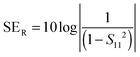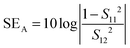Phase specific dispersion of functional nanoparticles in soft nanocomposites resulting in enhanced electromagnetic screening ability dominated by absorption
Aishwarya V.
Menon
a,
Giridhar
Madras
 b and
Suryasarathi
Bose
b and
Suryasarathi
Bose
 *c
*c
aCenter for Nano Science and Engineering, Indian Institute of Science, Bangalore-560012, India
bDepartment of Chemical Engineering, Indian Institute of Science, Bangalore-560012, India
cDepartment of Materials Engineering, Indian Institute of Science, Bangalore-560012, India. E-mail: sbose@materials.iisc.ernet.in
First published on 22nd November 2016
Abstract
The effect of phase specific localisation of MWNTs (multiwalled carbon nanotubes) and magnetic FeNi (iron–nickel) alloy particles on bulk electrical conductivity and electromagnetic (EM) wave attenuation was investigated in biphasic co-continuous blends of PVDF/SMA (polyvinylidene fluoride/styrene maleic anhydride). It is envisaged that packing different functional nanoparticles in a given phase of a co-continuous blend can impede the charge transport phenomenon and the overall dispersion state. Therefore, phase specific localisation can facilitate the tuning of the functional properties in biphasic blends. This strategy was adopted here wherein conducting MWNTs and magnetic FeNi particles were surface tailored to position them in different phases during processing. As the functional particles prefer the PVDF phase by virtue of thermodynamics, by harnessing amine functional moieties on the surface, their localisation can be tuned to position them in the SMA phase (due to amine–anhydride coupling). This was achieved by sequential mixing during processing. For the best combination, SET was observed to be −23 dB when MWNTs were localised in the SMA phase and magnetic particles in the PVDF phase of the blend with an impressive 92% absorption of the incident EM radiation.
Introduction
Electromagnetic interference (EMI) is a major concern due to the excessive usage of electronic equipment. They trigger electromagnetic induction which is manifested in the neighbouring electronic circuitry as stray fields or induced signals (voltage and current). EMI also has adverse effects on human health; therefore, several agencies have come up with standards and regulations for electromagnetic compatibility (EMC).1,2Over the years, several strategies have been used to reduce pollution caused due to EMI. Metals were the initial choices owing to their high conductivity. Metallic shields have been used to reflect EM waves due to the Faraday cage effect, which works on the principle of reorganisation of electric charges in the shield, that cancels the total electric field inside and outside the shield.3 But they posed several issues like weight, corrosion and processability issues.4 Faraday cages have no effect on magnetic fields. In order to avoid these difficulties polymer based nanocomposites containing functional nanoparticles were designed since they possess unique properties like easy processing, flexibility, economical advantage etc. These advantages make polymeric shields very accessible in the commercial sector. By dispersing various conducting fillers in the polymer matrix, it is possible to attenuate EM waves. However, conducting materials attenuate EMI by reflection, which is not desirable since it causes EMI pollution.5,6 In order to achieve attenuation by absorption, the shield should possess impedance very close to that of air. Therefore, various magnetic particles have been used to absorb EM waves.7
The most important properties to look for in a shield in order to attenuate EM waves are conductivity, permeability and permittivity. Several carbonaceous materials like carbon black, MWNTs, carbon fibers, GO (graphene oxide) etc. have been used for EMI shielding owing to their high conductivity/dielectric loss. MWNTs are a great choice owing to their high aspect ratio and capability to form a network-like structure even at low loadings. However, it is difficult to disperse them in the polymer matrix without getting agglomerated owing to van der Waals interaction between them.8 Several strategies like covalent and non-covalent functionalization techniques have been used to improve MWNT dispersion.9–11 Non-covalent techniques provide the advantage of preserving the integrity and functionalization of MWNTs without destroying their structure. Due to the double percolation effect, polymer blends have been used to provide shielding at low loadings of MWNTs.12,13
Several attempts have been made to introduce magnetic inclusion along with MWNTs in biphasic blends in order to tailor electromagnetic properties. Several ferromagnetic and dielectric materials have been used in this regard. However, it was observed that these particles tend to interfere with the MWNT network and therefore reduce the overall shielding effectiveness.14–16 Therefore, various other strategies like decoration of particles onto MWNTs or GO was also explored.17–20 This not only helped in the proper dispersion of nanoinclusions in the polymeric matrix but also helped to attenuate EM radiation by absorption owing to eddy current losses and natural resonance.21–23
In order to realise the effect of both MWNTs and magnetic nanoparticles in blends, it is required that the nanoparticles should not interfere with the MWNT network and also do not agglomerate due to interaction with each other. This will help in multiple scattering of EM waves because polarisation is caused when there are inclusions having different dielectric constants. This helps to absorb EM waves very efficiently.24–27
Therefore, keeping in mind the above-mentioned facts, we have made an attempt to incorporate both magnetic particles and MWNTs in the blend using a phase specific localization approach, wherein the localisation of these inclusions is tweaked by suitable surface chemistry to introduce functional moieties that can react with the available functional groups on the polymer during processing. This approach results even in the localization of particles in the less preferred phase. In order to assess how the properties and mechanism of shielding are affected due to incorporation of a particular nanoparticle in a polymer, we have surface functionalised the particles to restrict its localisation in the phase of our interest. PVDF/SMA is an immiscible blend system where due to high polarity of PVDF it is very difficult to restrict the migration of nanoparticles or MWNTs toward the PVDF phase.28 Therefore several approaches like the sequential mixing protocol and surface functionalization have been used to localise these inclusions in the SMA phase and observe its effect on EM wave attenuation.
