Designing hierarchical hollow nanostructures of Cu2MoS4 for improved hydrogen evolution reaction†
Ke
Zhang
a,
Yongli
Zheng
b,
Yunxiang
Lin
a,
Changda
Wang
a,
Hengjie
Liu
a,
Daobin
Liu
a,
Chuanqiang
Wu
a,
Shuangming
Chen
a,
Yanxia
Chen
*b and
Li
Song
 *a
*a
aNational Synchrotron Radiation Laboratory, CAS Center for Excellence in Nanoscience, University of Science and Technology of China, Hefei, Anhui 230029, China. E-mail: song2012@ ustc.edu.cn
bHefei National Laboratory for Physical Science at Microscale and Department of Chemical Physics, University of Science and Technology of China, Hefei, Anhui 230026, China. E-mail: yachen@ustc.edu.cn
First published on 29th November 2016
Abstract
Layered Cu2MoS4, consisting of earth-abundant elements, is regarded as a potential catalyst for the hydrogen evolution reaction (HER). Herein, we demonstrate a Cu2O-based template strategy to synthesise hierarchical hollow nanostructures of Cu2MoS4. The characterizations reveal that the electrochemically active surface of the hollow Cu2MoS4 is largely enhanced, in contrast to the nanosheet or nanoparticle structures. As the direct outcome, the designed hierarchical hollow structures display excellent HER activities with a low overvoltage and small Tafel slope. This study may provide new inspiration for the research of other ternary sulphide materials as well as subsequently accelerating their applications in the field of catalysis.
Introduction
The overuse of fossil fuels and the continuous deterioration of the environment have seriously hindered the sustainable development of the modern society.1 It is urgent to explore new ways to face these challenges. Hydrogen, as a clean energy source, has been considered as one of the most promising candidates to replace traditional energies and to help human beings to solve the above problems in the future.2 In the past few decades, tremendous efforts had been carried out to develop effective approaches for practical hydrogen production.3 Among these, the electrocatalytic hydrogen evolution reaction (HER) is treated as a feasible and important method. Pt and its alloys are the best known HER catalysts with low overpotentials and high current densities.4 However, high costs and scarce resources makes it difficult for them to be widely used in practical applications. So it's necessary to explore new noble-metal-free HER electrocatalysts. Up to now, much attention has been focused on exploiting earth-abundant solid-state inorganic materials for HER; for example, transition metal alloys, carbides, nitrides, phosphides, sulphides, and so on.5–9Recently, increasing interest has been devoted to two dimensional (2D) transition metal chalcogenide (TMC) nanomaterials which display exceptional mechanical, catalytic, optical and electronic properties, due to both their layered structure and the presence of transition metal d-electrons.10 Many binary TMCs have been widely investigated, such as MoS2, TiS2, WS2, MoSe2, etc.11 The above TMCs play important roles for HER. It was demonstrated that MoS2 nanoparticles exhibit excellent HER activities, mainly owing to the number of exposed edge sites which were suggested to be catalytically active.12 But as far as we know, there is little research about the synthesis and applications for ternary layered TMCs.13 Inspired by the great success of binary TMCs, it is significant to develop new methods to prepare ternary layered TMCs for further applications. Cu2MoS4 is a new ternary layered TMC material, which has two allotropes with the space groups: P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m and I
2m and I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m, respectively.14 Previous reports have shown that Cu2MoS4 would be a promising HER electrocatalyst because of its low overvoltage requirement and good stability in an aqueous solution with a wide pH range.15,16 However, just like MoS2 or its analogues, the exposure of the catalytically active sites of Cu2MoS4 is the bottleneck. Herein, we develop a new hard-template strategy to controllably fabricate Cu2MoS4 hierarchical hollow nanostructures (HHNSs), and it is found that the synthetic Cu2MoS4 HHNSs perform well for HER catalysis.
2m, respectively.14 Previous reports have shown that Cu2MoS4 would be a promising HER electrocatalyst because of its low overvoltage requirement and good stability in an aqueous solution with a wide pH range.15,16 However, just like MoS2 or its analogues, the exposure of the catalytically active sites of Cu2MoS4 is the bottleneck. Herein, we develop a new hard-template strategy to controllably fabricate Cu2MoS4 hierarchical hollow nanostructures (HHNSs), and it is found that the synthetic Cu2MoS4 HHNSs perform well for HER catalysis.
