The role of the binding salt sodium salicylate in semidilute ionic cetylpyridinium chloride micellar solutions: a rheological and scattering study†
D.
Gaudino
*a,
R.
Pasquino
a,
J.
Stellbrink
b,
N.
Szekely
c,
M.
Krutyeva
b,
A.
Radulescu
c,
W.
Pyckhout-Hintzen
b and
N.
Grizzuti
a
aDICMaPI, Università degli Studi di Napoli Federico II, P.le Tecchio 80, 80125 Napoli, Italy. E-mail: danila.gaudino@unina.it
bJülich Centre for Neutron Science JCNS (JCNS-1), Forschungszentrum Jülich GmbH, 52425 Jülich, Germany
cJülich Centre for Neutron Science outstation at MLZ, Lichtenbergstrasse 1, 85747 Garching, Germany
First published on 22nd November 2016
Abstract
The micellar system based on cetylpyridinium chloride (CPyCl) and sodium salicylate (NaSal) in brine solution is investigated on both macro- and micro-length scales through rheology and scattering measurements. The linear viscoelasticity of the system and its structural parameters are explored by systematically changing the amount of NaSal over an extremely wide range of concentrations, thus producing salt-to-surfactant molar ratios from zero to about 8.5. As a result, the well-known non-monotonic behaviour of the zero-shear rate viscosity as a function of salinity can be connected to micellar morphological changes, whose driving force is represented by the simultaneous binding and screening actions of NaSal. The viscosity behaviour can be seen as a direct consequence of consecutive lengthening/shortening of the contour length, where the micelles attempt to minimize the electrostatic charge density on their surface. Along similar lines, the scattering measurements of the semidilute solutions show that the local stiffness of the micellar chain changes with increasing salt content influencing the elasticity of the resulting network. Within this general view, the branching of the micelles can be seen as a side effect attributable to the main character of the play, namely, the binding salt NaSal, whereas the overall dynamics of the system is driven by the considerable changes in the entanglement density of the micellar network.
1 Introduction
Micellar solutions are made of amphiphilic molecules, with a hydrophilic head and a hydrophobic tail, dispersed in an aqueous medium. In order to reduce the contact between the hydrophobic part and water, amphiphiles form different morphological structures, the micelles, where molecules are tied together through simple physico-chemical interactions.1 The micellar morphology strongly affects the macroscopic behaviour of the solution. In particular, variations in the local morphology of the micelles can generate dramatic changes in the rheological response, making such systems suitable for a wide range of applications.2–4 The scission energy, Esciss, which is responsible for the dynamical breaking and recombination of the micellar units represents the driving force determining the micellar structure as they tend to assume the most favourable shape in order to minimize the free energy of the system.5,6The micellar structure can be easily modified by tuning several physical parameters of the system such as surfactant concentration, salinity, pH and temperature.1 In particular, adding a binding salt to the solution is one efficient way to change Esciss and determine structural changes.5 It is worth noticing that the dependence of Esciss upon salt concentration is very similar to that of the zero shear rate viscosity, η0. Hence, rheology is an effective tool to detect structural transitions occurring in micellar systems.6–8
In the absence of salt, if the amount of surfactant is larger than the Critical Micellar Concentration (cmc), surfactant molecules generally self-assemble into spherical objects. As the salinity is increased, micelles undergo several transitions: from spherical to rodlike objects, and eventually to long flexible chains, often referred to as “living polymers” due to their relentless attitude to continuously break and reform.9 Correspondingly, rheological transitions from a Newtonian to a viscoelastic, Maxwellian behaviour are observed.
The linear rheology of well entangled long wormlike micelles is well described by Cates' model9 and is accompanied by an increase in viscosity of many orders of magnitude.10 Upon further increasing the salt concentration, a considerable decrease of viscosity is observed.11 Cryo-TEM measurements12–15 indicate that in this region branched wormlike structures arise.12,16,17
A relaxation process faster than that found in the unbranched wormlike region characterizes the dynamics of branched micelles. This behaviour has been explained as the occurrence of an extra relaxation process, suggesting the sliding of the branched points along the micellar body.18 More recently, a novel explanation which attributes the faster relaxation to an increase in the end-cap concentration and therefore to a decrease in the average micellar length has been proposed.12
In some surfactant systems at higher salt concentrations it is possible to observe a second maximum in the viscosity vs. salinity curve.7,10,16 This second peak is explained as an increase of the micellar contour length and a decrease of the branching density, whereas the subsequent second drop of viscosity is suggested to be related to the coexistence of several different structures such as linear, branched and ring micelles.7 No experimental evidence, however, is available to support the occurrence of these somewhat “exotic” microstructures and a definitive physical explanation for the observed rheological response still remains an unresolved issue.
Micelle microstructural parameters, such as the micellar radius and the persistence and the contour length of the wormlike chains, can be obtained by combining different scattering methods, provided that the overall volume fraction of the species building up the system is low enough (dilute regime). The use of different scattering wavelengths and angles allows investigating a wide range of length scales of the structures present in the solution.19–21
Static light scattering (SLS) is a technique typically limited to relatively low values of the scattering vector, q, corresponding to large length scales. The contrast is given by difference in the index of refraction between the solute and the solvent. In order to investigate smaller length scale structures, higher scattering vector values are required. They can be accessed through Small Angle Neutron Scattering (SANS) measurements, whose contrast comes from the difference between scattering length densities of both solute and solvent, which strongly depend upon the nucleus structure. Since the accessible q-range and length scales of SANS match those of the typical morphologies of micellar systems, it is also possible to observe some micellar transitions such as the above mentioned spherical-to-rodlike-to-wormlike sequence. SANS, however, is not able to discriminate between entanglements and branch points.11,22
Although micellar solutions have been widely investigated for about thirty years,23–25 they still represent a challenge since a direct correlation between their microstructure and their macroscopic dynamics (as revealed, for instance, by rheology) as a function of the salt/surfactant molar ratio is not completely understood. In particular, the dilute regime26 has been investigated much more extensively than the semi-dilute one.