Experimental section
Materials
PVDF (Kynar-761, Mw – 440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) was procured from Arkema. SMA (XIRAN SZ26080, Mw − 80
000 g mol−1) was procured from Arkema. SMA (XIRAN SZ26080, Mw − 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, maleic anhydride content – 26%) was kindly provided by Polyscope. Pristine MWNTs were procured from Reinste nanoventures. Commercial iron–nickel (FeNi), 80/20 alloy powder (having particle size of <5 μm) was procured from Industrial Metal Powders. 1-Aminopyrene, 3-aminopropyl triethoxy silane (APTS) and H2O2 (30% in water) were procured from Sigma. Other solvents used were obtained from commercial sources.
000, maleic anhydride content – 26%) was kindly provided by Polyscope. Pristine MWNTs were procured from Reinste nanoventures. Commercial iron–nickel (FeNi), 80/20 alloy powder (having particle size of <5 μm) was procured from Industrial Metal Powders. 1-Aminopyrene, 3-aminopropyl triethoxy silane (APTS) and H2O2 (30% in water) were procured from Sigma. Other solvents used were obtained from commercial sources.
Preparation of 1-aminopyrene modified MWNTs (APCNTs)
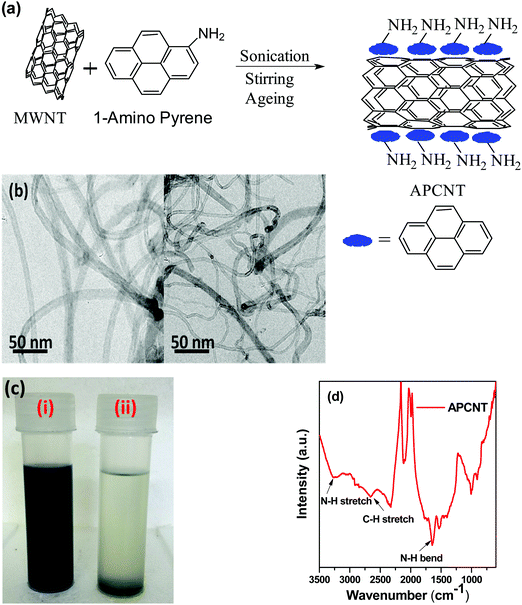
MWNTs were modified with 1-aminopyrene (1-AP) as illustrated in Fig. 1(a). In order to ensure good dispersion of unprocessed MWNTs, it is important to sonicate them in the presence of 1-aminopyrene so that modified MWNTs (APCNTs) exhibit no agglomeration. 500 mg of MWNTs was sonicated in 500 ml of ethanol along with 50 mg of 1-AP in a beaker for 1 h. The solution was then stirred for 12 h followed by ageing for another 8 h. The mixture was then washed with ethanol at least five times to remove the unbound 1-AP. The 1-AP modified MWNTs (APCNTs) so obtained were dried under vacuum overnight at 80 °C.Preparation of 3-aminopropyl triethoxy silane (APTS) coated FeNi particles
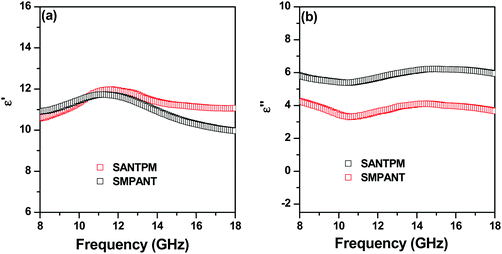
FeNi particles were coated with APTS using a two-step procedure as shown in Fig. 2(a).29 5 g of particles was added to 50 ml of H2O2. The mixture was kept in a hot water bath at 80 °C, followed by mechanical stirring for 3 h. The particles were then transferred to an ethanol/water mixture (95/5, v/v). To this mixture 5 ml of APTS solution was added, followed by mechanical stirring with sonication at 80 °C for another 4 h. Since the density of the particles was very high, mechanical stirring was opted as opposed to sonication. The modified particles were then washed repeatedly with ethanol five times to remove any unbound APTS. The APTS modified FeNi particles (APTS-FeNi) were then dried under vacuum at 60 °C for 8 h. | ||
| Fig. 2 (a) Schematic showing the preparation of APTS-FeNi; (b) VSM of FeNi particles; FT-IR spectra of (c) APTS-FeNi, (d) FeNi particles and (e) APTS-FeNi particles. | ||
Blend preparation
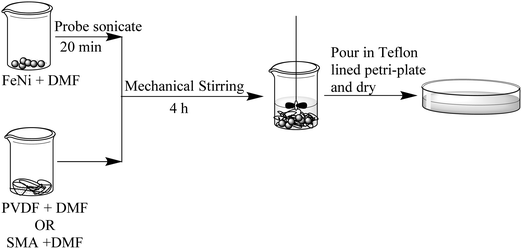
PVDF/SMA (60/40, w/w) was blended along with MWNTs and FeNi particles in a Haake minilab-II melt mixer at 250 °C at 60 rpm for 20 min under a nitrogen atmosphere. Initially MWNTs were added along with both the polymers and mixed for 20 min. A sequential mixing approach was used in order to localise MWNTs in the desired blend phase, wherein the desired polymer along with MWNTs or APCNTs was added into the mixer followed by shearing for 10 min. The other blend component was introduced into the mixer after 10 min and mixed for remaining 10 min without interrupting the mixing process.We have tried to localise the two particles, i.e. MWNTs and FeNi individually in each of the two blend phases. In order to localise FeNi in one of the phases and MWNTs in the other phase, we have adopted a two-step approach, as shown in Scheme 1, wherein PVDF or SMA was dissolved in DMF to make a viscous solution. FeNi particles were dispersed in DMF separately by probe sonication for 20 min. Both the solutions were mixed together followed by mechanical stirring for 4 h. This resulted in a viscous and well-dispersed solution of FeNi particles in a polymer matrix. The solution was thoroughly dried to remove DMF completely. This process ensured that FeNi particles were embedded in the polymer matrix. This was then introduced into the melt mixer during a sequential mixing approach. Various compositions that were prepared are listed in Table 1.