Cu2MoS4 HHNSs are prepared by a solvothermal method where Cu2O nanocrystals are used as sacrificial templates. Cu2O, a p-type semiconductor (2.2 eV), has been widely used in photocatalysis, solar cells or other areas by the controllable synthesis of Cu2O.17,18 What's more, as excellent sacrificial templates, Cu2O nanocrystals have been widely adopted to prepare many kinds of hollow nanostructures for various applications.19 We adopt Cu2O as the copper source, as well as the template, with sodium molybdate (Na2MoO4) and thioacetamide (C2H5NS) as the molybdenum and sulfur source, respectively. All three chemicals are environmentally-friendly and low-priced. In addition, we use ethylene glycol (EG) as a solvent, which is widely adopted to synthesize a variety of nanocrystals, such as noble metals, transition metal oxides and sulfides.20 On the other hand, the reducibility of EG can prevent the oxidation of Cu+ effectively. It has been demonstrated that the proposed approach is practical to obtain high quality Cu2MoS4 nanostructures.21
Experimental
Preparation of Cu2MoS4 hierarchical hollow nanostructures
To prepare Cu2MoS4 hierarchical hollow nanostructures, the Cu2O template was firstly prepared according to previous research.22 3.3 mg the Cu2O powder was dispersed in 20 mL ethylene glycol with stirring for 10 min. Then 10 mg thioacetamide and 5 mg sodium molybdate were added to the mixed solution. After 30 min stirring, the solution was transferred to a 45 mL Teflon-lined autoclave and heated at 160 °C for 10 h. The products were centrifuged and washed with deionized water and pure ethanol several times. Finally, the samples were dried at 60 °C under vacuum for further use.Characterization of the synthetic samples
X-ray powder diffraction (XRD) patterns were carried out by a RigakuTTR-III X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å). The elemental valence of the as-prepared products were characterized by X-ray photoelectron spectroscopy (XPS, ThermoESCALAB250). The morphologies of the samples were obtained by using scanning electron microscopy (SEM, JSM-6700F, 5 kV) and transmission electron microscopy (TEM, JEOL JEM-2100F microscope, 200 kV).Electrochemical measurements
A conventional three-electrode cell with a workstation (CHI 660E) was used to carried out the electrochemical measurements. In a typical process, 4 mg of the sample mixed with 30 μL Nafion solution (Sigma Aldrich, 5 wt%) was dispersed in a mixture of 750 μL Milli-Q water and 250 μL ethanol for at least 1 h. Then, 5 μL of this ink was dropped onto a glassy carbon electrode (GCE) with a diameter of 3 mm to form a catalyst loading of 0.285 mg cm−2. The reference electrode was an Ag/AgCl (3.0 M KCl) electrode and we used a graphite rod as the counter electrode. Linear sweep voltammetry was recorded with a scan rate of 5 mV s−1 in 0.5 M H2SO4 (purged with pure N2 for 30 min). All the potentials in this work were quoted against a reversible hydrogen electrode (RHE) unless noted otherwise. The electrochemical active surface areas (ECSA) were determined by taking cyclic voltammetry measurements at various scan rates ranging from 20 to 120 mV s−1 in the potential window of −0.10 to 0.10 V versus Ag/AgCl. Then, linear fitting of the charging current density differences (Δj = ja − jc) at the potential of 0.00 V versus Ag/AgCl was carried out. The linear slope is twice that of the double-layer capacitance Cdl. All the above measurements were carried out without ohmic-drop correction.Results and discussion
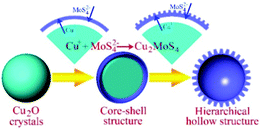
The synthetic procedure for Cu2MoS4 HHNSs is shown in Fig. 1. In our design, the Kirkendall effect plays an important role in the formation process. It means that the MoS42− ions in the solution diffuse onto the surface of the Cu2O nanocrystals, while the Cu+ cations migrate from Cu2O to the forming Cu2MoS4 interface, and this exchange reaction is thermodynamically favourable. The lasting supply of MoS42− dianions from the solution phase is an important factor for the formation of Cu2MoS4 HHNSs. In the solvothermal reaction system, MoS42− is derived according to the following reaction:| MoO42− + C2H5NS → MoS42− + NH4+ + OH− |
The concentration of MoS42− is controlled by the reaction above. Because the reaction itself takes time, a longer presence of this anion is anticipated for the formation of the shell. Owing to the simultaneous etching effect in the reaction solution, hierarchical structures are obtained.
