In vitro study of the host responses to model biomaterials via a fibroblast/macrophage co-culture system†
Guoying
Zhou
a,
Andrea
Liedmann
a,
Chandralekha
Chatterjee
a and
Thomas
Groth
*ab
aBiomedical Materials Group, Institute of Pharmacy, Martin Luther University Halle-Wittenberg, Heinrich-Damerow-Strasse 4, 06120 Halle, Saale, Germany. E-mail: thomas.groth@pharmazie.uni-halle.de; Fax: +49 (0345)55 27379; Tel: +49 (0345)55 28460
bInterdsciplinary Centre of Materials Science, Martin Luther University Halle-Wittenberg, Heinrich-Damerow-Strasse 4, 06120 Halle, Saale, Germany
First published on 2nd December 2016
Abstract
Surface properties are believed to play important roles in initial inflammatory and subsequent wound healing/fibrotic responses after implantation of biomaterials. To investigate the surface property effect in mediating these host responses, we used an in vitro fibroblast/macrophage co-culture model established with a cell migration chamber, and a series of self-assembling monolayers (SAMs) bearing different terminal groups as model surfaces to study the effect of surface properties on macrophage fusion, fibroblast attachment, spreading morphology, proliferation, outgrowth, as well as pro-(interleukin-6) and anti-(interleukin-10) inflammatory cytokine production, expression of ED-A fibronectin (FN) and alpha-smooth muscle actin (α-SMA). The obtained results show that the hydrophobic CH3 surface caused high levels of inflammatory but low levels of wound healing/fibrotic responses, while the hydrophilic/anionic COOH surface resulted in both low levels of inflammatory and wound healing/fibrotic responses. Interestingly, the hydrophilic OH surface was found to possess a low potential of inducing inflammatory responses but high potential of inducing wound healing/fibrotic responses. These results reveal that the extent of inflammation and wound healing/fibrosis might not be always related in vitro. However, more important is the observation of the macrophage contributions in facilitating the wound healing and fibrotic responses by up-regulation of fibroblast outgrowth, cytokine production as well as ED-A FN and α-SMA expression. Overall, by linking the surface properties to cell activities with our established fibroblast/macrophage co-culture system, we could provide an useful model system for in vitro studies to design more biocompatible biomaterials for various biomedical and tissue engineering applications.
1. Introduction
The implantation of biomaterials often triggers a series of host responses, among which chronic inflammation and fibrotic encapsulation at the end stage are important concerns for the design of biocompatible materials.1 Macrophages and fibroblasts are two dominating effector cells involved in the cascade of host reactions.2 Although macrophages are more dominant during the inflammatory responses while fibroblasts are more crucial during wound healing/fibrotic responses, the regulation of the whole host reactions needs the orchestration of both cell types by soluble signals such as cytokines, chemokines, and growth factors, but also fixed cues through direct cell–cell contacts.3 Additionally, we and many others have shown that macrophages, in the presence of foreign materials, can produce significant amounts of pro-inflammatory and pro-fibrotic cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β), which are believed to affect the ensuing fibrotic reactions.4–6 Acting on these signals, fibroblasts can be activated in terms of up-regulation of fibroblast proliferation, migration, expansion, and the synthesis of extracellular matrices (ECM) such as collagens, and finally leading to implant encapsulation.7 Thereupon, fibroblasts can differentiate into myofibroblasts, characterized by expression of an ED-A splice variant of fibronectin (ED-A FN) and α-smooth muscle actin (α-SMA), which are incorporated into stress fibres and thereby produce contractile forces to promote wound healing, but also scar formation.8 The normal wound healing processes are desired after implantation; however, the prolonged presence of myofibroblasts can lead to excessive collagen production and tissue contraction, which turns into fibrosis ultimately.9 Since fibrosis is one of the key factors leading to the failure of medical implants, it has become a ubiquitous problem and global burden.In order to know more about fibrosis and improve the implant function and longevity, intensive research work has been done to study the host responses by using different in vitro and in vivo models.10–13 Due to the key effects of macrophages and fibroblasts in the regulation of inflammatory and wound healing/fibrotic reactions, different in vitro macrophage/fibroblast co-culture models have been used to study inflammation and fibrosis.14,15 However, most of the studies focused only on the pro-inflammatory responses while the studies of the fibrotic reactions are largely missing.16 On the other hand, self-assembling monolayers (SAMs) or micron-sized particles with different functional groups, were implanted in subcutaneous air pouches of a mice model to investigate the surface chemistry effect on fibrous capsule formation.17 But a systematic study to correlate the surface properties with a wide scale of inflammatory reactions and fibrotic processes is still lacking. Furthermore, macrophages and fibroblasts were used to correlate the effect of surface properties on cellular behaviour, during which conditioned medium from one cell type was used to challenge the other one.18 This leads to the lack of in situ signal exchanges, and also a more accurate method to mimic the complexity of the in vivo situations is missing.
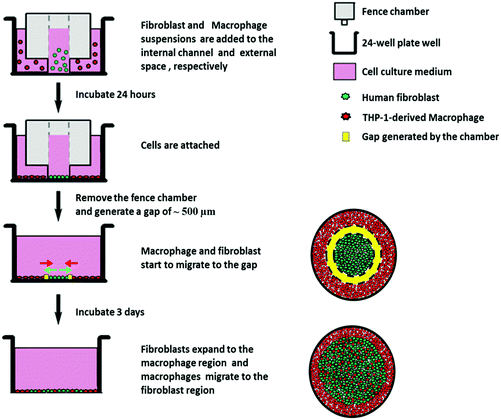
To overcome the limitations of the previous models, we present here an in vitro fibroblast/macrophage co-culture system with the use of migration fence chambers, which possess an internal and an external compartment, to allow seeding of fibroblast and macrophages separately on model biomaterials. After 24 h incubation, the fence chambers were removed to allow the exchange of soluble signals, and at the same time, for fibroblast outgrowth, macrophage migration and interaction between the two cell types. THP-1 cells were applied and differentiated into macrophage-like cells by treatment with phorbol-12-myristate-13-acetate (PMA)19 due to their uniform genetic background with the advantage of no donor variation, and also these are more easily obtained from cell cultures than the monocytes isolated from blood.20 Here, PMA-induced, but not M1 or M2 polarized macrophages were used due to the flexibility of the PMA-induced macrophage model, which can allow the switch from one to another phenotype when responding to different stimuli.21 On the other hand, SAMs possess a well-defined chemistry to tailor the material surfaces to obtain specific surface properties and are suitable to study the interactions with proteins and cells.22,23 Therefore, SAMs with methyl (CH3), amine (NH2), hydroxyl (OH) and carboxyl (COOH) terminated groups having different wettabilities and surface potentials were used here as model surfaces to compare the surface chemistry effect on the host responses by fibroblasts and macrophages. The host reactions on different SAM surfaces were studied with respect to macrophage fusion, fibroblast attachment, spreading morphology, fibroblast proliferation, outgrowth, as well as pro-(IL-6) and anti-(IL-10) inflammatory cytokine production and expression of two markers for myofibroblasts, namely ED-A FN and α-SMA. The results are reported herein.