| Serial no. | Compositions (P: PVDF; S: SMA; MWNTs: NTs; aminopyrine modified CNTs: APCNTs; FeNi magnetic particles: M) |
|---|---|
| 1 | 60/40/1 wt% MWNTs (PSNT1) |
| 2 | 60/40/1 wt% APCNTs (PSANT1) |
| 3 | 60/40/1 wt% APCNTs (APCNTs + SMA mixed first) (SANT1P) |
| 4 | 60/40/3 wt% APCNTs (APCNTs + SMA mixed first) (SANT3P) |
| 5 | 60/40/5 wt% APCNTs (APCNTs + SMA mixed first) (SANT5P) |
| 6 | 60/40/1 wt% APCNTs (APCNTs + PVDF mixed first) (PANT1S) |
| 7 | 60/40/3 wt% APCNTs (APCNTs + PVDF mixed first) (PANT3S) |
| 8 | 60/40/5 wt% APCNTs (APCNTs + PVDF mixed first) (PANT5S) |
| 9 | 60/40/5 wt% APCNTs/30 wt% FeNi (APCNTs + SMA mixed first) (SANTPM) |
| 10 | 60/40/5 wt% APCNTs/30 wt% FeNi (APTS-FeNi + SMA mixed first) (SMPANT) |
Characterisation
Fourier transform infrared (FT-IR) spectroscopy was carried out using an UATR set up with a Perkin-Elmer GX in the range of 4000–400 cm−1 having a resolution of 4 cm−1. Morphology of the blends was analysed using a ZEISS ULTRA 55 field emission scanning electron microscope (FESEM) with an accelerating voltage of 5 kV. Prior to imaging, the blends were etched for 2 days in chloroform in order to remove the SMA phase. After that they were dried at 80 °C under vacuum. This increased the contrast in the images.In order to characterise MWNTs, transmission electron microscopy (TEM) images were acquired using a Tecnai T20 at 200 kV. The rheology of the blends was performed using a DHR-3 rheometer from TA instruments using parallel plate geometry (25 mm in diameter). All tests were performed under a nitrogen atmosphere to prevent any degradation.
Room temperature electrical conductivity of the blends was measured on 10 mm compression moulded discs using an Alpha-N Analyser, Novocontrol (Germany), in a frequency range of 0.1 Hz to 10 MHz. The EMI shielding characteristics of the as-prepared blends were studied in the X- and Ku-band frequency range using an Anritsu MS4642A vector network analyser (VNA) coupled to a coax (Damaskos M07T) set up on 5.5 mm thick toroidal specimens, obtained by compression moulding. The as-prepared samples had an outer diameter of 7 mm and an inner diameter of 3 mm.
Results and discussion
Characterisation of APCNTs and APTS coated FeNi
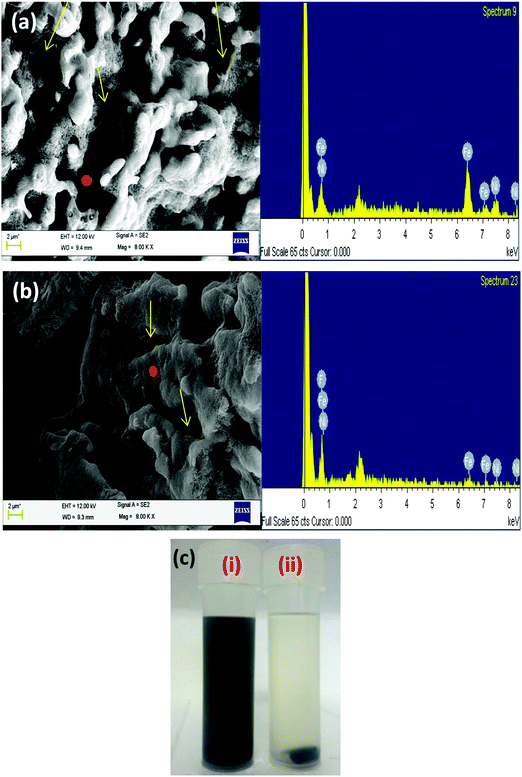
In order to localise MWNTs in the SMA phase of the blend, MWNTs were modified with aminopyrene since PVDF is the preferred phase for localisation of MWNTs. Amine functionalised MWNTs can chemically react with maleic anhydride of SMA during melt mixing and thereby facilitate the localization in the SMA phase rather than PVDF. Chemical modification of MWNTs can induce defects in them thereby reducing their conductivity. Therefore non-covalent functionalization was adopted in our study. Pyrene ring in 1-aminopyrene consists of a six membered aromatic ring which can interact with MWNTs via π–π interaction and therefore help reduce the van der Waals' interaction between MWNTs.30Fig. 1(b) presents the TEM images of pristine MWNTs (left panel) and APCNTs (right panel) which show the binding of aminopyrene to the surface of MWNTs. The TEM image clearly shows the binding of 1-AP moieties to MWNTs. Due to the hydrophobicity of MWNT side walls, they tend to form very poor dispersion in aqueous solutions. However, after modification with the amine functionality, the dispersion ability of MWNTs in solvent increases.31 This can be observed in Fig. 1(c) where both MWNTs and APCNTs were dispersed in THF by sonicating for 15 min. After 1 h of sonication, pristine MWNTs in vial (ii) settled at the bottom whereas the APCNTs in vial (i) formed a stable solution even after 2 weeks. Fig. 1(d) shows the FT-IR spectra of APCNTs. The peak at 3266 cm−1 represents the N–H stretching and the peak at 1640 cm−1 represents the N–H bending vibrations of the amine functionality of aminopyrene. The peak at 2659 cm−1 represents C–H stretching vibrations of alkanes arising from the pyrene interlinking chain of the amine group. The peak at 1522 cm−1 represents the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching frequency of both MWNTs and the pyrene moiety.31
C stretching frequency of both MWNTs and the pyrene moiety.31
As mentioned above, in order to tweak the localization of functional nanoparticles in the blends, suitable surface functional groups were introduced. In order to harness amine functional groups on FeNi particles, APTS was grafted onto the FeNi particles (as shown in Fig. 2a). Fig. 2(b) shows the VSM plot of FeNi magnetic particles. It is evident from the plot that these particles are highly magnetic in nature since they have a saturation magnetisation (Ms) of 120.9 emu g−1. Fig. 2(c) presents the FT-IR spectra of APTS modified FeNi particles. The peaks at 3301 cm−1 and 1012 cm−1 represent the N–H stretching vibrations of the amine functionality and the Si–O stretching vibrations, respectively, of APTS. The peaks at 2219 cm−1 and 2846 cm−1 represent the C–H stretching vibrations and the peak at 1109 cm−1 represents the C–N stretching vibrations of APTS.29,32 This confirms the binding of APTS to FeNi particles. APTS treatment is further confirmed in the TEM images shown in Fig. 2(d and e).