Several attempts to link the dynamics to the micellar morphologies by techniques such as H-Nuclear Magnetic Resonance spectroscopy (H-NMR) have also been published.27–32
However, a better understanding of the microstructure–dynamics connection is still missing.
In view of the above, the present work is devoted to an extensive study of the macroscopic (rheological) and microstructural behaviour (scattering) of a well-known semidilute surfactant solution based on Cetylpyridinium Chloride and Sodium Salicylate (CPyCl-NaSal) in brine solution (with NaCl) as a function of NaSal concentration. The rheology, in the low-to-middle salt regime, has already been largely investigated,10,12,23,24 while that in the high salt range remains less explored. Moreover, it is worth noticing that scattering measurements were performed on semi-dilute micellar systems and were directly compared with independent information coming from the corresponding mechanical spectra. As it will be shown, the salt plays a fundamental role in determining both the micellar microstructure and the solution dynamics.
The paper is structured as follows: after this introduction, materials and methods are presented. The main results of rheological and scattering measurements are reported in the third section, which is followed by a discussion and comparison of the main outcomes. The main conclusions of the work are given in the last section.
2 Materials and methods
Semidilute solutions of 100 mM Cetylpyridinium Chloride (CPyCl, purity 96%, cmc = 0.12 mM at 25 °C) in 100 mM Sodium Chloride (NaCl, purity 99.5%) brine solution were prepared at different Sodium Salicylate (NaSal, purity 99%) concentrations, ranging from 20.5 to 855 mM. As often reported in the literature, NaCl is used in order to screen the long-range electrostatic repulsions among surfactant molecules, whereas the more significant and interesting role is played by the binding salt, NaSal, owing to its ability to build up π bonds with the surfactant molecules.5,33 All components were obtained from Applichem Panreac and used as received.Samples were prepared by gently stirring the surfactant and the salts in deuterated water for a day at room temperature, followed by storage for at least two days in order to reach the equilibrium state. Deuterated water is required to enhance the nuclear contrast and to reduce the incoherent background, thus improving the quality and sensitivity of neutron scattering measurements.
Rheological experiments were conducted on deuterated solutions in order to reduce possible extra differences in the comparison between the microscopic and the macroscopic results. Preliminary rheological measurements were performed also on protonated aqueous solutions and showed the same qualitative results as displayed by the deuterated systems. Stress controlled rheometers (MCR 702 TwinDrive (Anton Paar) and AR G2 (TA Instruments)) were used to investigate the linear viscoelastic response of the 100 mM CPyCl solutions (in the frequency range 0.01–200 rad s−1), after having checked the linear regime. 50 mm–0.017 rad and 60 mm–0.017 rad cone-plate geometries (for MCR 702 and AR G2, respectively) were used. The temperature of 23 °C was controlled by a Peltier-lower-plate geometry. A solvent trap was used to reduce water evaporation. Reproducibility was guaranteed from repeating the measurements six times on fresh samples. A delay time of 200 seconds was applied before each experiment.
Small Angle Neutron Scattering (SANS) measurements were carried out at the MLZ, Garching Research Centre (Münich, Germany), using the KWS-2 small angle scattering diffractometer. All the prepared samples were held in quartz cells of 1 mm thickness, with the scattering vector, q, ranging between 2 × 10−3 and 0.4 Å−1 and with a wavelength of 5 Å and detector distances of 1, 2, 8 and 20 m. The intensities were corrected for transmission, empty cell scattering and background scattering using standard procedures and converted to absolute differential scattering cross sections. Test experiments were also performed using the Ultra-SANS KWS3 diffractometer, covering the q-range of 10−4–10−2 Å−1 to verify whether further q-dependent scattering, in view of the expected large size of the scattering objects, was present. The U-SANS data were essentially flat and are not shown in this work.
Static Light Scattering (SLS) measurements were carried out on selected samples in order to extend the scattering vector range to values one order of magnitude smaller than the SANS KWS-2 q-range. SLS experiments were performed on a compact goniometer (ALV SP-125 Langen, Germany) using an argon ion laser (Coherent, Innova 90-4) operating with vertically polarized light at λ0 = 514.5 nm and I0 = 100–300 mW. Samples were filtered through 0.2 μm Anotop membrane filters into cylindrical cells (Hellma, Germany) with 10 mm outer diameter, in order to reduce the parasitic scattering of large impurities or dust. Comparison of filtered and unfiltered solutions clearly revealed that only parasitic scattering was affected by filtration. Likewise, corrections for solvent and empty cell scattering were performed using standard procedures.