2. Materials and methods
2.1 Preparation of self-assembling monolayers (SAMs)
Round glass cover slides (∅ 15 mm, Menzel, Germany) were cleaned with 0.5 M NaOH in 96% ethanol (Roth, Germany) for 2 h. Subsequently, the slides were extensively rinsed with double-distilled water (10 × 5 min) and dried under nitrogen flow.SAMs with different terminated groups were obtained by immersing the cleaned glass slides in different silane solutions. Chlorodimethyloctadecylsilane (ODS), 3-aminopropyltriethoxysilane (APTES), glycidoxypropyl trimethoxysilane (GPTMS) and triethoxysilylpropyl succinic anhydride (TESPSA) were all obtained from ABCR (Karlsruhe, Germany). The formation of SAMs was performed according to the previously described report24 with slight modifications, and is also reported in our previous work.15 Briefly, CH3-terminated SAMs were generated by immersion of clean glass in a 5% (v/v) solution of ODS in n-hexane for 1 h at room temperature. Then the surfaces were washed with n-hexane (2 × 5 min), ethanol (2 × 5 min), and double-distilled water (6 × 5 min) and dried under nitrogen flow. NH2-, epoxy- and COOH-terminated surfaces were obtained by immersion of clean glass in 1% (v/v) solution of APTES, GPTMS and TESPSA, respectively, in ethanol for 16 h at room temperature. After immersion, the surfaces were rinsed extensively with ethanol, washed with double-distilled water (10 × 5 min) and dried with nitrogen. The OH-terminated surfaces were obtained by further treatment of epoxy-terminated surfaces in 100 mM HCl at 80 °C for 1 h.24
2.2 Characterization of the surface properties of SAMs
2.3 Cell experiments
The cells of the human monocytic cell line THP-1 (DSMZ, Braunschweig, Germany) were cultured in RPMI-1640 medium (Biochrom AG, Germany) supplemented with 10% (v/v) FBS (Biochrom AG) and 1% (v/v) AAS (Sigma-Aldrich, Germany) at 37 °C in a humidified 5% CO2/95% air atmosphere. The suspended cells were separated by centrifugation. The old medium was removed and the cell pellet was resuspended in fresh medium every second day in order to maintain a cell density of 0.5–1.0 × 106 cells per mL. The macrophages were obtained by incubation of THP-1 cells with 200 nM phorbol-12-myristate-13-acetate (PMA, Sigma-Aldrich, Germany) for 48 h in T75 cell culture flasks (Greiner Bio-One, Frickenhausen, Germany).25 Afterwards, the macrophages were detached by 0.25% trypsin/0.02% EDTA (Biochrom AG) and used for seeding in the co-culture systems. The characterization of macrophages by immunofluorescence and flow cytometry was performed in separate experiments and is outlined in more detail in the ESI.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, Sigma, Germany) for 30 min at room temperature. Subsequently, after washing with PBS twice, the secondary goat-anti-mouse antibody conjugated with CY2 (1
100, Sigma, Germany) for 30 min at room temperature. Subsequently, after washing with PBS twice, the secondary goat-anti-mouse antibody conjugated with CY2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, Dianova, Germany) were incubated with the cells for another 30 min. Actin and nuclei were stained by using Bodipy® Phalloidin (1
100, Dianova, Germany) were incubated with the cells for another 30 min. Actin and nuclei were stained by using Bodipy® Phalloidin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, Invitrogen, Germany) and TO-PRO3 (1
50, Invitrogen, Germany) and TO-PRO3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Invitrogen, Germany) by 30 min incubation each. The samples were then washed, mounted with Mowiol (Calbiochem, Darmstadt, Germany) and examined with confocal laser scanning microscopy (CLSM, LSM 710, Carl Zeiss, Oberkochen, Germany) using a 63× oil immersion objective. Images were processed with the ZEN2011 software (Carl Zeiss, Oberkochen, Germany).
500, Invitrogen, Germany) by 30 min incubation each. The samples were then washed, mounted with Mowiol (Calbiochem, Darmstadt, Germany) and examined with confocal laser scanning microscopy (CLSM, LSM 710, Carl Zeiss, Oberkochen, Germany) using a 63× oil immersion objective. Images were processed with the ZEN2011 software (Carl Zeiss, Oberkochen, Germany).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were added to each well and incubated at 37 °C for 2 h. Thereafter, 100 μL of the supernatant from each well was transferred to a black 96-well plate, and the fluorescence intensities were measured at an excitation wavelength of 544 nm and emission wavelength of 590 nm with a plate reader (FLUOstar, BMG LabTech, Offenburg, Germany).
