Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Two decades of antifilarial drug discovery: a review
Jaiprakash N. Sangshetti
 *a,
Devanand B. Shinde
b,
Abhishek Kulkarni
a and
Rohidas Arote
c
*a,
Devanand B. Shinde
b,
Abhishek Kulkarni
a and
Rohidas Arote
c
aY. B. Chavan College of Pharmacy, Dr. Rafiq Zakaria Campus, Rauza Baugh, Aurangabad-(MS), India. E-mail: jnsangshetti@rediffmail.com
bShivaji University, Kolhapur (MS), India
cDepartment of Molecular Genetics, School of Dentistry, Seoul National University, Seoul, Republic of Korea
First published on 11th April 2017
Abstract
Filariasis is one of the oldest, most debilitating, disabling, and disfiguring neglected tropical diseases with various clinical manifestations and a low rate of mortality, but has a high morbidity rate, which results in social stigma. According to the WHO estimation, about 120 million people from 81 countries are infected at present and an estimated 1.34 billion people live in areas endemic to filariasis and are at risk of infection. In this review, we focus on Lymphatic Filariasis (LF) and provide brief insights on some other filarial conditions. Current drug treatments have beneficial effects in the elimination of only the larval stage of the worms. Very few drugs are available for treatment of filariasis but their repetitive use may give rise to drug resistance. They are also found to be fairly ineffective towards eliminating adult worms. Moreover, no effective vaccine is available for treatment of filariasis. Because of these limitations, development of new antifilarials has been of utmost importance, a fact which encourages researchers to discover new drugs having antifilarial activity. Herein we extensively review developments in the field of antifilarials including the types of filariasis, their historical perspectives, eradication programs, different classes of drug (i.e. synthetic as well as natural), explored product-derived different targets, patents and clinical trials.
Introduction
“Overcoming neglected tropical diseases (NTDs) make sense both for economies and for development. The prospects for success have never been so strong. Millions of people are being freed from the misery and disability that have kept populations mired in poverty, generation after generation, for centuries”, according to Dr Margaret Chan, Director-General, World Health Organization.1A variety of NTDs have received less attention globally. Among these, vector-borne filariasis is one of the oldest, most debilitating, disabling and disfiguring of the NTDs. Vector-borne filariasis has a low rate of mortality, but a high morbidity rate, which results in social stigma.2–4 According to estimates, about 120 million people in 81 countries are currently infected, and 1.34 billion people who live in areas endemic to filariasis are at risk of infection.2 Its two major forms are lymphatic filariasis (LF) (responsible vector: mosquito species) and onchocerciasis, also known as river blindness (responsible vector: black fly). In human beings these parasites reside in lymph nodes, in deep connective, cutaneous and subcutaneous tissues or in mesenteries.1,4 Severe symptoms generally start to appear during adulthood and are found more commonly in men than in women. These symptoms include damage to various tissues such as lymphatic system, arms, legs or genitals, causing significant pain, loss of productivity and social isolation.5,6
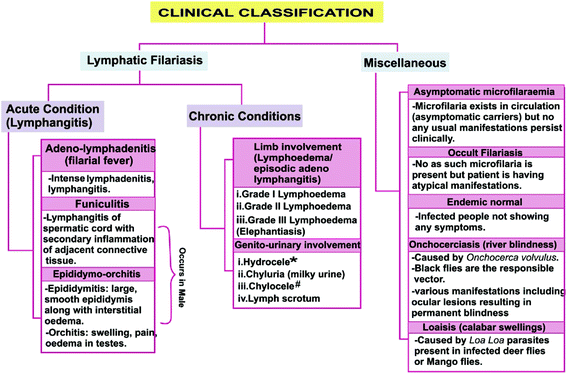
Filariasis exhibits various clinical manifestations depending on various factors such as vector, host immune response, associated microbes or affected site of the host (Fig. 1).7–17 Approximately 40 million people suffer from the stigmatizing and disabling clinical manifestations of the LF among whom 15 million have lymphoedema (elephantiasis), and particularly 25 million men have urogenital swelling, principally scrotal hydrocele.2
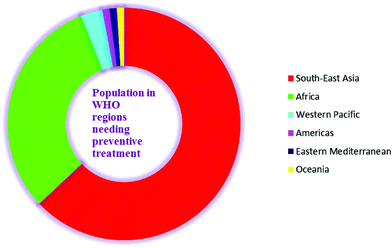