3 Results
3.1 Rheology
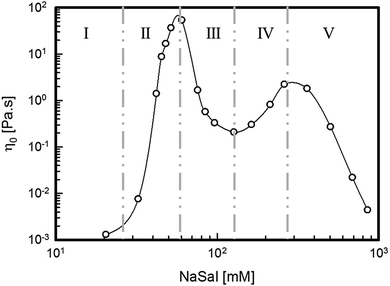
Fig. 1 shows the zero shear rate viscosity, η0, of the solutions as a function of the amount of NaSal. The values of η0 have been obtained as the long-time-steady-state measurement of start-up experiments at low shear rates. A non-monotonic behaviour, amply advertised in the literature since the late 80s,10 is recorded as the salinity of the system is increased and five different concentration regions can be identified. In region I, η0 is roughly comparable to the viscosity of water. In region II, η0 grows by several orders of magnitude, reaching a maximum at [NaSal] ≈ 60 mM. A further increase in the salt concentration leads first to a viscosity decrease and then to a second viscosity peak (regions III and IV, respectively). Eventually, at higher salicylate concentrations, η0 decreases again (region V). It must be noticed that the dramatic changes in the rheology response are only due to the two-orders-of-magnitude change of the salt to surfactant molar ratio.The viscoelastic moduli of selected solutions corresponding to the different regions described in Fig. 1 are shown in Fig. 2 through the Cole–Cole representation.34 With the exception of region I, for which the samples behave like Newtonian fluids (see ESI†), the beginning of region II and the very high salt concentration samples in region V, for which a Newtonian behaviour is found, all the surfactant systems show significant viscoelastic features. In particular, the Maxwellian behaviour, represented by a well-defined semi-circular Cole–Cole plot,34 clearly appears for samples at [NaSal] corresponding to the two viscosity peaks in Fig. 1 ([NaSal] ≈ 60 mM and [NaSal] ≈ 250 mM). Strong deviations from this simple behaviour can be observed for samples belonging to the saddle region of the same plot.
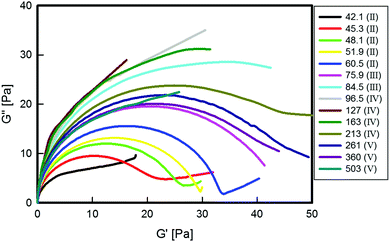 | ||
| Fig. 2 Cole–Cole plots of the surfactant solutions at different [NaSal] in mM (see the legend, where the corresponding salt regions are shown). | ||
The maximum value of G′′, Gmax′′, obtained from Fig. 2, can be used to extract both the main relaxation time, τ, and the elastic modulus plateau, G0, of the viscoelastic systems. The former is obtained as the inverse of the frequency at which the maximum occurs, whereas G0 is calculated as traditionally done in the rheological literature,12,34,35 as G0 = 2Gmax′′. The main relaxation time and the plateau modulus are plotted as functions of [NaSal] in Fig. 3. By comparing Fig. 1 and 2 it is easy to deduce that τ follows the same, non-monotonic behavior of the zero-shear rate viscosity (see Fig. 1), whereas G0 reaches a maximum at the border between regions III and IV, where the zero shear viscosity local minimum is attained.
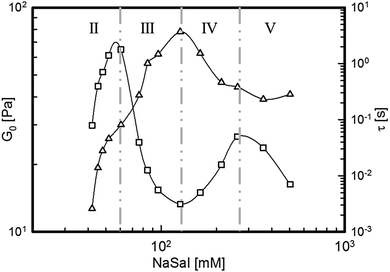 | ||
| Fig. 3 Elastic modulus plateau, G0 (empty triangles), and main relaxation time, τ (empty squares), of the surfactant solutions as a function of [NaSal]. | ||
3.2 Scattering
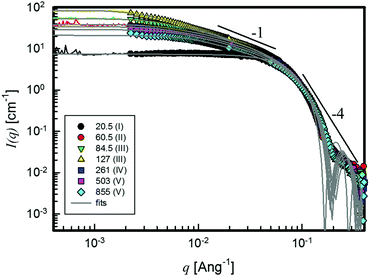
The results of SLS and SANS measurements on selected samples belonging to different salinity regions are summarized in Fig. 4. The background corrected SLS data were shifted vertically to fit the absolute neutron scattering intensities, I(q).36Qualitatively, several regions can be distinguished. At high values of the scattering vector, q > 0.1 Å−1, all I(q) data overlap, showing an envelope with roughly q−4 dependence. At intermediate q values (10−2 < q < 10−1 Å−1), the q-dependence of I(q) changes, approaching a slope close to −1, which is more or less extended depending on salt concentration. At the lowest q values, I(q) becomes essentially independent of the scattering vector and reaches almost a Guinier-like plateau in the SANS data, from which the size of the scattering objects can be estimated.21 The presence of the plateau-like regime and the simultaneous linearity of the intensity in a Guinier representation (ln[I(q)] vs. q2) are further corroborated by the overlap with the SLS data on identical samples.
This behaviour is displayed by all samples, except for the lowest salt concentration system, where only the plateau and the q−4 dependence show-up.21
The scattering curves show strong indications of the appearance of two different morphologies: the q−1 slope is typical of 1D rod-like architectures of characteristic length l, whereas at high q the single q−4 slope indicates a clear interface between the objects and the solvent.
The overlap of SANS and SLS data suggests that there is no other q-dependence in between the two domains. Furthermore, preliminary Ultra-SANS measurements did not show any contributions in the size range larger than 100 nm. It is therefore expected that the plateau level of the SLS measurement sets a limit to the form factor, which can be obtained from classical SANS experiments. In addition, it has to be considered that the systems are semidilute.37 Inter-particle interactions, therefore, exist and a structure factor, S(q), cannot be neglected in a more thorough data evaluation. This means that the I(q) response contains information about both the structure factor and the form factor, thus hiding the direct microstructure information related to the real size of the micelles,38 which are known to be in some cases even micrometres long.12,16
To further verify the influence of concentration, also some dilute surfactant solutions have been analysed through SANS measurements. Inspired by the pioneering work of Rehage and Hoffmann,10 roughly 13 millimoles of NaSal have been added to a 5 mM CPyCl solution. This molar ratio allows one to obtain the corresponding “viscosity peak” for this dilute regime.