1) were added to each well and incubated at 37 °C for 2 h. Thereafter, 100 μL of the supernatant from each well was transferred to a black 96-well plate, and the fluorescence intensities were measured at an excitation wavelength of 544 nm and emission wavelength of 590 nm with a plate reader (FLUOstar, BMG LabTech, Offenburg, Germany).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, Santa Cruz Biotechnology, Germany), secondary antibody CY2 (1
100, Santa Cruz Biotechnology, Germany), secondary antibody CY2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, Dianova, Germany), CY3-conjugated anti-α-SMA (1
100, Dianova, Germany), CY3-conjugated anti-α-SMA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, Sigma-Aldrich, Germany) and TO-PRO3 (1
200, Sigma-Aldrich, Germany) and TO-PRO3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Invitrogen, Germany) one after another, by 30 min incubation each. The samples were then washed, mounted with Mowiol and examined with CLSM (Carl Zeiss, Oberkochen, Germany) using a 20× objective. The images were processed with the ZEN2011 software (Carl Zeiss, Germany).
500, Invitrogen, Germany) one after another, by 30 min incubation each. The samples were then washed, mounted with Mowiol and examined with CLSM (Carl Zeiss, Oberkochen, Germany) using a 20× objective. The images were processed with the ZEN2011 software (Carl Zeiss, Germany).
2.4 Statistics
All data are represented as mean values ± standard deviations (SD). Statistical examination was performed using one-way analysis of variance (ANOVA) followed by post-hoc Tukey testing. The significance level was set at p < 0.05 and indicated by an asterisk. The number of samples has been indicated in the respective figure caption.3. Results and discussion
3.1 Surface properties of SAMs
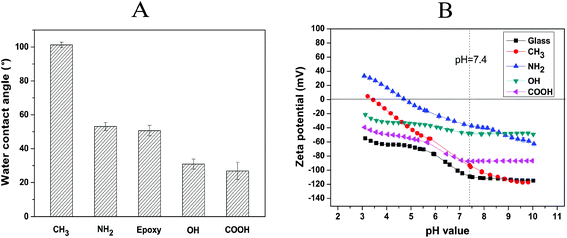
The wettability of the SAM surfaces was evaluated by static water contact angle (WCA) measurements (Fig. 2A). As expected, –CH3 terminated SAMs provided an extremely hydrophobic substrate with WCA ∼ 101°, while the –NH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 and –epoxy28 terminated SAMs formed moderately wettable substrates with WCA ∼ 53° and 51°, respectively. By contrast, SAMs terminated with –COOH groups exhibited highly wettable surfaces with WCA ∼ 27°, which is in good agreement with the published values.29 Furthermore, the WCA of epoxy-terminated surfaces decreased from 51° to 31° after modification with HCl, indicating the successful conversion of epoxy to OH groups according to previous protocols.24 In general, the WCA values of the –CH3, –NH2, –epoxy, –OH and –COOH terminated SAMs corresponded well with previous studies.22,30
27 and –epoxy28 terminated SAMs formed moderately wettable substrates with WCA ∼ 53° and 51°, respectively. By contrast, SAMs terminated with –COOH groups exhibited highly wettable surfaces with WCA ∼ 27°, which is in good agreement with the published values.29 Furthermore, the WCA of epoxy-terminated surfaces decreased from 51° to 31° after modification with HCl, indicating the successful conversion of epoxy to OH groups according to previous protocols.24 In general, the WCA values of the –CH3, –NH2, –epoxy, –OH and –COOH terminated SAMs corresponded well with previous studies.22,30
Fig. 2B shows the zeta potentials versus the pH value of a standard electrolyte solution (1 mM KCl) for bare glass and different SAM surfaces. The bare glass, OH, and COOH SAMs exhibited negative surface potentials throughout the whole measured pH range. By contrast, CH3 and NH2 SAMs were found to have a point of zero charge (PZC) at pH 3.4 and 4.8, respectively. The highest potential at pH 7.4 was found on the –NH2 terminated SAM surface, which is attributed to the partial protonation of amino groups in the low pH region.31 The neutral –OH and –CH3 terminated surfaces displayed negative zeta potentials at pH 7.4 because of a preferential anion adsorption on the non-charged surfaces.32 The curve for the –COOH terminated surface possessed a negative surface potential from pH 3–10 and a plateau in the basic pH region arising from the presence of dissociable acidic groups on this surface.31 Additionally, the zeta potential values of all SAM-modified surfaces were higher compared to bare glass at pH 7.4, which indicates the successful immobilization of the organosilanes and the formation of SAMs on the bare glass substrate.
Physical characterization studies confirmed that the SAMs possessed different wetting and surface charge properties, which made them applicable as model surfaces to study the effect of surface properties on fibroblast and macrophage reactions as described in the following sections.
3.2 HF adhesion, spreading and proliferation
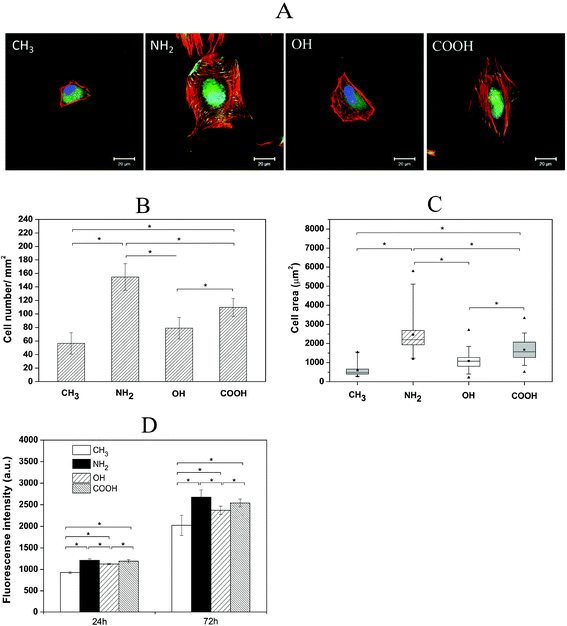
As fibroblasts are one of the key effector cells during wound healing and fibrotic processes and also the initial inflammatory phase after a biomaterial is implanted, the initial adhesion, spreading as well as the following proliferation and outgrowth of fibroblasts crucial for the potential wound healing and potential development of fibrosis were studied here.We firstly evaluated the HF morphology during initial cell adhesion on different SAMs by analysis of the focal adhesion complexes using vinculin (green) and actin (red) staining as shown in Fig. 3A. After 4 h incubation in the presence of serum, HFs adhering to NH2 and COOH SAMs were well spread and contained short focal adhesion plaques at the cell periphery. Strong actin stress fibres were also formed. Additionally, a colocalization of actin and vinculin was observed, illustrated by the yellow colour. By contrast, adhesion and spreading of HFs on CH3 and OH SAMs were largely suppressed. No focal adhesions or actin stress fibres were observed on these surfaces. Considering the presence of 10% serum in the medium, it can be anticipated that NH2 and COOH SAMs are favourable to bind adhesion-promoting proteins such as fibronectin and vitronectin from serum,33,34 which promoted fibroblast adhesion and spreading. By contrast, the OH surface was reported to inhibit protein adsorption, and thus impair cell adhesion.29 On the other hand, although CH3 surfaces can bind larger quantities of proteins, the conformation of the adsorbed proteins can easily change due to the hydrophobic interactions between the surfaces and the hydrophobic domains of proteins.35 As a result, fibroblast adhesion and spreading were also supressed on this surface.