Morphology, dispersion and selective localisation
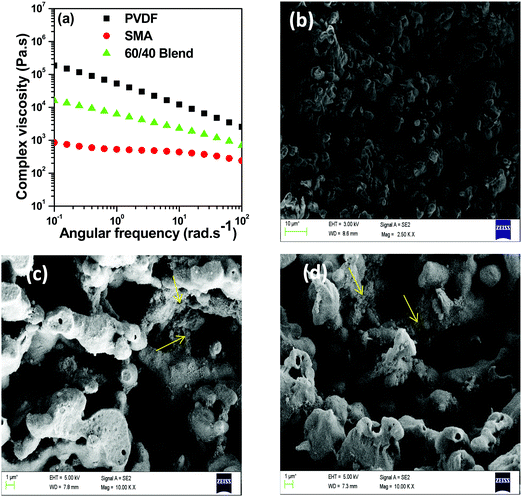
Table 2 shows the surface free energy data for PVDF, SMA and MWNTs at the processing temperature (250 °C). Evaluation of the wetting coefficient from the data shows that SMA is the preferred phase for localisation of MWNTs. Even from the complex viscosity data in Fig. 3(a) it can be seen that due to the lower viscosity of SMA compared to PVDF, SMA is the preferred phase for localisation of MWNTs. However, interestingly, it can be noted that the MWNTs localise in the PVDF phase of the blend (see SEM analysis). This is attributed to the high polarity of PVDF as compared to SMA.24,25 Localisation of MWNTs in PVDF can be well supported from the SEM images shown in Fig. 3b.In order to localise MWNTs in the SMA phase, AP modified MWNTs were used, since the amine moiety in APCNTs can react with the anhydride functional groups of SMA during melt mixing at 250 °C. This could help in arresting the MWNTs in the SMA phase of the blend. However, it was observed that few MWNTs migrated to the PVDF phase, which can be seen in the SEM images in Fig. 3(c). This can be attributed to the specific interaction between the amine group and PVDF.33 This specific interaction coupled with high polarity of PVDF drives some of the APCNTs to the PVDF phase; nevertheless, most of the APCNTs were positioned in the SMA phase. The rationale behind this approach was to fill the PVDF phase with magnetic particles (which is also the thermodynamically preferred phase) and the conducting MWNTs can be positioned in the SMA phase. This phase specific localization can help in better charge transport in the MWNT filled phase, especially the nomadic charges at the interface of the soft polymer phase and the conducting MWNT phase.
Therefore, in order to tune the localisation of APCNTs in SMA a sequential mixing protocol was employed. It has been reported earlier that sequential mixing can help in tuning the localisation of MWNTs in the phase of interest.37 This would ensure that the amine group in APCNTs reacted with the anhydride functionality in SMA. Similarly APCNTs were initially mixed with the PVDF phase and SMA was introduced later to assess this phase specific localization of MWNTs on the AC electrical conductivity and EM screening ability of the blends.
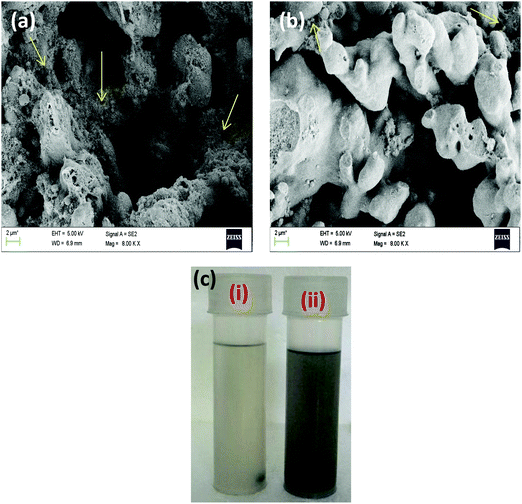
It was seen that sequential mixing was successful in localising MWNTs in the SMA phase, which otherwise migrates to the PVDF phase driven by thermodynamic factors. This can be seen from the SEM micrographs (Fig. 4a and b) and the selective dissolution experiment as shown in Fig. 4(c). However, in the SEM images it was seen that some APCNTs still migrated to the PVDF phase. Since maleic anhydride content per chain of SMA is only 26%, all of the APCNTs cannot react with SMA; therefore the unreacted APCNTs possibly migrated to the PVDF phase. The migration increases as the APCNT loading increases. When PVDF was added along with APCNTs in the first step of sequential mixing followed by SMA addition, it was seen that APCNTs were very well dispersed in the PVDF due to the specific interaction between them and the SMA phase was free of nanotubes.