Fig. 5 displays the scattering intensity for the above-mentioned sample. Due to the dilute regime (the overall concentration is lower than 0.4% wt), the different q-dependencies of I(q) (q−1 and q−1.5 up to roughly 10−2 and 3 × 10−3 Å−1, respectively) are now clearly shown; thus, approximate values of the characteristic dimensions can be extracted from the changes of the I(q)-slope.
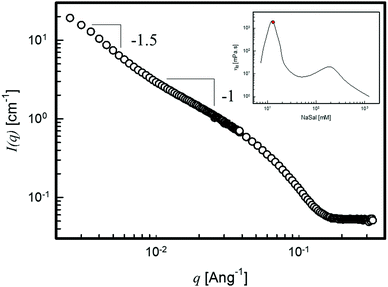 | ||
| Fig. 5 The small angle neutron scattered intensity of the surfactant solution of 5 mM CPyCl and 13 mM NaSal. The inset shows the data taken from ref. 10, with the red filled circle representing the measured sample. | ||
In this case, a persistence length of roughly 10 nm and a micellar radius of gyration, Rg, of about 35 nm are obtained.11 This value of Rg is consistent both with q-dependence I(q) ∼ q−1/ν=−1.5, with ν ≈ 0.58 the Flory exponent, suggesting wormlike micelles with excluded volume interactions in a good solvent and with the length of worm-like micelles composing a 1 Pa s-surfactant solution (see the inset in Fig. 5). Hence, Fig. 5 confirms the crucial role played by the overall concentration in the analysis of the scattering curves and serves as an internal consistency check.
Coming back to our surfactant systems, from a literature screening of the models usually applied to the scattering data treatment of similar systems,39–44 the Random Phase Approximation (RPA) model45,46 is the one that seems to best fit the observed experimental behaviour, as previously seen in similar semi-dilute systems.47 The model takes into account the interactions between scatterers through an empirical parameter, Y, and depends on the concentration of the components (NaSal + CPyCl), which build up the micelles, leading to the following expression for the scattering intensity:
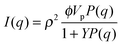 | (1) |
Eqn (1) can be particularized by selecting P(q), corresponding to the geometry of the supposed scatterers under investigation. In view of the above observations the well-known form factors of spheres of radius r and cylinders of radius r and length l have been considered.46 The P(q) of orientational-averaged cylinders used to fit the data is
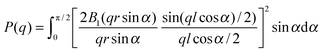 | (2) |
The predictions of the model are compared to the experimental data in Fig. 4. The position of the first form factor minimum at high q allows one to estimate the radius, r, of the scatterers, which assumes a value roughly equal to 2 nm for all samples. This agrees with the molecular length of the surfactant molecule.49 The first minimum for a cylinder-like system shows up at q·r ≈ 3.83 approximately. For the case of spherical micelles, this minimum shifts to q·r ≈ 4.49.
The agreement between the experimental data and the model is remarkably good. The values of the fitting parameters for all samples investigated are reported in Table 1.
| NaSal [mM] | l ± Δl [nm] | r ± Δr [nm] | Y ± ΔY | ρ 2 ± Δρ2 [1019 cm−4] |
|---|---|---|---|---|
| 20.5 | — | 2.72 ± 0.02 | — | 109 ± 2 |
| 32.5 | 46 ± 4 | 2.17 ± 0.01 | 2.2 ± 0.3 | 277 ± 3 |
| 42.1 | 63 ± 7 | 2.18 ± 0.01 | 2.4 ± 0.3 | 263 ± 4 |
| 45.3 | 62.3 ± 1.8 | 2.14 ± 0.01 | 2.2 ± 0.1 | 256 ± 3 |
| 48.1 | 72.4 ± 0.1 | 2.18 ± 0.01 | 2.2 ± 0.1 | 255 ± 3 |
| 51.9 | 75 ± 10 | 2.16 ± 0.01 | 2.6 ± 0.4 | 250 ± 3 |
| 60.5 | 74.8 ± 0.1 | 2.15 ± 0.01 | 2.3 ± 0.1 | 245 ± 3 |
| 75.9 | 90 ± 12 | 2.13 ± 0.01 | 2.3 ± 0.4 | 226 ± 2 |
| 84.5 | 120 ± 22 | 2.12 ± 0.01 | 2.4 ± 0.5 | 218 ± 2 |
| 96.5 | 136 ± 1 | 2.07 ± 0.01 | 1.6 ± 0.1 | 205 ± 1 |
| 127 | 139 ± 1 | 2.02 ± 0.01 | 1.4 ± 0.1 | 178 ± 1 |
| 163 | 102 ± 13 | 2.03 ± 0.01 | 1.8 ± 0.3 | 171 ± 2 |
| 213 | 94 ± 11 | 2.02 ± 0.01 | 2.4 ± 0.3 | 159 ± 2 |
| 261 | 102 ± 16 | 2.01 ± 0.01 | 2.9 ± 0.5 | 144 ± 1 |
| 360 | 102 ± 8 | 1.97 ± 0.01 | 3.2 ± 0.3 | 121 ± 1 |
| 503 | 77 ± 9 | 1.92 ± 0.01 | 2.1 ± 0.3 | 97 ± 1 |
| 689 | 83 ± 9 | 1.89 ± 0.01 | 2.3 ± 0.3 | 76.30 ± 1 |
| 855 | 51 ± 3 | 1.86 ± 0.01 | 1.5 ± 0.2 | 64.20 ± 1 |
Some objective comments apply to the data in Table 1:
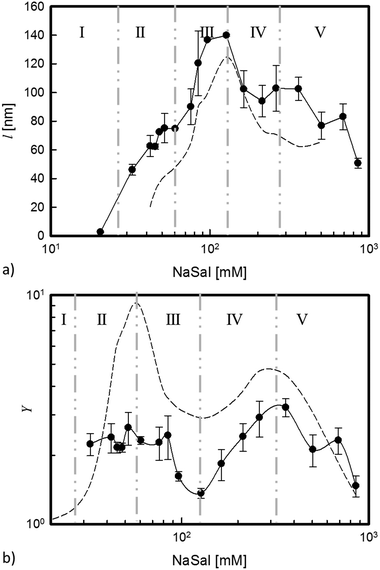
– The effective cylinder length, l, increases with increasing salinity and reaches a maximum, which is roughly three times larger than the one at low salt concentrations. Beyond the maximum, l decreases monotonically upon further addition of salt.