Apart from the HF morphology studies, a quantitative estimation of cell adhesion (cell count) and spreading (cell area) were conducted on different SAM surfaces after 4 h incubation in the presence of serum (Fig. 3B and C). The results show that there were significantly more cells adhering on NH2 and COOH SAMs than on OH and CH3 SAMs. The analysis of cell spreading data also revealed significantly a higher spreading area of HFs on NH2 and COOH surfaces in comparison to OH and CH3 surfaces. These findings are well in line with previous observations showing that cells prefer to adhere and spread more on moderately hydrophilic and hydrophilic/anionic surfaces compared to hydrophobic and non-ionic hydrophilic surfaces.22,29,36
The proliferation of HFs on different SAM surfaces was studied after the incubation periods of 24 and 72 h. Fig. 3D shows the metabolic activity of HFs measured from the QBlue assay that represents an indication of the quantity of metabolically active cells on the different SAMs. The obtained data illustrate that cell growth was higher on NH2 and COOH surfaces compared to OH and CH3 surfaces after both 24 and 72 h, which is in line with the observed HF adhesion and spreading results. In general, the surface properties dictate the protein adsorption from serum-containing medium, which affects the ligand binding to adhesion receptors of cells like integrins.33,37 Such a ligand–receptor binding mediates cell adhesion, signal transduction followed by cytoskeleton reorganization, protein synthesis and cell proliferation.38
3.3 HF outgrowth studies
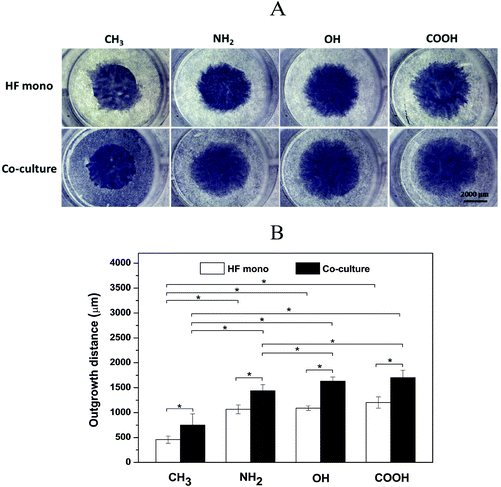
HF migration and growth are very important during the processes associated with wound healing and fibrosis and strongly affected by cytokine release from macrophages.39 Therefore, we further evaluated the effects of surfaces on HF growth by a specific outgrowth study. Fig. 4A shows the crystal violet staining images of HF outgrowth after 3 days in the presence (co-culture) or absence (HF mono) of macrophages. The macrophages derived from the THP-1 monocyte cell line after induction with PMA used here represent an early phenotype positive for CD 68, a common macrophage marker, but not already committed to M1 or M2 phenotypes as found out by immunofluorescence studies and flow cytometry (see the ESI† for more details). During these experiments, the macrophages were seeded at the same time as that for HFs, but in the external compartment of the chamber, which separated them by a 500 μm gap after lifting up the chamber. Fig. 4B depicts the quantitative outgrowth distances on different SAMs after subtracting the original diameter of the HF region. It was observed that the CH3 SAM induced the smallest outgrowth distance in both HF mono- and co-cultures. This can be explained by the impaired HF adhesion and proliferation due to the high hydrophobicity of this surface, leading to conformational changes of adsorbed adhesive proteins and impaired cell responses.29,40 Contradictorily, the NH2 SAM with the highest cell adhesion and proliferation in single cell cultures shown before, generated only an intermediate outgrowth, less than OH and COOH SAMs. This might be due to the strong interactions between the HFs and the NH2 SAM, illustrated by the multiple, extended focal adhesion complexes (see Fig. 3A), which caused an impaired ability of cell migration,41 although with higher cell proliferation. The migration of HFs on NH2 and COOH surfaces has been reported by Faucheux et al. previously, also revealing that HFs on the FN-coated COOH surface migrated faster than that on the NH2 surface.29 Additionally, it is notable that the OH SAM caused a comparable outgrowth distance like that of the COOH SAM. This might be probably due to the weak adhesion of HFs on the OH SAM, resulting in a higher migration ability.30,39 Besides, the OH SAM provoked a moderate proliferation of HFs as observed in the proliferation studies shown here and by others.42 However, the most important finding of these experiments was that the outgrowth distance was significantly increased in co-cultures compared to fibroblast mono-cultures on all SAMs. During these experiments it was also observed that the effect of surface chemistry on HF outgrowth was the same as observed in the HF monoculture experiments. The increased outgrowth distance is obviously attributed to the presence of the surrounding macrophages. It was reported previously that cytokines such as IL-1β and IL-6 secreted by macrophages promote HF migration and proliferation,43,44 which supports the findings made here.3.4 Macrophage fusion studies
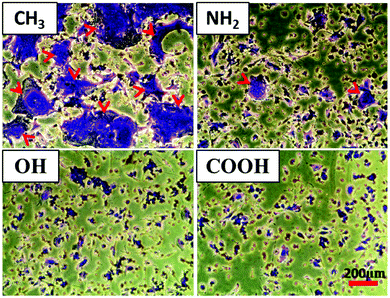
In addition to the analysis of the HF outgrowth distance, the macrophage fusion on different SAM surfaces was also evaluated in the macrophage regions of the co-culture system (Fig. 5). It can be seen that large quantities of foreign body giant cells (FBGCs) were formed on the CH3 SAM, illustrated by the large size of cells shown by crystal violet staining (red arrows). By contrast, fewer FBGCs with lower size were found on NH2, OH and COOH SAMs. Macrophages fuse to form FBGCs in order to increase their phagocytosis ability when facing foreign materials of a larger size than themselves.45 It was also found that the fusion extent represents a measure of biocompatibility of a given material.46 Hence, the difference in FBGC formation classifies the CH3 SAM as more pro-inflammatory than the other SAMs.3.5 Pro- and anti-inflammatory cytokine production