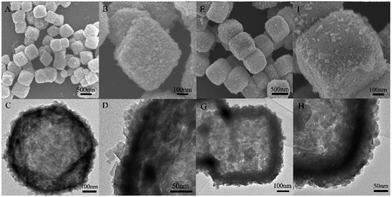
As expected, the morphology and size of the Cu2O template have a significant effect on the final product of Cu2MoS4 HHNSs. We prepared different Cu2O nanocrystals by tuning the reaction conditions (ESI,† Table S1). The SEM and TEM images of the synthetic Cu2O samples are shown in Fig. S1 (ESI†). It is observed that hollow nanoflowers are obtained when Cu2O nanospheres with a size of about 60–80 nm are used (shown in Fig. 2). These hollow nanoflowers have a diameter of about 100–150 nm and they are composed of many primary nanocrystals with a size of about 20–30 nm, according to the SEM (Fig. 2A) and TEM (Fig. 2B and C) images. The HRTEM image is also given in Fig. 2D and the measured lattice distance is 5.39 Å, corresponding to the (010) face of Cu2MoS4 with a P-phase. Cu2MoS4 HHNSs with other morphologies and sizes were also constructed, such as octahedral HHNSs and cubic HHNSs, which are shown in Fig. 3. Herein, we select the hollow nanoflower to be the reference, in order to investigate the features and potential applications of the series of Cu2MoS4 HHNSs. In addition, Cu2MoS4 nanosheets and nanoparticles were also prepared as comparative samples by a method that was proposed in a previous work (ESI,† Fig. S2).21,23
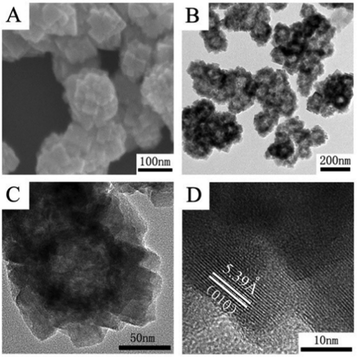 | ||
| Fig. 2 The morphologies and structures of Cu2MoS4 hollow nanoflowers. (A) SEM image; (B and C) TEM images with different magnifications; (D) high resolution TEM image. | ||
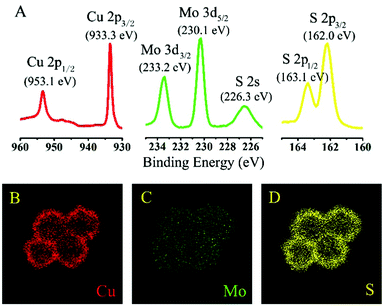
The composition and elemental valence state of the synthetic flower-like sample were obtained using X-ray photoelectron spectroscopy (XPS). Fig. 4A gives the binding energies of Cu 2p, Mo 3d and S 2p, and the data suggests that the elemental valence states of Cu, Mo and S are +1, +6 and −2 valences, respectively. The energy dispersive X-ray (EDX) elemental mapping results (Fig. 3B–D and ESI,† Fig. S3) for Cu2MoS4 hollow nanoflowers suggest that these three elements in the samples are homogeneously distributed.
The structures of the Cu2MoS4 samples (hollow nanoflower, nanosheet and nanoparticle) were analyzed by XRD and the patterns are shown in Fig. 5. It reveals that the as-prepared Cu2MoS4 hollow nanoflower and nanoparticle structures are both P-phase, while the nanosheet is I-phase. The diffraction peaks of the nanosheet pattern are strong and sharp, suggesting that the nanosheets are well-crystalline. The accurate structure of the I-Cu2MoS4 nanosheets has been defined in previous work.21 Owing to the reduced size all of the diffraction peaks of the Cu2MoS4 nanoparticle, and especially those of the hollow nanoflower, are broadened to some degree. Combined with the SEM and TEM data, the XRD results indicate that the primary particles of the hollow nanoflowers are smaller than the simple nanoparticles. In addition, it can be seen that the (001) peak of the nanoparticle sample is stronger than the (010) peak. However, it is quite different for the hollow nanoflower, whose (001) peak almost disappears, while the (010) feature is obvious. A similar result was also found in previous work.23
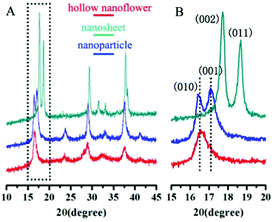 | ||
| Fig. 5 XRD patterns of Cu2MoS4 nanostructures: hollow nanoflower (P phase), nanosheet (I phase) and nanoparticle (P phase). | ||
The hydrogen evolution reaction (HER) was carried out to demonstrate the potential application of these Cu2MoS4 HHNSs in the H2 energy field. The HER activities of the Cu2MoS4 hollow nanoflower, nanosheet and nanoparticle structures were investigated in 0.5 M H2SO4 solution using a typical three-electrode setup. The result is shown in Fig. 6 and Table 1. The polarization curve from the Cu2MoS4 hollow nanoflower displays an onset overpotential of −165 mV for HER, and this is lower than that of the nanoparticle and nanosheet. What's more, the Tafel slope of the hollow nanoflower is also the smallest among the three samples, and is only 64 mV dec−1. The electrochemical results indicate that the Cu2MoS4 hollow nanoflower exhibits better HER activity than the nanosheet and the nanoparticle. In addition, the durability of the Cu2MoS4 samples was assessed by an accelerated cyclic test, from −0.3 V to 0.2 V vs. RHE for 1000 cycles. It was found (shown in the ESI,† Fig. S4) that the polarization curve of the nanosheet catalyst shows no evident change from the initial test after continuous long-term cycling in an acidic condition. However, the current density of both the nanoflower and the nanoparticle degrades to some degree after 1000 CV cycles. Nevertheless, the nanoflower showed better stability than the nanoparticle.