– The radius r of the scatterers does not change significantly. However, a faint decrease can be perceived as salinity increases, similar to previous– investigations,50 which may be due to the action of the salicylate anion determining a denser structure of the rod.
– The interaction parameter Y changes slightly with [NaSal] as has already been observed for other similar systems subjected to equivalent interactions.42 Nevertheless, a significant and interesting behaviour as a function of salicylate content can still be detected: at low salt concentrations, Y is almost constant and, after a first drop, it reaches a maximum at a higher salt concentration.
– With the exception of the sample with the lowest salt concentration, the contrast factor, ρ2, is a monotonic decreasing function of [NaSal]. This is in agreement with the salt incorporation. The fitted values of the contrast factor agree fairly well with the predicted ones21 (see ESI† for details).
4 Discussion
4.1 LVE: dynamics investigation
The non-monotonic behaviour of the zero shear rate viscosity clearly indicates that changes in the binding salt concentration induce relevant morphological transitions, as already shown elsewhere.11,12 With increasing salt content, wormlike micelles form and entangle just like polymer chains. The first increasing viscosity branch (going from region I to region II) and the relative viscoelastic response highlight the transition from spherical to cylindrical, linear entangled micelles. In particular, region II is characterized by samples whose microstructure is built up with longer linear worm-like structures with contour lengths in the micron size range7 and which show a well-defined Maxwellian behaviour when the first viscosity maximum is reached. This first transition is clearly shown in Fig. 2, where an almost perfect semi-circle appears at [NaSal] = 60.5. A further addition of salt induces a sudden decrease of η0 and τ (region III) with a non-Maxwellian viscoelastic response. In the same salinity region, cryoTEM images, carried out on the same system, have shown the formation of branched micelles.12 Thus, a morphological transition from linear to branched wormlike micelles occurs between regions II and III. The occurrence of this microstructural transition has been usually addressed as being responsible for an additional relaxation process and, therefore, to an overall faster process to release the stress.18 Recently, however, the decrease of τ in this salt regime has been alternatively justified by a strong shortening of the micellar contour length, due to an increase of the end-cap concentration.12Regions IV and V display an almost mirror-like behaviour as displayed by regions II and III. The second viscosity peak is characterized by the almost perfect Cole–Cole semi-circle shown also at the first peak. Again, by further increasing the NaSal concentration, the Maxwellian behaviour progressively fades away, eventually recovering the Newtonian features at the highest NaSal/CPyCl molar ratios. Following arguments similar to those applied for regions II and III, it is assumed that micelles become longer (region IV) and shorter (region V) again.
The hypothesis that the system dynamics is controlled by the variation of the micellar contour length rather than by the “exotic” branch sliding effect, and actually already mentioned in the literature,7,12 is here strongly confirmed by an estimate of the number of entanglements per chain, nν, as a function of salt concentration. According to Cates' model and subsequent refinements, nν can be calculated from the mechanical spectrum of the solutions as51
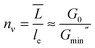 | (3) |
![[L with combining macron]](https://www.rsc.org/images/entities/i_char_004c_0304.gif) is the micellar contour length and le is the distance between entanglements. The increase of nν for samples in region II as well as its decrease in region III can be qualitatively seen in Fig. 6, where the normalized loss modulus of the surfactant solutions is presented as a function of the Deborah number, De. De is obtained as the product between the angular frequency, ω, and the relaxation time of each solution, whereas G′′ is normalized by the crossover modulus, Gcr.
is the micellar contour length and le is the distance between entanglements. The increase of nν for samples in region II as well as its decrease in region III can be qualitatively seen in Fig. 6, where the normalized loss modulus of the surfactant solutions is presented as a function of the Deborah number, De. De is obtained as the product between the angular frequency, ω, and the relaxation time of each solution, whereas G′′ is normalized by the crossover modulus, Gcr.
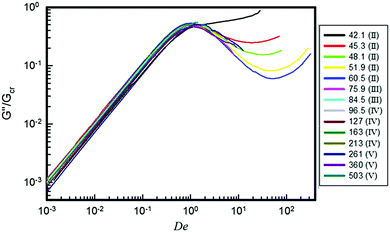 | ||
| Fig. 6 Normalized viscous modulus as a function of De of the surfactant solutions at different [NaSal] in mM (see the legend, where the corresponding salt regions are shown). | ||
A second lengthening/shortening of the micellar contour length would induce a new increase/decrease of the number of entanglements for samples in regions IV and V (Fig. 6). Quantitatively, nν can be derived from the rheological data using eqn (3). For NaSal concentrations where the minimum in G′′ could not be measured, data published on the same system by Oelschlaeger and co-workers7 were used. The results are reported in Fig. 7.