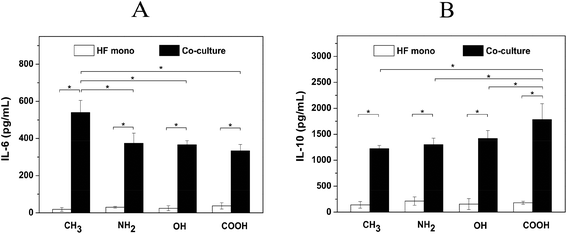
The soluble signals including pro- and anti-inflammatory cytokines are important mediators during inflammatory and wound healing/fibrotic processes. Macrophages have a high degree of functional plasticity, which can act as both M1 inflammatory and M2 wound healing phenotype in response to different stimuli.21 M1 macrophages display more pro-inflammatory function by secretion of cytokines such as IL-1β, IL-6 and TNF-α, while M2 macrophages have anti-inflammatory function and promote wound healing by secretion of cytokines like IL-10 and TGF-β1.47 Besides, fibroblasts not only can modulate the cytokine production by macrophages but also can generate both pro- and anti-inflammatory cytokines including IL-1β, IL-6, TGF-β and IL-10.48,49 The balance between the pro- and anti-inflammatory mediators is critical for orchestrating the outcome of the inflammatory and wound healing/fibrotic responses. Therefore, a premature type of macrophage still not committed to the M1 or M2 phenotype was used here as generated by the use of PMA, only. We tested here both the pro-inflammatory cytokine IL-6 and anti-inflammatory cytokine IL-10 production on different SAMs.Fig. 6A shows that there was no significant difference of IL-6 release among the various SAMs in HF mono-cultures. This might be related to the limited HF population due to the low seeding density in the internal channel of the chambers (0.15 mL of HFs at 2.5 × 104 cells per mL) and limited proliferation of HFs in the absence of serum. By contrast, IL-6 released from cells adherent to different SAMs in co-cultures showed a surface-dependent behavior. It was found that the cells adhering on the CH3 SAM produced significantly higher levels of IL-6 in comparison to the other SAMs. This result indicates that IL-6 production is provoked by the hydrophobic nature of the CH3 SAM, which is in consistency with the previous findings that hydrophobic surfaces induced the highest levels of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α.15,18,50 On the other hand, IL-10 production showed an opposite surface-dependent effect compared to IL-6 in co-cultures (Fig. 6B). The results show that the cells cultured on the COOH SAM produced significantly higher amounts of IL-10 than on CH3, NH2 and OH SAMs. This is also in line with other reports documenting that the hydrophilic/anionic surfaces reduce pro-inflammatory responses and promote the wound healing processes.4 Additionally, the production of both IL-6 and IL-10 increased significantly in fibroblast/macrophage co-cultures compared to HF mono-cultures on all SAMs. This indicates that macrophages contribute largely to both pro- and anti- inflammatory signal production. Moreover, a much higher amount of IL-10 compared to IL-6 was observed for the same samples. This might imply a reduced inflammatory response and an increased wound healing response under the measuring conditions.
3.6 ED-A FN and α-SMA expression
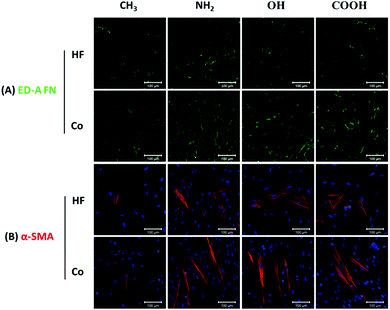
Myofibroblast phenotype transformation is an essential step during wound healing and fibrotic processes, which is normally accompanied by abundant ED-A FN and α-SMA expression in these cells.8 TGF-β1 can stimulate fibroblast differentiation into myofibroblasts and is known as an important contributor to fibrosis both in vitro and in vivo.51 Hence, the levels of ED-A FN and α-SMA expression on different SAMs were studied in the presence of TGF-β1 as also done in other studies52,53 to mimic the in vivo situation to some extent.Fig. 7A presents the ED-A FN expression on different SAM surfaces in both HF mono- and co-cultures. A comparison of the staining revealed obvious surface chemistry-dependent differences with the NH2 SAM expressing the highest level of ED-A FN in both mono- and co-cultures. By contrast, on the CH3 surface considerably lower levels of ED-A FN were detected, while COOH and OH surfaces exhibited intermediated expression compared to NH2 and CH3 SAMs. Overall, the ED-A FN expression results corresponded well to the HF adhesion, proliferation and outgrowth experiments and showed an order of NH2 > COOH ∼ OH > CH3. Besides, the markedly increased expression levels of ED-A FN in all co-cultures revealed that macrophages amplified the ED-A FN matrix synthesis by HFs. Indeed, a large body of research studies have confirmed that macrophage contributes to wound healing and fibrosis by promoting fibroblast activation, proliferation and differentiation into myofibroblasts.43,44
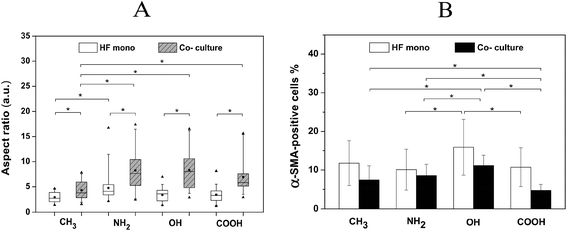