In the next step magnetic particles were introduced into the blend along with APCNTs. Commercial FeNi particles were used for this purpose. It has been reported earlier that localisation of magnetic particles and MWNTs in different phases of the blend leads to better EM wave attenuation.24 Sequential mixing was used to localise the particles and MWNTs in different blend phases. It is well known that particles have high affinity for the highly polar PVDF phase; therefore, FeNi was modified with APTS in order to localise FeNi in the SMA phase of the blend, since the amine functionality in APTS-FeNi can react with maleic anhydride.38 Therefore, two compositions were developed wherein particles were localised in PVDF and APCNTs in SMA and vice versa.
As seen in Fig. 5(a and b) it was observed that sequential mixing was successful in localising the particles and APCNTs in the phase of interest. A selective dissolution experiment was carried out to etch SMA using chloroform, as shown in Fig. 5(c), which also proves that the particles and APCNTs were selectively localised in the required phase.
Effect of selective localisation on AC electrical conductivity
The AC electrical conductivity of blends was analysed as shown in Fig. 6. The AC electrical conductivity of composites gives information about the connectivity of the MWNT network. The electrical conductivity plot shows three types of behaviour.39 Below the percolation threshold, conductivity increases linearly as frequency increases. Below percolation, the composite behaves as a dielectric where conductivity follows the equation| σ = ωε′′ε0 | (1) |
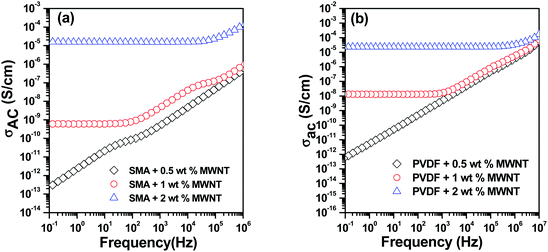
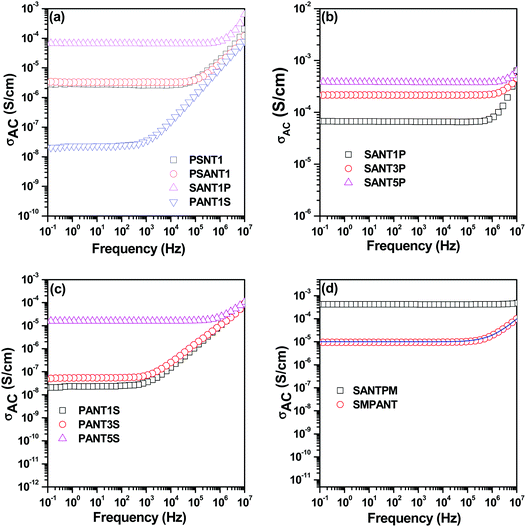
The surface modification of MWNTs by a covalent route creates defects on the MWNT surface which reduces the electrical conductivity. Therefore non-covalent attachment was preferred in order to retain the conductivity of MWNTs. From Fig. 7(a) it can be seen that conductivity values are similar when both APCNTs and MWNTs are added directly into the blend due to re-distribution of MWNNTs during mixing. However it can be seen that sequential mixing has a pronounced effect on dispersion of MWNTs in a particular phase. Localisation of APCNTs in the SMA phase leads to enhancement in conductivity values whereas localisation in PVDF leads to a slight reduction in conductivity. This is because of the difference in the dispersion state of MWNTs in both the blend phases. PVDF being a crystalline polymer results in additional complexity as the MWNNTs are rejected from the growing crystalline fronts whereas SMA being an amorphous polymer, the MWNTs are relatively well dispersed.40,41 Moreover the connectivity between MWNTs at 1 wt% MWNT concentration is much better in SMA compared to PVDF.
It is understood that increasing the MWNT concentration reduces the inter-tube distance, thereby facilitating efficient charge transport. It can be seen in Fig. 7(b) that increasing APCNT concentration in the SMA phase initially leads to remarkable improvement in conductivity but on further increasing the APCNT concentration the conductivity remains unaltered. This may be due to complete saturation of the SMA phase, which is in minor fraction with APCNTs or due to the possible migration of APCNTs to the PVDF phase. However, from Fig. 7(c) it can be seen that AC electrical conductivity scales with APCNT addition when APCNTs are localised in PVDF. A remarkable improvement in conductivity can be noticed at 5 wt% APCNT.
The addition of magnetic particles had no significant effect on the electrical conductivity of blends as can be analysed from Fig. 7(d). However, it can be seen that when particles were localised in the PVDF phase and APCNTs in the SMA phase the DC conductivity was not affected. Therefore, it can be said that the particles did not impede the conducting MWNT network. Moreover, it can be seen that the mechanism of charge transport was through tunnelling, since the conductivity plot shows a frequency independent plateau in the whole frequency range.