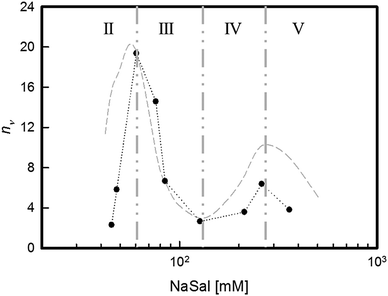 | ||
| Fig. 7 Number of entanglements per chain, nν, as a function of [NaSal]. The grey dashed line represents the behaviour of the main relaxation time, τ, in arbitrary units. | ||
It is fair to stress that, when the entanglement density drops to very low values, such as in the saddle region at intermediate NaSal concentrations, the validity of Cates' model is at least questionable. Although not quantitatively reliable, however, the results reported in Fig. 7 unequivocally confirm that the salt-driven contour length of the micelles is responsible for all the observed behaviours. It is thus proven that the overall relaxation mechanism of the surfactant system is governed by the entanglement network, whose nν determines the τ for each solution.
4.2 Salt effects on the micellar structural parameters
Experiments carried out through scattering techniques normally represent a suitable method to obtain microstructural information on different length scales. In particular, the combination of neutron and light scattering measurements (SANS and SLS respectively) can provide information up to the micron length scale.21In the used approach (see above) we have resorted to include a structure factor, S(q), referring to the interactions among the scatterers in the more concentrated case. The RPA-like structure factor was used ad hoc due to the similarity with concentrated polymer solutions, where it can be shown that for semi-dilute concentrations, an apparent 2nd virial coefficient is effectively obtained.46,52 Indeed, the derived average 2nd virial coefficient from Y ≈ 3 × 10−5 is of the same order of magnitude as the average virial coefficient, which can be calculated for dilute rod solutions,52 confirming somehow the reliability of our approach. From Table 1 the difference in the length of the cylinders is 25% with respect to the average value. The form factor, P(q), on the other hand, contains all the information concerning the shape of the particles.21
As said, the surfactant solutions studied in the present work are all in the semidilute regime, due to the overall concentration of all species (CPyCl, NaSal and NaCl).37 As a direct consequence, strong inter-micellar interactions are expected, S(q) cannot be neglected and the scattering intensity can provide only a hint of the complete microstructure.
The above-mentioned situation is clearly shown in Fig. 4, from which a size of about 100 nm can be extracted from the Guinier-like plateau.21 In addition, the I(q) dependencies on the scattering vector suggest that scatterers are of cylindrical shape, with the only exception of the lowest salt concentration sample, where the SANS/SLS curve clearly indicates spherical objects.39 In other words, scattering techniques see the microstructure forming in these micellar systems as short, interacting, stiff cylinders.
However, it is worth noticing that such systems are demonstrated to be long entangled wormlike micelles,7,35 as suggested also by the rheological data presented in this work. Hence, interactions occur and, at low q, where the contribution of S(q) is stronger, part of the structural information is hidden.38
Fig. 8 shows the dependence of the main parameters of the RPA model fit, l and Y, as functions of the salt concentration.
We notice that the cylinder length, l, follows a trend that is comparable to that of the elastic plateau modulus, G0 (Fig. 8a).
It must be said that the fitted characteristic length of Fig. 8a cannot be identified with the well-established micelle persistence length proposed in the literature. For example, independent measurements on less concentrated micellar systems, but similar to the one used in the current work, indicate a value of the persistent length lp ≈ 25 nm.35 More recently, cryoTEM measurements on non-deuterated samples of the same micellar system used here confirmed the above-mentioned lp values, even in the semi-dilute regime.12
In comparison to the above reported value of the persistence length, the characteristic length obtained from the model fit is larger and maybe extends over a few segments. However, the surprising agreement between l and the main plateau modulus suggests that in any case the fitted length provides an indication of the stiffness characterizing the interacting micellar structures. In particular, in region II, l assumes a roughly constant value of about 70 nm. Correspondingly, the rheological measurements show a slight increase of G0. In region III, where branched connections form, l increases, assuming a maximum value (twice larger than that attained in region II). As seen in Fig. 8a, G0 increases and reaches a maximum value at the same critical salt concentration. This higher rigidity, indicated by the macroscopic rheological measurements, fits remarkably well with the microstructure information coming from SANS measurements, which indicate a corresponding increasing stiffness of the microstructure.
Analogously, the further decrease of G0 and its more or less constant value at higher salt content (regions IV and V, respectively) agree with the SANS description of the local system rigidity, as demonstrated by a roughly constant value of the fitted length in the same salinity regimes.
The behaviour assumed by Y, which is a local structural parameter, represents a key factor in our analysis, since it confirms experimentally the speculation previously asserted and agrees with recent results obtained through numerical simulations.6
Y is connected with the screening action carried out by the salicylate ions and it is also strictly related to the behaviour assumed by the zero shear rate viscosity, a macroscopic experimental parameter, as shown in Fig. 8b.
A constant value of Y in region II reflects the more pronounced binding action of the salt compared to its screening force. Micelles elongate because the packing parameter is geometrically changed by the insertion of Sal− ions between the surfactant heads.53 The decrease of Y and the corresponding decrease of the interactions among the micelles (region III) can be seen as the effect of the two-fold screening action of the salt: at the macroscopic level, the number of entanglements and the relaxation time decrease as evidenced by the linear viscoelastic response12 (see Fig. 3); at the microscopic level, the local rigidity of the structures increases. When the viscosity reaches its local minimum (end of region III), also Y goes through a minimum. In this particular condition, the micellar surface is electrostatically neutral7,10,54 since all the CPy+ are saturated by Sal−, their ratio being almost equimolar.