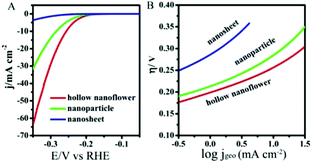 | ||
| Fig. 6 The polarization (A) and Tafel (B) curves of HER electrodes composed of Cu2MoS4 hollow nanoflowers, nanosheets and nanoparticles. Electrolyte: 0.5 M H2SO4, scan rate: 5 mV s−1. | ||
| Catalyst | Onset potential [mV] | Tafel slope [mV dec−1] |
|---|---|---|
| Hollow nanoflower | −165 | 64 |
| Nanoparticle | −175 | 71 |
| Nanosheet | −240 | 98 |
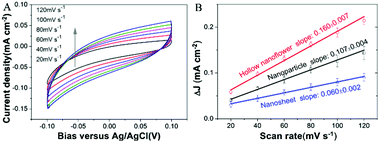
Based on Tran's research,15 Cu2MoS4 is confirmed to act as an active catalyst, not as a precursor of MoS2 or MoS3, and Cu2MoS4 remains intact under the catalytic hydrogen evolution condition. Regardless of the two potential intermediate states for Cu2MoS4: [H-MoVI(μ-SH)CuI] and [MoV{(μ-SH)CuI}2], the key point lies in the exposure of potential active sites, which induces the activity difference of the Cu2MoS4 hollow nanoflower, nanosheet and nanoparticle. To better understand the different catalytic performances of these three types of Cu2MoS4 nanostructure, we further compared the ECSA (electrochemically active surface areas) of these three samples using their double-layer capacitances (Cdl), as shown in Fig. 7. The ECSA of the hollow nanoflower is 0.160 ± 0.007 mF cm−2, which is 1.5-fold the ECSA of the nanoparticle and 2.7-fold the ECSA of the nanosheet, which does not account for the dramatic catalytic improvement in HER. Therefore, we later compared the nanostructures of the three samples. Among the three samples, although the thickness of the Cu2MoS4 nanosheet is in the nanoscale, both the length and width are on a micrometer scale. So we can treat the nanosheet as the so-called bulk material, compared to the hollow nanoflower and nanoparticle, and it is easy to understand that the large nanosheet is less active through the size effect, which says that a nanomaterial with a smaller size usually has a better activity than the larger one. However, it is hard to compare the HER activity of the Cu2MoS4 hollow nanoflower and nanoparticle simply using the size effect, although the primary particle of the hollow nanoflower is actually smaller than the nanoparticle, which is certified by both electron microscopy (SEM, TEM) and XRD data. In contrast, smaller particles aggregate easier and this hinders further enhancement of the catalytic activity. So the excellent performance of the Cu2MoS4 hollow nanoflower may derive from its unique hierarchical hollow structure. The three-dimensional (3D) feature of the hollow nanoflower with high surface area not only gives more active sites for catalytic reaction, but also prevents the disadvantageous effect of particle aggregation effectively. What's more, the robust hollow frame is also beneficial to maintain the stability. So, the construction of Cu2MoS4 HHNSs provides a new opportunity to enhance the HER performance. However, up to now many questions about the accurate mechanisms of Cu2MoS4-based HER catalysts are still unanswered. More theoretical and experimental research should be carried out in the future.