Furthermore, the expression of α-SMA, a key feature of their transformation into myofibroblast during wound healing and fibrosis, was also analysed on the different SAMs (Fig. 7B). Notably more α-SMA positively stained HFs (α-SMA-positive HFs) were observed on the NH2, OH and COOH surfaces in comparison to the CH3 surface. Moreover, the α-SMA-positive HFs on all co-culture surfaces were much more elongated than those in the corresponding mono-cultures. Fig. 8A depicts the quantitative evaluation of the cell aspect ratio of the α-SMA-positive HFs on the different SAMs. First, no significant difference of the aspect ratio was observed among NH2, OH and COOH SAMs in both HF mono- and co-cultures. By contrast, the cells on the CH3 SAM exhibited a significantly lower aspect ratio than NH2, OH and COOH SAMs in co-cultures. The elongation extent of the cells represents their contractile capability, which plays important roles in wound healing and fibrotic processes. Therefore, the in vitro data obtained here may suggest that the hydrophobic CH3 SAM had lower potential of promoting wound healing and fibrosis. Furthermore, the aspect ratio of the α-SMA-positive HFs in all co-cultures significantly increased compared to the corresponding mono-cultures. This can be attributed to the pro-wound healing and pro-fibrotic activities of the macrophages in co-cultures.21
The determination of the percentage of α-SMA-positive cells shown in Fig. 8B reveals that the OH surface caused the highest percentage of α-SMA-positive cells, while the COOH surface induced the lowest percentage, following the order OH > NH2 ∼ CH3 > COOH. Here, the higher adhesion and outgrowth of the fibroblasts on the hydrophilic/anionic COOH SAM resulted in the lowest percentage of α-SMA-positive cells, indicating a lower potential of inducing wound healing/fibrosis. This might also be related to the highest IL-10 cytokine production on this surface, which was reported to have both anti-inflammatory and anti-fibrotic effects.54 By contrast, the hydrophilic OH surface provoked a lower extent of pro-inflammatory response in terms of FBGC formation and pro-inflammatory cytokine IL-6 production, but more myofibroblasts than the other surfaces. This indicates that OH-functionalized surfaces have a higher potential of inducing wound healing/fibrosis. These findings are generally consistent with previous work using polypropylene microspheres with different functional groups as also used here to study the extent of fibrotic tissue reactions elicited by the different functionalities, and their data show that the OH surface triggers the strongest capsule formation while the COOH surface promotes the least capsule formation.55 It was suggested that the complement activation can affect the recruitment of inflammatory cells and the following fibrotic tissue reactions.56 The OH groups are reported to bind with the C3b component and activate the alternative complement pathway to a much greater extent than COOH groups,57 which may partly explain the findings here.
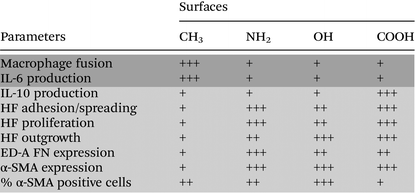
A summary of the measured parameters involved in the inflammatory and wound healing/fibrotic responses on CH3, NH2, OH and COOH SAMs is shown in Table 1. Firstly, we observed that the hydrophilic/anionic COOH SAM caused lower inflammatory reactions in comparison to the CH3 SAM. Furthermore, although the highest fibroblast adhesion, proliferation, outgrowth, as well as strong expression of ED-A FN and α-SMA were found on the COOH SAM, the percentage of the α-SMA positive cells was the lowest, indicating a lower extent of inducing wound healing/fibrosis. Therefore, it can be concluded that the hydrophilic/anionic COOH SAM has both anti-inflammatory and anti-fibrotic potential. Secondly, it was observed that the hydrophobic CH3 SAM with the highest potential of inducing inflammatory responses illustrated by the highest level of FBGC formation and IL-6 production revealed a low potential of inducing wound healing/fibrotic responses through the observation of fibroblast activations. Here, the low wound healing/fibrotic potential of the CH3 SAM seems to be contradictory to some in vivo findings that hydrophobic surfaces induced thicker fibrous capsules.58 However, we wanted to clarify that we have seeded fibroblasts at a very low density here, which is different from the in vivo situations that a full coverage of fibroblasts on the surfaces can be achieved. Thus we suggest that the impaired fibroblast adhesion, spreading, proliferation and outgrowth on the hydrophobic CH3 surface here should be responsible for the reduced potential of inducing the wound healing/fibrotic reactions. On the other hand, the hydrophilic OH SAM triggered a low level of inflammatory responses but indicated a higher potential of inducing wound healing/fibrotic reactions, which is consistent with the poor biocompatibility of this surface reported also by others.55 Furthermore, the results also reveal that the inflammatory and wound healing/fibrotic responses might not always be consistent.59 Further in-depth inflammation–wound healing/fibrosis link studies will help to design biomaterials with appropriate fibrotic tissue responses.