A further addition of salt (region IV) induces a new change in the charge density of the micelles, due to an excess of Sal− ions that cannot be any longer balanced by the already saturated CPy+ surfactant cations. This new electrostatic configuration induces again an increase of the relaxation time, which suggests longer micelles (η0 increases), in order to reduce the surface charge density. However, a negative electrostatic shell now surrounds the micelles, inducing repulsive interactions (Y increases). As a direct consequence, the network becomes sparser, hence G0 decreases.
Region V is characterized by samples that are approaching again the Newtonian regime. This is due, basically, to a new shortening of the micellar contour length led by an increase in the end-cap concentration and, therefore, in the surface available to reduce the charge density. Eventually, micelles become so short that the elastic component is lost and the system becomes purely viscous.
In concluding this section, it should be noticed that also the trend of the other two parameters of the RPA model fit is consistent with the above picture. As Table 1 shows, the micellar radius, r, is found to decrease with increasing salt concentration. This is expected because, as [NaSal] increases, the screened repulsions around the surfactant head groups lead to a smaller effective size. The presence of Sal− around the micelles is confirmed by the fact that the contrast factor, ρ2, decreases as the salt concentration increases (see ESI† for more details).
5 Conclusions
In this work, the dynamics and the structure of 100 mM CPyCl surfactant brine micellar solutions at different NaSal concentrations have been investigated through rheology, Small Angle Neutron Scattering (SANS) and Static Light Scattering (SLS).The micellar solutions have been systematically analyzed over an extremely wide range of the salt-to-surfactant molar ratio, in order to uncover the key role of the strong binding salt, NaSal, and its effects on both the macroscopic scale, through rheological investigations, and the microscopic scale, via scattering techniques.
The agreement between the rheological and scattering parameters (in particular G0 − l and η0 − Y in Fig. 8a and b respectively) confirms the following physical picture: the salt binding action at lower concentrations and the subsequent screening action at higher concentrations determine both the macroscopic solution dynamics and the local rigidity of the micelles.
Within this general framework, the experimental results seem to indicate that micellar branching is a side effect attributable to salinity changes, which induces the formation of interconnected points in order to reach an energy compromise.12 Branching however does not seem to determine relevant effects on the macroscopic behaviour of the system. In fact, the evolution of the dynamical response of the micellar system can be explained by only considering the variations of the electrostatic charge density on the micellar surface. This induces changes in the micellar repulsive action and, more significantly, in the micellar contour length and, thus, in the number of entanglements.
Furthermore, the change in the local rigidity of the micelles, detected by independent experiments, i.e., SANS and rheology, represents an additional consequence of the strong action of the binding salt.
In conclusion, rheology and scattering measurements converge on the crucial role played by the salt in the dynamical and morphological evolution of micellar structures.
Acknowledgements
The authors are grateful for helpful comments and discussions with Dr Francesco Greco (CNR, Italy) and Gunther Arnold (Anton Paar) and thank Dr Vitaly Pipich for the measurements at the Ultra-SANS KWS3 diffractometer. DG kindly thanks the University of Naples Federico II for a fellowship program (Coinor-Programma Star) and the JCNS of the Jülich Forschungszentrum for financial support.References
-
R. G. Larson, The Structure and Rheology of Complex Fluids, Oxford University, New York, 1999 Search PubMed
.
- S. Kefi, J. Lee, T. Pope, P. Sullivan, E. Nelson, A. Hernandez, T. Olsen, M. Parlar, B. Powers, A. Roy, A. Wilson and A. Twynam, Oilfield Rev., 2004, 16, 10 CAS
.
- R. Pasquino, M. Di Domenico, F. Izzo, D. Gaudino, V. Vanzanella, N. Grizzuti and B. de Gennaro, Colloids Surf., B, 2016, 146, 938 CrossRef CAS PubMed
.
-
R. Zana and E. Kaler, Giant Micelles, Properties and Applications, CRC, Florida, 2007 Search PubMed
.
- G. Porte, R. Gomati, O. El Haitamy, J. Appell and J. Marignan, J. Phys. Chem., 1986, 90, 5746 CrossRef CAS
.
- S. Dhakal and R. Sureshkumar, J. Chem. Phys., 2015, 143, 024905 Search PubMed
.
- C. Oelschlaeger, M. Schopferer, F. Scheffold and N. Willenbacher, Langmuir, 2008, 25, 716 CrossRef PubMed
.
- M. E. Helgeson, T. K. Hodgdon, E. W. Kaler and N. J. Wagner, J. Colloid Interface Sci., 2010, 349, 1 CrossRef CAS PubMed
.
- M. E. Cates, Macromolecules, 1987, 20, 2289 CrossRef CAS
.
- H. Rehage and H. Hoffmann, J. Phys. Chem., 1988, 92, 4712 CrossRef CAS
.
- C. A. Dreiss, Soft Matter, 2007, 3, 956 RSC
.
- D. Gaudino, R. Pasquino and N. Grizzuti, J. Rheol., 2015, 59, 1363 CrossRef CAS
.
- Y. I. Gonzàlez and E. W. Kaler, Curr. Opin. Colloid Interface Sci., 2005, 10, 256 CrossRef
.
- T. M. Clausen, P. K. Vinson, J. R. Minter, H. T. Davis, Y. Talmon and W. G. Miller, J. Phys. Chem., 1992, 96, 474 CrossRef CAS
.
- I. Harwigsson, O. Soderman and O. Regev, Langmuir, 1994, 10, 4731 CrossRef CAS
.
- C. Oelschlaeger, P. Suwita and N. Willenbacher, Langmuir, 2010, 26, 7045 CrossRef CAS PubMed
.