Lymphatic filariasis (LF) is endemic in five of the six WHO regions. Worldwide, 1.39 billion people require preventive chemotherapy. Currently, 877 million people from 9 countries (63–65% of the global population at risk) in the South-East Asia Region and 432 million people from 39 countries (31% of the global population at risk) in the Africa Region account for the maximum global burden of this disease and require preventive treatment. The South-East Asia Region also accounts for approximately 57% of total global burden of disability adjusted life years (DALYs) lost due to LF. From the Western Pacific Region, almost 40 million people are at a risk of infection and account for 3% of the global population at risk. This region is logistically divided into two regions viz. the Mekong-Plus region and the Pacific region. Brunei Darussalam, the Philippines, Lao People's Democratic Republic, Malaysia, Cambodia, and Vietnam are 6 endemic countries in the Mekong-Plus sub region that are at risk of infection. However, in the same region, countries such as China and the Republic of Korea have successfully eliminated this disease. Four endemic countries (Brazil, the Dominican Republic, Haiti and Guyana) in the America Regions account for another 1% with the largest at risk population located in Haiti. Three endemic countries (Egypt, Sudan and Yemen) from the Eastern Mediterranean region account for 1% of the global disease burden, and 16 endemic countries in Oceania account for another 1% (Fig. 2).2,18
Historical perspective on filariasis
Ancient artifacts suggest that filarial disease may have been found as early as 2000 B.C.19 First, ancient documented evidence of filariasis in India was recorded in Sushruta Samhita (approximately 600 B.C.) by the renowned Indian physician Sushruta.20 According to one report, the first reliable documentation of filariasis was found in the late 15th and early 16th centuries.21 Chyluria (passage of lymph in urine) a common condition associated with lymphatic filariasis, was documented by William Prout in 1849. In Paris, the French surgeon Jean-Nicolas was the first person to describe the microfilariae observed in the fluid extracted from hydrocele (1863).22 These microfilariae were first observed by Timothy Lewis in human blood (1872), who was working in India.23 In 1876, the Australian scientist Joseph Bancroft recovered female adult worms of filariasis and named them Filaria bancrofti, and this species was later included in the genus Wuchereria.24 In 1877, Sir Patrick Manson observed some parasitic development in the stomach of mosquito that was fed on the blood of a microfilaremic gardener and thus indicated that filariasis is transmitted by mosquitos.25 Two other species that cause filariasis were first identified in 1960 (Brugia malayi) and in 1977 (B. timori).26Filariasis – an overview
Background, epidemiology and need
The main species of thread like filarial spirunid nematode worms responsible for human LF are Wuchereria bancrofti (W. bancrofti causes almost 90% of infection), Brugia malayi (B. malayi) or B. timori, each of which is transmitted by their own specific insect vector, i.e., through the bite of an infected mosquito.From various species of the vector that causes LF, Anopheles, Culex, Aedes and Mansonia are the leading genera. According to the WHO, the increased numbers of infected hosts, the density of microfilariae in peripheral blood and the biting rate of the vector result in greater chances of microfilarial uptake by mosquito vectors and transmission to healthy people.5
Another worm, i.e., Onchocerca volvulus (an African eye worm), which is a cutaneous and subcutaneous parasite, is responsible for onchocerciasis. Loa loa is another species of worm that causes loaisis. It has also been reported that Wuchereria bancrofti and Onchocerca volvulus do not require an animal reservoir.1,27–32
According to the WHO, filarial transmission in a community is influenced by various factors, such as the number of infected persons, frequency of human-vector contact, density of vector mosquitoes, and the characteristics of the vectors, among others.33 Although W. bancrofti selectively infects humans, other species of filaria may also infect animals. As discussed, approximately two-thirds of the infected peoples are residents of Asia, among which approximately 40% live in India. Data suggests that depressive illness is very common in individuals with LF and accounts for an estimated 5.09 million DALYs (disability-adjusted life years).34–36 After infection, microfilaremia usually start to appear in children with an approximate age of 5 years, but the actual signs of disease generally appear during puberty. Hydroceles also begin to develop in this stage. A 26 year survey conducted over the previous decade indicated that once the infection has been acquired, the chance of becoming naturally free from infection is low.37
