. Faraday Soc., 1971, 67, 3166–3175 RSC.
- W. H. Fisher, T. Carrington, C. M. Sadowski and C. H. Dugan, Chem. Phys., 1985, 97, 433–448 CrossRef CAS.
- D. Husain and L. J. Kirsch, Chem. Phys. Lett., 1971, 9, 412–415 CrossRef CAS.
- K. Sato, N. Ishida, T. Kurakata, A. Iwasaki and S. Tsunashima, Chem. Phys., 1998, 237, 195–204 CrossRef CAS.
- K. M. Hickson, J. C. Loison, H. Guo and Y. V. Suleimanov, J. Phys. Chem. Lett., 2015, 6, 4194–4199 CrossRef CAS PubMed.
- K. M. Hickson, J.-C. Loison, F. Lique and J. Kłos, J. Phys. Chem. A, 2016, 120, 2504–2513 CrossRef CAS PubMed.
- I. R. Craig and D. E. Manolopoulos, J. Chem. Phys., 2004, 121, 3368 CrossRef CAS PubMed.
- Y. V. Suleimanov, F. J. Aoiz and H. Guo, J. Phys. Chem. A, 2016, 120, 8488–8502 CrossRef CAS PubMed.
- N. Daugey, P. Caubet, B. Retail, M. Costes, A. Bergeat and G. Dorthe, Phys. Chem. Chem. Phys., 2005, 7, 2921–2927 RSC.
- N. Daugey, P. Caubet, A. Bergeat, M. Costes and K. M. Hickson, Phys. Chem. Chem. Phys., 2008, 10, 729–737 RSC.
- R. Grondin, J.-C. Loison and K. M. Hickson, J. Phys. Chem. A, 2016, 120, 4838–4844 CrossRef CAS PubMed.
- S. Habershon, D. E. Manolopoulos, T. E. Markland and T. F. Miller, Annu. Rev. Phys. Chem., 2013, 64, 387 CrossRef CAS PubMed.
- I. R. Craig and D. E. Manolopoulos, J. Chem. Phys., 2005, 122, 084106 CrossRef PubMed.
- I. R. Craig and D. E. Manolopoulos, J. Chem. Phys., 2005, 123, 034102 CrossRef PubMed.
- J. O. Richardson and S. C. Althorpe, J. Chem. Phys., 2009, 131, 214106 CrossRef PubMed.
- Y. V. Suleimanov, R. Collepardo-Guevara and D. E. Manolopoulos, J. Chem. Phys., 2011, 134, 044131 CrossRef PubMed.
- Y. Li, Y. V. Suleimanov, M. Yang, W. H. Green and H. Guo, J. Phys. Chem. Lett., 2013, 4, 48 CrossRef CAS PubMed.
- Y. Li, Y. V. Suleimanov, W. H. Green and H. Guo, J. Phys. Chem. A, 2014, 118, 1989 CrossRef CAS PubMed.
- R. Collepardo-Guevara, Y. V. Suleimanov and D. E. Manolopoulos, J. Chem. Phys., 2009, 130, 174713 CrossRef PubMed; R. Collepardo-Guevara, Y. V. Suleimanov and D. E. Manolopoulos, J. Chem. Phys., 2010, 133, 049902 CrossRef.
- R. Perez de Tudela, F. J. Aoiz, Y. V. Suleimanov and D. E. Manolopoulos, J. Phys. Chem. Lett., 2012, 3, 493 CrossRef CAS PubMed.
- Y. V. Suleimanov, R. Perez de Tudela, P. G. Jambrina, J. F. Castillo, V. Saez-Rabanos, D. E. Manolopoulos and F. J. Aoiz, Phys. Chem. Chem. Phys., 2013, 15, 3655 RSC.
- Y. Li, Y. V. Suleimanov and H. Guo, J. Phys. Chem. Lett., 2014, 5, 700 CrossRef CAS PubMed.
- Y. V. Suleimanov, W. J. Kong, H. Guo and W. H. Green, J. Chem. Phys., 2014, 141, 244103 CrossRef PubMed.
- Y. Li, Y. V. Suleimanov, J. Li, W. H. Green and H. Guo, J. Chem. Phys., 2013, 138, 094307 CrossRef PubMed.
- R. Pérez de Tudela, Y. V. Suleimanov, M. Menéndez, J. F. Castillo and F. J. Aoiz, Phys. Chem. Chem. Phys., 2014, 16, 2920 RSC.
- J. Espinosa-Garcia, A. Fernández-Ramos, Y. V. Suleimanov and J. C. Corchado, J. Phys. Chem. A, 2014, 118, 554 CrossRef CAS PubMed.
- Y. V. Suleimanov and J. Espinosa-Garcia, J. Phys. Chem. B, 2016, 120, 1418 CrossRef CAS PubMed.
- D. J. Arseneau, D. G. Fleming, Y. Li, J. Li, Y. V. Suleimanov and H. Guo, J. Phys. Chem. B, 2016, 120, 1641–1648 CrossRef CAS PubMed.
- Q. Meng, J. Chen and D. H. Zhang, J. Chem. Phys., 2015, 143, 101102 CrossRef PubMed.
- Q. Meng, J. Chen and D. H. Zhang, J. Chem. Phys., 2016, 144, 154312 CrossRef PubMed.
- J. Zuo, Y. Li, H. Guo and D. Xie, J. Phys. Chem. A, 2016, 120, 3433 CrossRef CAS PubMed.
- Q. Meng, K. M. Hickson, K. Shao, J.-C. Loison and D. H. Zhang, Phys. Chem. Chem. Phys., 2016, 18, 29286–29292 RSC.
- Y. V. Suleimanov, J. W. Allen and W. H. Green, Comput. Phys. Commun., 2013, 184, 833 CrossRef CAS.
- J. R. Duke and N. Ananth, J. Phys. Chem. Lett., 2015, 6, 4219–4223 CrossRef CAS PubMed.
- J. O. Richardson, P. Meyer, M.-O. Pleinert and M. Thoss, Chem. Phys., 2016 DOI:10.1016/j.chemphys.2016.09.036.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cp07381f |
| This journal is © the Owner Societies 2017 |