- S. R. Raghavan, G. Fritz and E. W. Kaler, Langmuir, 2002, 18, 3797 CrossRef CAS
.
- F. Lequeux, Europhys. Lett., 1992, 19, 675 CrossRef CAS
.
- P. Schurtenberger, L. J. Magid, S. M. King and P. Lindner, J. Phys. Chem., 1991, 95, 4173 CrossRef CAS
.
- P. Schurtenberger, G. Jerke, C. Cavaco and J. S. Pedersen, Langmuir, 1996, 12, 2433 CrossRef CAS
.
-
B. Hammouda, Probing nanoscale structures-the sans toolbox, Natl. Institute Standards Technology Center for Neutron Research, Gaithersburg, MD, 2008 Search PubMed
.
- V. Croce, T. Cosgrove, G. Maitland, T. Hughes and G. Karlsson, Langmuir, 2003, 19, 8536 CrossRef CAS
.
-
H. Hoffmann, Structure and Flow in Surfactant Solutions, ACS Symposium Series, America Society, Washington, DC, 1994, ch. 1, pp. 2–31 Search PubMed
.
-
J. P. Rothstein, Rheology Review, The British Society of Rheology, 2008 Search PubMed
.
- S. A. Rogers, M. A. Calabrese and N. J. Wagner, Curr. Opin. Colloid Interface Sci., 2014, 19, 530 CrossRef CAS
.
- F. Nettesheim and N. J. Wagner, Langmuir, 2007, 23, 5267 CrossRef CAS PubMed
.
- P. G. Nilsson, H. Wennerstroem and B. Lindman, J. Phys. Chem., 1983, 87, 1377 CrossRef CAS
.
- T. Shikata, H. Hirata and T. Kotaka, Langmuir, 1988, 4, 354 CrossRef CAS
.
- K. Bijma and J. B. Engberts, Langmuir, 1997, 13, 4843 CrossRef CAS
.
- D. Varade, T. Joshi, V. K. Aswal, P. S. Goyal, P. A. Hassan and P. Bahadur, Colloids Surf., A, 2005, 259, 95 CrossRef CAS
.
- I. Furó, J. Mol. Liq., 2005, 117, 117 CrossRef
.
- M. Blesic, M. H. Marques, N. V. Plechkova, K. R. Seddon, L. P. N. Rebelo and A. Lopes, Green Chem., 2007, 9, 481 RSC
.
- S. J. Haward and G. H. McKinley, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2012, 85, 031502 CrossRef PubMed
.
- F. Kern, F. Lequeux, R. Zana and S. J. Candau, Langmuir, 1994, 10, 1714 CrossRef CAS
.
- R. G. Larson, J. Rheol., 2012, 56, 1363 CrossRef CAS
.
- V. M. Garamus, J. S. Pedersen, H. Maeda and P. Schurtenberger, Langmuir, 2003, 19, 3656 CrossRef CAS
.
- J.-F. Berret, J. Appell and G. Porte, Langmuir, 1993, 9, 2851 CrossRef CAS
.
- N. C. Das, H. Cao, H. Kaiser, G. T. Warren, J. R. Gladden and P. E. Sokol, Langmuir, 2012, 28, 11962 CrossRef CAS PubMed
.
-
L. A. Feigin and D. I. Svergun, Structure Analysis by Small-Angle X-Ray and Neutron Scattering, Plenum Press, New York, 1987 Search PubMed
.
- M. Takeda, T. Kusano, T. Matsunaga, H. Endo, M. Shibayama and T. Shikata, Langmuir, 2011, 27, 1731 CrossRef CAS PubMed
.
- P. Van der Schoot, Macromolecules, 1992, 25, 2923 CrossRef CAS
.
- W.-R. Chen, P. D. Butler and L. J. Magid, Langmuir, 2006, 22, 6539 CrossRef CAS PubMed
.
- L. Arleth, M. Bergstöm and J. S. Pedersen, Langmuir, 2002, 18, 5343 CrossRef CAS
.
- V. M. Garamus, J. S. Pedersen, H. Kawasaki and H. Maeda, Langmuir, 2000, 16, 6431 CrossRef CAS
.
- T. Shimada, M. Doi and K. J. Okano, Chem. Phys., 1988, 88, 2815 CAS
.
- J. S. Pedersen, Adv. Colloid Interface Sci., 1997, 70, 171 CrossRef CAS
.
- M. Y. Lin, H. J. M. Hanley, S. K. Sinha, G. C. Straty, D. G. Peiffer and M. W. Kim, Phys. B, 1995, 213, 613 CrossRef
.
- P. Van der Schoot and T. Odijk, J. Chem. Phys., 1992, 97, 515 CrossRef CAS
.
- L. Abezgauz, K. Kuperkar, P. A. Hassan, O. Ramon, P. Bahadur and D. Danino, J. Colloid Interface Sci., 2010, 342, 83 CrossRef CAS PubMed
.
- B. A. Schubert, E. W. Kaler and N. J. Wagner, Langmuir, 2003, 19, 4079 CrossRef CAS
.
- A. Khatory, F. Kern, F. Lequeux, J. Appell, G. Porte, N. Morie, A. Ott and W. Urbach, Langmuir, 1993, 9, 933 CrossRef CAS
.
- H. Benoit, and M. Benmoun, Polymer, 1984, 25, 1059 CrossRef CAS
.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninham, J. Chem. Soc., Faraday Trans. 2, 1976, 72, 1525 RSC
.
- T.-H. Kim, S.-M. Choi and S. R. Kline, Langmuir, 2006, 22, 2844 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cp06964a |
| This journal is © the Owner Societies 2017 |