Filarial worms that cause lymphatic filariasis by colonizing the lymphatic system live up to 8 years and release millions of microfilariae into the blood. Regarding the elimination of the disease, the Global Alliance to Eliminate Lymphatic Filariasis (GPELF) was created in 2000 with the goal of interrupting the transmission of this disease by 2020 through the use of drugs distribution worldwide.38 Although annual drug treatments may prove helpful in reducing the levels of circulating microfilariae in circulation (thereby disrupting disease transmission), these treatments fail to kill adult worms. These drug treatments include doses of albendazole plus ivermectin, diethylcarbamazine (DEC) fortified salt or DEC plus albendazole.31,39 Because these drugs are only effective against the early stage of parasite (microfilariae) development, they provide only a partial benefit to infected individuals. In addition, their repetitive use may result in drug resistance. They are also fairly ineffective toward adult worms (macrofilariae). Traditional or conventional approaches have provided relatively few classes of agents and no vaccine treatments, which are some of the limitations underlying the pressing need for advancements in antifilarial drug discovery. Thus, researchers are searching for new classes of leads to comfort patients as well as to combat and alleviate this disease on a global scale.39
Diagnosis
LF is primarily diagnosed using the immunochromatographic card test kit via antigen detection methods (which also detects latent infections). The traditional diagnosis of LF is performed by microscopy to detect circulating microfilariae. Molecular xenomonitoring of parasites in mosquitoes, serological testing, ultrasonography, PCR tests, lymphoscintigraphy, detection of exposure to transmission in children via antibody detection and the recently introduced filariasis test strip (FTS) are some of the other diagnostic approaches that are currently used.42–48Life cycle of the parasite-biological process
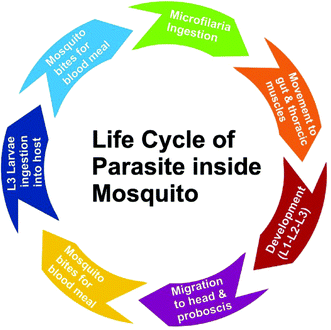
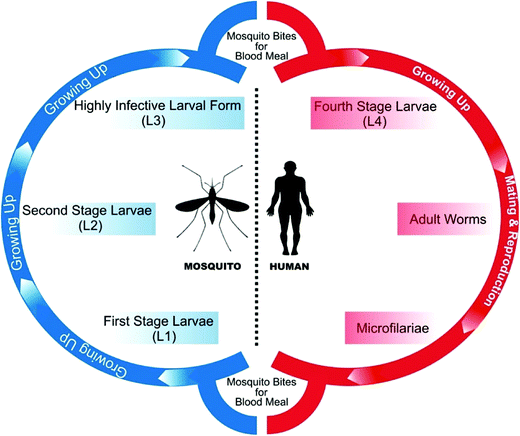
All species of parasitic worms generally have similar life cycles. When a female mosquito bites an infected human for a blood meal, the microfilariae (mf) from its blood circulation are also taken up through bite wound by the mosquito. Inside the stomach of the mosquito, microfilariae lose their sheaths and migrates through the wall of the proventriculus and cardiac portion of the midgut to reach the thoracic muscles where they develop into sluggish first-stage larvae (L1), which subsequently (within 08–12 days) develop into second-stage larvae (L2) and finally into the highly infective larval form (L3) which further migrate through the hemocoel to the mosquito's proboscis. When the infected mosquito has a blood meal from a healthy human, these infectious larvae (male and female) enter the human through the bite wound, migrate into the lymph system of the healthy human and develop into numerous fourth-stage larvae (L4), which are finally converted into adult worms that normally resides in lymphatics. These adults matures in about 9 months and mate in lymph vessels and nodes where they produce microfilariae (177 to 230 μm in length and 5 to 7 μm in width for B. malayi whereas 244 to 296 μm in length by 7.5 to 10 μm in diameter for W. bancrofti) which are sheathed and have nocturnal periodicity except the South Pacific microfilariae which shows the absence of marked periodicity. These microfilariae then migrate into lymph and enter the blood stream reaching the peripheral blood (Fig. 3 and 4). According to morphological point of view, for Brugia malayi female worms generally measure 43 to 55 mm in length by 130 to 170 μm in width, and males generally measure 13 to 23 mm in length by 70 to 80 μm in width whereas for W. bancrofti, female measures about 80 to 100 mm in length and 0.24 to 0.30 mm in diameter and that of male measures 40 mm by 1 mm. Unlike the transmission of malaria, large numbers of bites from infected mosquitoes are generally required to initiate a new infection in a healthy person.5,49Designing a drug to counter filariasis in biological point of view