Conclusions
In summary, we constructed a novel hierarchical hollow nanostructure of Cu2MoS4, which is earth-abundant and inexpensive. A facile and environmentally-friendly solvothermal method with Cu2O nanocrystals as hard templates was used in the synthetic process. With unique structural features and highly exposed reactive sites, the designed Cu2MoS4 hierarchical hollow nanostructures exhibited an excellent activity for HER. In addition, the synthetic strategies may give new inspiration for the research of other ternary sulphide materials, which will largely accelerate their applications in the fields of catalysis and clean energy.Acknowledgements
We acknowledge the financial support from the ministry of science and technology of China (2014CB848900, 2015CB932301), the National Natural Science Foundation of China (U1232131, U1532112, 11375198, 11574280), the Fundamental Research Funds for the Central Universities (WK2310000053, WK6030000031), User with Potential from CAS Hefei Science Center (2015HSC-UP020). L. S. thanks the recruitment program of global experts and the CAS Hundred Talent Program of China. The authors thank Dr Hongliang Jiang for useful discussions, MCD and Photoemission Endstations in NSRL for helps in X-ray measurements.References
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- J. K. Norskov and C. H. Christensen, Science, 2006, 312, 1322–1323 CrossRef CAS PubMed.
- J. A. Turner, Science, 2004, 305, 972–974 CrossRef CAS PubMed.
- J. Greeley, T. F. Jaramillo, J. Bonde, I. Chorkendorff and J. K. Norskov, Nat. Mater., 2006, 5, 909–913 CrossRef CAS PubMed.
- S. K. Singh, A. K. Singh, K. Aranishi and Q. Xu, J. Am. Chem. Soc., 2011, 133, 19638–19641 CrossRef CAS PubMed.
- W. F. Chen, J. T. Muckerman and E. Fujita, Chem. Commun., 2013, 49, 8896–8909 RSC.
- W. F. Chen, K. Sasaki, C. Ma, A. I. Frenkel, N. Marinkovic, J. T. Muckerman, Y. M. Zhu and R. R. Adzic, Angew. Chem., Int. Ed., 2012, 51, 6131–6135 CrossRef CAS PubMed.
- Y. Xu, R. Wu, J. F. Zhang, Y. M. Shi and B. Zhang, Chem. Commun., 2013, 49, 6656–6658 RSC.
- Y. J. Sun, C. Liu, D. C. Grauer, J. Yano, J. R. Long, P. D. Yang and C. J. Chang, J. Am. Chem. Soc., 2013, 135, 17699–17702 CrossRef CAS PubMed.
- Q. H. Wang, K. K. Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- J. F. Xie, H. Zhang, S. Li, R. X. Wang, X. Sun, M. Zhou, J. F. Zhou, X. W. Lou and Y. Xie, Adv. Mater., 2013, 25, 5807–5813 CrossRef CAS PubMed.
- Y. G. Li, H. L. Wang, L. M. Xie, Y. Y. Liang, G. S. Hong and H. J. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- K. Ramasamy, H. Sims, W. H. Butler and A. Gupta, J. Am. Chem. Soc., 2014, 136, 1587–1598 CrossRef CAS PubMed.
- C. J. Crossland and J. S. O. Evans, Chem. Commun., 2003, 2292–2293 RSC.
- P. D. Tran, Y. Chen, P. P. Boix, P. S. Bassi, N. Yantara, L. H. Wong and J. Barder, Nanoscale, 2014, 6, 6506–6510 RSC.
- B. B. Chen, D. K. Ma, Q. P. Ke, W. Chen and S. M. Huang, Phys. Chem. Chem. Phys., 2016, 18, 6713–6721 RSC.
- Y. Shang, D. Sun, Y. M. Shao, D. F. Zhang, L. Guo and S. H. Yang, Chem. – Eur. J., 2012, 18, 14261–14266 CrossRef CAS PubMed.
- Y. Shang, D. F. Zhang and L. Guo, J. Mater. Chem., 2012, 22, 856–861 RSC.
- Z. Y. Wang, D. Y. Luan, C. M. Li, F. B. Su, S. Madhavi, F. Y. C. Boey and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 16271–16277 CrossRef CAS PubMed.
- Y. G. Sun and Y. N. Xia, Science, 2002, 298, 2176–2179 CrossRef CAS PubMed.
- W. X. Chen, H. P. Zhu, H. T. Zhu, Q. Q. Gao, J. Luo, Y. Wang, S. Zhang, K. Zhang, C. M. Wang, Y. J. Xiong, Y. F. Wu, X. S. Zheng, W. S. Chu, L. Song and Z. Y. Wu, Small, 2014, 10, 4637–4644 CrossRef CAS PubMed.
- D. F. Zhang, H. Zhang, L. Guo, K. Zheng, X. D. Han and Z. Zhang, J. Mater. Chem. A, 2009, 19, 5220–5225 RSC.
- K. Zhang, W. X. Chen, Y. Wang, J. Li, H. P. Chen, Z. Y. Gong, S. Chang, F. Ye, T. X. Wang, W. S. Chu, C. W. Zou and L. Song, AIP Adv., 2015, 5, 077130 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cp07269k |
| This journal is © the Owner Societies 2017 |