4. Conclusion
In the present study, we have established an in vitro fibroblast/macrophage co-culture system to study the host responses ranging from inflammatory to wound healing/fibrotic responses of a series of SAMs with different terminating groups as model materials. It was found that the hydrophilic/anionic COOH SAM caused low levels of both inflammatory and wound healing/fibrotic responses while the hydrophobic CH3 surfaces possess the highest potential of inducing inflammatory responses but induced a low level of wound healing/fibrotic responses. By contrast, the OH SAM which evoked a lower extent of pro-inflammatory responses, but revealed a higher potential of inducing wound healing/fibrosis. These observations can provide useful clues for the design of biomaterials by triggering appropriate host responses for different biomedical and clinical applications. More important however is the obvious advantage of the presented in vitro fibroblast/macrophage co-culture system, which can generate a spatially controlled interaction between the macrophages and the fibroblasts to study the host responses of biomaterials in vitro. The results showed that macrophages can promote wound healing/fibrotic responses by up-regulation of fibroblast outgrowth, cytokine production as well as ED-A FN and α-SMA expression in co-cultures. Lastly but importantly, one can also study here the in vitro effects of additional factors like cytokines and other bioactive molecules on the interplay between the two cell types using our co-culture system.Disclosure
No conflicts of interests are declared.Acknowledgements
G. Zhou thanks the China Scholarship Council (CSC) and the Prorectorate for Structur and strategic development of Martin Luther University Halle-Wittenberg together with the Federal Ministry of Education and Research (BMBF) for offering the scholarships to work in Germany. Mrs M. Porobin is gratefully acknowledged for the assistance of zeta potential measurement. We are also very grateful to Dr A. Navarrete Santos from the University Medical Centre, Core Facility Centre of Martin Luther University Halle-Wittenberg for the kind support to perform the flow cytometry measurements for cell analysis and cell sorting.Notes and references
- J. M. Anderson, A. Rodriguez and D. T. Chang, Semin. Immunol., 2008, 20, 86–100 CrossRef CAS PubMed.
- T. Glaros, M. Larsen and L. W. Li, Front. Biosci., 2009, 14, 3988–3993 CrossRef CAS.
- S. Franz, S. Rammelt, D. Scharnweber and J. C. Simon, Biomaterials, 2011, 32, 6692–6709 CrossRef CAS PubMed.
- W. G. Brodbeck, Y. Nakayama, T. Matsuda, E. Colton, N. P. Ziats and J. M. Anderson, Cytokine, 2002, 18, 311–319 CrossRef CAS PubMed.
- G. Zhou, M. S. Niepel, S. Saretia and T. Groth, J. Biomed. Mater. Res., Part A, 2016, 104, 493–502 CrossRef CAS PubMed.
- G. Zhou, H. Al-Khoury and T. Groth, Int. J. Artif. Organs, 2016, 39, 37–44 CrossRef CAS PubMed.
- T. A. Wynn, J. Pathol., 2008, 214, 199–210 CrossRef CAS PubMed.
- G. Serini, M. L. Bochaton-Piallat, P. Ropraz, A. Geinoz, L. Borsi, L. Zardi and G. Gabbiani, J. Cell Biol., 1998, 142, 873–881 CrossRef CAS PubMed.
- S. E. Mutsaers, J. E. Bishop, G. McGrouther and G. J. Laurent, Int. J. Biochem. Cell Biol., 1997, 29, 5–17 CrossRef CAS PubMed.
- Z. Yuan, J. Zhao, W. Zhu, Z. Yang, B. Li, H. Yang, Q. Zheng and W. Cui, Biomater. Sci., 2014, 2, 502–511 RSC.
- C. Helary, A. Abed, G. Mosser, L. Louedec, D. Letourneur, T. Coradin, M. M. Giraud-Guille and A. Meddahi-Pellé, Biomater. Sci., 2015, 3, 373–382 RSC.
- W. J. Hu, J. W. Eaton and L. P. Tang, Blood, 2001, 98, 1231–1238 CrossRef CAS PubMed.
- A. P. Marques, R. L. Reis and J. A. Hunt, Macromol. Biosci., 2005, 5, 775–785 CrossRef CAS PubMed.
- K. G. Battiston, R. S. Labow and J. P. Santerre, Biomaterials, 2012, 33, 8316–8328 CrossRef CAS PubMed.
- G. Zhou, H. Loppnow and T. Groth, Acta Biomater., 2015, 26, 54–63 CrossRef CAS PubMed.
- M. R. MacEwan, W. G. Brodbeck, T. Matsuda and J. M. Anderson, J. Biomed. Mater. Res., Part A, 2005, 74A, 285–293 CrossRef CAS.
- J. N. Barbosa, P. Madureira, M. A. Barbosa and A. P. Aguas, J. Biomed. Mater. Res., Part A, 2006, 76A, 737–743 CrossRef CAS PubMed.
- F. F. R. Damanik, T. C. Rothuizen, C. van Blitterswijk, J. I. Rotmans and L. Moroni, Sci. Rep., 2014, 4, 6325 CrossRef CAS PubMed.
- E. K. Park, H. S. Jung, H. I. Yang, M. C. Yoo, C. Kim and K. S. Kim, Inflammation Res., 2007, 56, 45–50 CrossRef CAS PubMed.
- A. Schildberger, E. Rossmanith, T. Eichhorn, K. Strassl and V. Weber, Mediators Inflammation, 2013, 2013, 697972 Search PubMed.
- P. J. Murray and T. A. Wynn, Nat. Rev. Immunol., 2011, 11, 723–737 CrossRef CAS PubMed.
- G. Altankov, F. Grinnell and T. Groth, J. Biomed. Mater. Res., 1996, 30, 385–391 CrossRef CAS PubMed.
- V. Llopis-Hernandez, P. Rico, J. Ballester-Beltran, D. Moratal and M. Salmeron-Sanchez, PLoS One, 2011, 6, e19610 CAS.
- G. K. Toworfe, R. J. Composto, I. M. Shapiro and P. Ducheyne, Biomaterials, 2006, 27, 631–642 CrossRef CAS PubMed.
- J.-W. Tjiu, J.-S. Chen, C.-T. Shun, S.-J. Lin, Y.-H. Liao, C.-Y. Chu, T.-F. Tsai, H.-C. Chiu, Y.-S. Dai, H. Inoue, P.-C. Yang, M.-L. Kuo and S.-H. Jee, J. Invest. Dermatol., 2009, 129, 1016–1025 CrossRef CAS PubMed.
- E. G. Fischer, A. Stingl and C. J. Kirkpatrick, J. Immunol. Methods, 1990, 128, 235–239 CrossRef CAS PubMed.
- V. Katzur, M. Eichler, E. Deigele, C. Stage, P. Karageorgiev, J. Geis-Gerstorfer, G. Schmalz, S. Ruhl, F. Rupp and R. Muller, J. Colloid Interface Sci., 2012, 366, 179–190 CrossRef CAS PubMed.