On the other hand, when particles were localised in SMA and MWNTs in PVDF, it was seen that the conductivity decreases. This may be attributed to the migration of particles to PVDF either due to high polarity of PVDF or saturation of the SMA phase. The universal power law42 can be employed in order to fit the frequency dependent AC conductivity to determine the equivalent network of resistors and capacitors in the blends.
| σ′(ω) = σ(0) + σac(ω) = σdc + Aωs | (2) |
EM shielding: effect of selective localisation on attenuation of EM radiation
EM shielding refers to the attenuation of EM waves (microwave radiation in this case) using electrically conducting materials or materials containing electric and/or magnetic dipoles. EM radiation consists of mutually perpendicular electric and magnetic components. Therefore, it is required that the material should be electrically conducting as well as magnetic in nature. There are three mechanisms by which a material can attenuate EM waves: reflection, multiple reflections and absorption. Total shielding effectiveness (SET) is the summation of shielding by reflection (SER), multiple reflections (SEMR) and absorption (SEA). It is expressed as| SET = SER + SEA + SEMR | (3) |
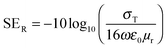 | (4) |
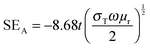 | (5) |
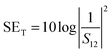 | (6) |
As shown in Fig. 8(a) when 1 wt% MWNTs and APCNTs were added directly into the blends it was observed that SET in both the cases was similar. However, when sequential mixing was done, the SET values showed a deviation. When APCNTs were localised in SMA, it was seen that SET was higher as compared to when APCNTs were localised in PVDF. This was evident from the conductivity data as well. This shows that dispersion of MWNTs in the blends plays a key role in attenuation of EM waves. As the APCNT concentration is increased from 1 wt% to 5 wt% in SMA, SET increases from −4.3 dB to −12.5 dB but when APCNT concentration is increased from 1 wt% to 5 wt% in PVDF, SET increases from −3.2 dB to −15.6 dB. This shows that when higher concentration of APCNTs is added to SMA there are chances of migration of APCNTs to the PVDF phase, thereby reducing shielding effectiveness. The increase in conductivity with MWNT loading is due to the formation of a strong inter-connecting network between MWNTs, which helps to easily carry mobile charges and thus attenuate EM waves by reflection. Also, as the MWNT concentration increases, there is a possibility of attenuation mechanism to be absorption due to the large specific surface area of MWNTs causing multiple scattering.24 Absorption arising from MWNTs is mainly due to polarisation, multiple scattering and ohmic losses.
On addition of particles using sequential mixing, it was seen that localisation of APCNTs in SMA and FeNi particles in PVDF leads to better EM wave attenuation as shown in Fig. 8(d). The presence of heterogeneities in the shield is a key factor influencing the scattering of microwave radiation, since they create multiple and large interfaces for scattering.47 When EM waves interact with macroscopic boundaries in the shield that are quite different in their electrical and magnetic properties, they eventually get absorbed. It is envisaged that absorption depends upon electric field intensity. Therefore, it can be said that localisation of both types of particles on different blend phases causes local field variation causing the EM wave to get absorbed. It is observed in Fig. 8(e and f) that the major mechanism of EM attenuation was absorption. The localisation of particles in PVDF and APCNTs in SMA gives a SET of −23 dB, while localisation of particles in SMA and APCNTs in PVDF gives a SET of only −17.5 dB. It was seen earlier that FeNi particles migrated to the PVDF phase in spite of sequential mixing. This causes these big particles to interfere with the MWNT network, causing a decrease in SET.
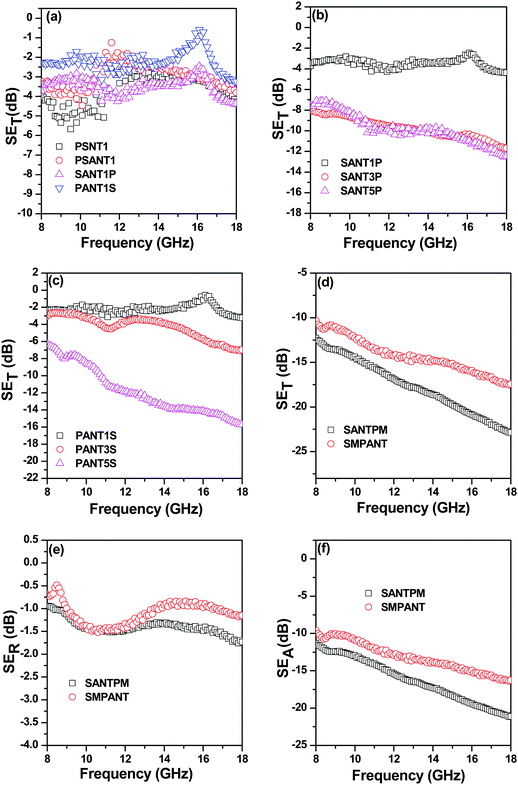
In order to obtain more insight into the mechanism of shielding, correlation between various electromagnetic attributes (complex permeability and complex permittivity) and the observed shielding response was established. The Nicolson Ross algorithm was used to evaluate complex permeability and permittivity from various scattering parameters. Fig. 9 represents complex permittivity (ε* = ε′ + jε′′) as a function of frequency. The real part of permittivity (ε′), also called the dielectric constant, represents the storage of electrical energy while the imaginary part (ε′′), also called dielectric loss, represents the dissipation of electrical energy. It is envisaged that both high dielectric loss values as well as dielectric constant values are desirable for absorption of EM waves. ε′ represents losses due to reorientation of the electric dipole as field changes direction, whereas ε′′ represents ohmic losses due to nomadic charge transfer through the shield.48 In Fig. 9 it is seen that both ε′ values are similar for both the composites; however ε′′ is higher for composites containing APCNTs in the SMA phase and FeNi particles in the PVDF phase. This is because in shields containing inclusions of varying dielectric constants, interfacial polarisation known as Maxwell–Wagner polarisation occurs due to accumulation of charges at the interface of two media having varying dielectric constants.49 In the other case when particles are in SMA and APCNTs in PVDF, migration of particles to the highly polar PVDF phase causes them to interfere with the MWNT network, increasing the contact resistance between neighbouring MWNTs.
Fig. 10 shows the complex permeability (μ* = μ′ + jμ′′) data for the composites as a function of frequency. In general, it is desirable that magnetic storage energy (μ′) should be low and magnetic dissipation energy (μ′′) should be high for the absorption of EM waves. High magnetic losses are responsible for EM wave attenuation.50 It can be seen that localisation of magnetic particles in either of the phases shows similar values of magnetic loss and magnetic storage energy.