- I. Luzinov, D. Julthongpiput, A. Liebmann-Vinson, T. Cregger, M. D. Foster and V. V. Tsukruk, Langmuir, 2000, 16, 504–516 CrossRef CAS.
- N. Faucheux, R. Schweiss, K. Lutzow, C. Werner and T. Groth, Biomaterials, 2004, 25, 2721–2730 CrossRef CAS PubMed.
- J. Benesch, J. F. Mano and R. L. Reis, Acta Biomater., 2010, 6, 3499–3505 CrossRef CAS PubMed.
- J. J. Shyue and M. R. De Guire, Langmuir, 2004, 20, 8693–8698 CrossRef CAS PubMed.
- C. Werner, H. Korber, R. Zimmermann, S. Dukhin and H. J. Jacobasch, J. Colloid Interface Sci., 1998, 208, 329–346 CrossRef CAS PubMed.
- B. G. Keselowsky, D. M. Collard and A. J. Garcia, Biomaterials, 2004, 25, 5947–5954 CrossRef CAS PubMed.
- F. Grinnell and M. K. Feld, J. Biol. Chem., 1982, 257, 4888–4893 CAS.
- B. G. Keselowsky, D. M. Collard and A. J. Garcia, J. Biomed. Mater. Res., Part A, 2003, 66A, 247–259 CrossRef CAS PubMed.
- Y. Arima and H. Iwata, Biomaterials, 2007, 28, 3074–3082 CrossRef CAS PubMed.
- N. M. Alves, I. Pashkuleva, R. L. Reis and J. F. Mano, Small, 2010, 6, 2208–2220 CrossRef CAS PubMed.
- S. Miyamoto, H. Teramoto, O. A. Coso, J. S. Gutkind, P. D. Burbelo, S. K. Akiyama and K. M. Yamada, J. Cell Biol., 1995, 131, 791–805 CrossRef CAS PubMed.
- J. J. Zhao, L. Hu, N. Y. Gong, Q. M. Tang, L. Y. Du and L. L. Chen, Tissue Eng., Part A, 2015, 21, 982–991 CrossRef CAS PubMed.
- G. Hummer, S. Garde, A. E. Garcia, A. Pohorille and L. R. Pratt, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 8951–8955 CrossRef CAS.
- A. Huttenlocher and A. R. Horwitz, Cold Spring Harbor Perspect. Biol., 2011, 3, a005074 Search PubMed.
- G. Altankov, K. Richau and T. Groth, Materialwiss. Werkstofftech., 2003, 34, 1120–1128 CrossRef CAS.
- F. Y. Chow, D. J. Nikolic-Paterson, R. C. Atkins and G. H. Tesch, Nephrol., Dial., Transplant., 2004, 19, 2987–2996 CrossRef CAS PubMed.
- S. Van Linthout, K. Miteva and C. Tschope, Cardiovasc. Res., 2014, 102, 258–269 CrossRef CAS PubMed.
- S. MacLauchlan, E. A. Skokos, N. Meznarich, D. H. Zhu, S. Raoof, J. M. Shipley, R. M. Senior, P. Bornstein and T. R. Kyriakides, J. Leukocyte Biol., 2009, 85, 617–626 CrossRef CAS PubMed.
- J. M. Anderson and K. M. Miller, Biomaterials, 1984, 5, 5–10 CrossRef CAS PubMed.
- A. Mantovani, S. Sozzani, M. Locati, P. Allavena and A. Sica, Trends Immunol., 2002, 23, 549–555 CrossRef CAS PubMed.
- H. Pan, H. L. Jiang, S. Kantharia and W. L. Chen, Biomed. Mater., 2011, 6, 065002 CrossRef PubMed.
- K. P. Sundararaj, D. J. Samuvel, Y. Li, J. J. Sanders, M. F. Lopes-Virella and Y. Huang, J. Biol. Chem., 2009, 284, 13714–13724 CrossRef CAS PubMed.
- S. Hamlet, M. Alfarsi, R. George and S. Ivanovski, Clin. Oral Implants Res., 2012, 23, 584–590 CrossRef PubMed.
- M. B. Vaughan, E. W. Howard and J. J. Tomasek, Exp. Cell Res., 2000, 257, 180–189 CrossRef CAS PubMed.
- A. Watarai, L. Schirmer, S. Thoenes, U. Freudenberg, C. Werner, J. C. Simon and U. Anderegg, Acta Biomater., 2015, 25, 65–75 CrossRef CAS PubMed.
- A. van der Smissen, S. Samsonov, V. Hintze, D. Scharnweber, S. Moeller, M. Schnabelrauch, M. T. Pisabarro and U. Anderegg, Acta Biomater., 2013, 9, 7775–7786 CrossRef CAS PubMed.
- J.-H. Shi, H. Guan, S. Shi, W.-X. Cai, X.-Z. Bai, X.-L. Hu, X.-B. Fang, J.-Q. Liu, K. Tao, X.-X. Zhu, C.-W. Tang and D.-H. Hu, Arch. Dermatol. Res., 2013, 305, 341–352 CrossRef CAS PubMed.
- A. Nair, L. Zou, D. Bhattacharyya, R. B. Timmons and L. Tang, Langmuir, 2008, 24, 2015–2024 CrossRef CAS PubMed.
- L. Liu and H. Elwing, J. Biomed. Mater. Res., 1994, 28, 767–773 CrossRef CAS PubMed.
- J. N. Barbosa, M. A. Barbosa and A. P. Aguas, Biomaterials, 2004, 25, 2557–2563 CrossRef CAS PubMed.
- J. N. Barbosa, P. Madureira, M. A. Barbosa and A. P. Águas, J. Biomed. Mater. Res., Part A, 2006, 76A, 737–743 CrossRef CAS PubMed.
- L. P. Tang, Y. L. Wu and R. B. Timmons, J. Biomed. Mater. Res., 1998, 42, 156–163 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6bm00247a |
| This journal is © The Royal Society of Chemistry 2017 |