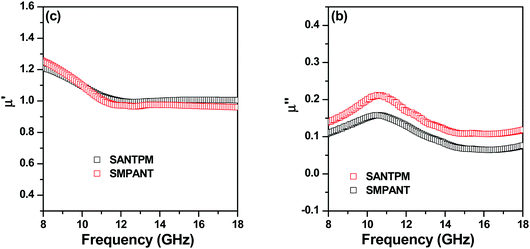 | ||
| Fig. 10 (a) Magnetic storage data. (b) Magnetic loss data for blends with 5 wt% APCNTs and 30 wt% FeNi. | ||
Taken together, our study clearly demonstrates that by adopting phase specific dispersion of nanoparticles (as shown schematically in Fig. 11), the EM shielding can be tuned and, moreover, the dominant mechanism is by absorption (93% in the case where magnetic nanoparticles are localized in the SMA phase and APNTs in the PVDF phase and 92% in the case wherein the magnetic particles are in the PVDF phase and APNTs in the SMA phase).
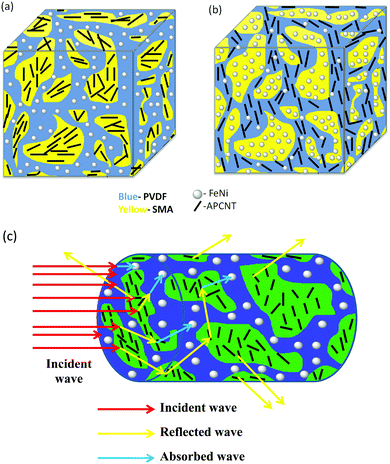 | ||
| Fig. 11 Schematic showing the (a) dispersion of MWNTs and FeNi in SANTPM. (b) Dispersion of MWNTs and FeNi in SMPANT. (c) Mechanism of shielding in blends. | ||
Conclusions
It can be summarised that selective localisation of various particles in appropriate blend phases has a profound influence on attenuation of EM waves. Due to crystalline morphology of PVDF, MWNTs agglomerate, thereby decreasing conductivity and SET values. Therefore, localising MWNTs by surface modification using a pyrene derivative would be appropriate to localise them in the SMA phase. However, due to inherent high polarity of PVDF, the APCNTs migrate to PVDF though only at higher concentrations. Therefore, a sequential mixing protocol was employed to selectively localise MWNTs in the phase of interest. This caused an improvement in SET and conductivity values. In order to attenuate EM waves, the presence of magnetic dipoles is a must; therefore commercial FeNi particles were employed. An attempt was made to compartmentalize the composites wherein different inclusions were selectively localised in each of the blend phases. It was seen that localisation of APCNTs in SMA and magnetic particles in PVDF would be the most desirable approach since interfacial polarisation causes absorption of EM waves. When magnetic particles are localised in SMA using APTS treatment, there is a chance that particles can still migrate to the more preferred PVDF phase. This causes them to disrupt the conducting MWNT network and reduce EM wave attenuation. Hence, by adopting phase specific dispersion of functional particles, the EM shielding can be tuned.Acknowledgements
The authors sincerely acknowledge Dr. Injamamul Arief for his insightful suggestions. The authors would like to acknowledge the Department of Science and Technology (DST), India, and JATP (IISc) for providing financial aid.References
- B. E. Keiser, Dedham, Mass., Artech House, Inc., 1979, p. 341 Search PubMed.
- M. Ramdani, E. Sicard, A. Boyer, S. B. Dhia, J. J. Whalen, T. H. Hubing, M. Coenen and O. Wada, IEEE Trans. Electromagn. Compat., 2009, 51, 78–100 CrossRef.
- J.-M. Thomassin, C. Jerome, T. Pardoen, C. Bailly, I. Huynen and C. Detrembleur, Mater. Sci. Eng., R, 2013, 74, 211–232 CrossRef.
- D. Chung, J. Mater. Eng. Perform., 2000, 9, 350–354 CrossRef CAS.
- R. Meena, S. Bhattachrya and R. Chatterjee, J. Magn. Magn. Mater., 2010, 322, 1923–1928 CrossRef CAS.
- H. Bayrakdar, J. Magn. Magn. Mater., 2011, 323, 1882–1885 CrossRef CAS.
- W.-L. Song, M.-S. Cao, B. Wen, Z.-L. Hou, J. Cheng and J. Yuan, Mater. Res. Bull., 2012, 47, 1747–1754 CrossRef CAS.
- N. Grossiord, J. Loos, O. Regev and C. E. Koning, Chem. Mater., 2006, 18, 1089–1099 CrossRef CAS.
- Y. Yang, M. C. Gupta, K. L. Dudley and R. W. Lawrence, Nano Lett., 2005, 5, 2131–2134 CrossRef CAS PubMed.
- N. Li, Y. Huang, F. Du, X. He, X. Lin, H. Gao, Y. Ma, F. Li, Y. Chen and P. C. Eklund, Nano Lett., 2006, 6, 1141–1145 CrossRef CAS PubMed.
- S. Bose, R. A. Khare and P. Moldenaers, Polymer, 2010, 51, 975–993 CrossRef CAS.
- M. Sumita, K. Sakata, Y. Hayakawa, S. Asai, K. Miyasaka and M. Tanemura, Colloid Polym. Sci., 1992, 270, 134–139 Search PubMed.
- C. Zhang, X.-S. Yi, H. Yui, S. Asai and M. Sumita, Mater. Lett., 1998, 36, 186–190 CrossRef CAS.
- H. Hekmatara, M. Seifi, K. Forooraghi and S. Mirzaee, Phys. Chem. Chem. Phys., 2014, 16, 24069–24075 RSC.
- J. Zhu, S. Wei, N. Haldolaarachchige, D. P. Young and Z. Guo, J. Phys. Chem. C, 2011, 115, 15304–15310 CrossRef CAS.
- G. P. Kar, S. Biswas, R. Rohini and S. Bose, J. Mater. Chem. A, 2015, 3, 7974–7985 RSC.
- J. WooáLee and S. BináKim, Nanoscale, 2011, 3, 3583–3585 RSC.
- B. Qu, C. Zhu, C. Li, X. Zhang and Y. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 3730–3735 Search PubMed.
- T. Wang, Z. Liu, M. Lu, B. Wen, Q. Ouyang, Y. Chen, C. Zhu, P. Gao, C. Li and M. Cao, J. Appl. Phys., 2013, 113, 024314 CrossRef.
- P.-B. Liu, Y. Huang and X. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 12355–12360 Search PubMed.
- S. P. Pawar, D. A. Marathe, K. Pattabhi and S. Bose, J. Mater. Chem. A, 2015, 3, 656–669 RSC.
- A. Kumar, A. P. Singh, S. Kumari, A. K. Srivastava, S. Bathula, S. K. Dhawan, P. K. Dutta and A. Dhar, J. Mater. Chem. A, 2015, 3, 13986–13993 RSC.
- Y. Ren, C. Zhu, S. Zhang, C. Li, Y. Chen, P. Gao, P. Yang and Q. Ouyang, Nanoscale, 2013, 5, 12296–12303 RSC.
- S. Biswas, G. P. Kar and S. Bose, Nanoscale, 2015, 7, 11334–11351 RSC.
- S. Biswas, G. P. Kar and S. Bose, J. Mater. Chem. A, 2015, 3, 12413–12426 RSC.
- Y.-L. Ren, H.-Y. Wu, M.-M. Lu, Y.-J. Chen, C.-L. Zhu, P. Gao, M.-S. Cao, C.-Y. Li and Q.-Y. Ouyang, ACS Appl. Mater. Interfaces, 2012, 4, 6436–6442 Search PubMed.
- P. Liu, Y. Huang, J. Yan and Y. Zhao, J. Mater. Chem. C, 2016, 4, 6362–6370 RSC.
- K. Goutam Prasanna, B. Sourav and B. Suryasarathi, Mater. Res. Express, 2016, 3, 064002 CrossRef.
- I. J. Bruce and T. Sen, Langmuir, 2005, 21, 7029–7035 CrossRef CAS PubMed.
- G. P. Kar, A. Karmakar and J. B. Baruah, J. Chem. Crystallogr., 2010, 40, 702–706 CrossRef CAS.
- H. Pang, J. Liu, D. Hu, X. Zhang and J. Chen, Electrochim. Acta, 2010, 55, 6611–6616 CrossRef CAS.
- C. Weigel and R. Kellner, Fresenius' Z. Anal. Chem., 1989, 335, 663–668 CrossRef CAS.
- M. Sharma, G. Madras and S. Bose, Phys. Chem. Chem. Phys., 2014, 16, 23421–23430 RSC.
- H. Kaczmarek, A. Felczak and A. Szalla, Polym. Degrad. Stab., 2008, 93, 1259–1266 CrossRef CAS.
- A. Pustak, M. Denac, M. Leskovac, I. Švab, V. Musil and I. Šmit, Polym.-Plast. Technol. Eng., 2015, 54, 647–660 CrossRef CAS.
- S. Nuriel, L. Liu, A. Barber and H. Wagner, Chem. Phys. Lett., 2005, 404, 263–266 CrossRef CAS.
- S. Bose, A. R. Bhattacharyya, A. R. Kulkarni and P. Pötschke, Compos. Sci. Technol., 2009, 69, 365–372 CrossRef CAS.
- S. Biswas, G. P. Kar and S. Bose, Phys. Chem. Chem. Phys., 2015, 17, 27698–27712 RSC.
- Y. J. Kim, T. S. Shin, H. Do Choi, J. H. Kwon, Y.-C. Chung and H. G. Yoon, Carbon, 2005, 43, 23–30 CrossRef CAS.
- X.-L. Xie, Y.-W. Mai and X.-P. Zhou, Mater. Sci. Eng., R, 2005, 49, 89–112 CrossRef.
- E. D. Laird and C. Y. Li, Macromolecules, 2013, 46, 2877–2891 CrossRef CAS.
- K. Ngai, C. White and A. Jonscher, Nature, 1979, 277, 185–189 CrossRef CAS.
- D. Chung, Carbon, 2001, 39, 279–285 CrossRef CAS.
- Z. Liu, G. Bai, Y. Huang, Y. Ma, F. Du, F. Li, T. Guo and Y. Chen, Carbon, 2007, 45, 821–827 CrossRef CAS.
- P. Saini, V. Choudhary, B. Singh, R. Mathur and S. Dhawan, Synth. Met., 2011, 161, 1522–1526 CrossRef CAS.
- S. P. Pawar, V. Bhingardive, A. Jadhav and S. Bose, RSC Adv., 2015, 5, 89461–89471 RSC.
- S. P. Pawar, M. Gandi, C. Saraf and S. Bose, J. Mater. Chem. C, 2016, 4, 4954–4966 RSC.
- S. P. Pawar, M. Gandi and S. Bose, RSC Adv., 2016, 6, 37633–37645 RSC.
- S. P. Pawar, S. Stephen, S. Bose and V. Mittal, Phys. Chem. Chem. Phys., 2015, 17, 14922–14930 RSC.
- S. Biswas, G. P. Kar and S. Bose, ACS Appl. Mater. Interfaces, 2015, 7, 25448–25463 Search PubMed.
| This journal is © the Owner Societies 2017 |