Drug discovery is an iterative process that generally involves discrete stages. Knowledge of basic biology and biochemistry of the parasite is often required to identify molecular targets of the parasite. In some cases compounds are also tested, without prior knowledge of the target.Compounds active against the whole parasite are defined as hits while compounds that are active in the animal models and are considered to be ‘drug like’ are termed as leads. Lead compounds generally require optimization for enhancing efficacy and good pharmaceutical properties. Once a compound reaches the stage at which it can be considered for testing in human patients, it is defined as a drug candidate. From there it enters the preclinical and then clinical trials (typical drug development pathway).50
Based on the biological process and symptoms, a drug could be designed and developed (according to drug development pathway) so as to counter the filariasis. Number of targets can be studied for developing new drugs viz. macrofilaricidal drugs (drugs killing adult worms), drugs directly targetting microfilariae, drugs preventing exsheathing of microfilariae, drugs preventing migration of microfilariae through cell wall, drugs targeting developmental stages of larvae (L1, L2, L3 and L4). On the other hand, vaccine development can also prove beneficial preventive measure and development of some mosquito repellents such as insecticide nets, body lotions, mosquito killing agents (insecticides) such as sprays, coils etc. along with good sanitization activities may also prevent mosquito vector development which together can help in combating filariasis. In one study by Babu et al., they reported that host immune responses, especially CD4+ T-cell responses clearly play a role in mediating pathological manifestations of LF, including hydrocele, elephantiasis and lymphedema. Hence, they suggested that failure to induce T-cell hyporesponsiveness is the main underlying defect in the development of clinical pathology.50 Therefore, study of immunology in the context of filariasis may further prove effective tool for the development of new antifilarials. Drugs can also be designed and developed to combat filariasis according to its symptoms (Fig. 1) viz. drugs used for the treatment of lymphatic filariasis (drugs effective against adeno lymphadenitis, funiculitis, epididymo-orchitis, lymphedema, hydrocele, chyluria, chylocele, lymph scrotum) and drugs used in the treatment of other manifestations like asymptomatic microfilaraemia, occult filariasis, endemic normal, onchocerciasis, loaisis.
Current antifilarial therapy
Diethylcarbamazine
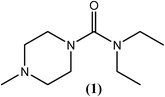
Diethylcarbamazine (DEC), a piperazine derivative (1), has been the most common and widely used orally administered drug over the previous 6 decades. The activity of DEC (1) was first time studied in cotton rats infected with Litomosoides carinii as well as in dogs with Dirofilaria immitis. The results revealed that DEC is an effective microfilaricide. In 1947, DEC was used for the first time to treat humans infected with filariasis. Later, strong antimicrofilarial activity was also shown against other filarial species that infect humans, such as W. bancrofti, B. malayi, O. volvulus and Loa loa.50–53 DEC (1) acts rapidly (within a few min), resulting in a rapid drop in the peripheral blood microfilarial count. It is also known to act by initiating the host immune system.54–57 Along with its antimicrofilarial action, it also exhibits varying degrees of macrofilaricidal activity.58–61 One report published in the last decade describing an in vitro and in vivo study on DEC (1) showed a direct mechanism of action (including apoptosis and organelle damage) on W. bancrofti microfilariae.62 For rapid killing of microfilariae by DEC (1), inducible nitric oxide was found to be an essential factor.63 However, combination therapy of DEC (1) with albendazole (4) revealed effective killing of W. bancrofti microfilariae, but this treatment was followed by the development of hydroceles and worsening of the pre-existing hydroceles.64In light of its pharmacokinetic studies, DEC (1) is almost completely absorbed after oral administration and is widely distributed in non-fatty tissues. Metabolism of DEC is extensive and rapid and the residuum is recovered unchanged in the urine within 48 hours of dosage. The plasma half-life is attained usually within the range of 6–12 hours. According to WHO, DEC (1) can be administered orally at a dose of 6 mg kg−1 daily for 12 days (preferably in divided doses after meals) in an individual treatment and 6 mg kg−1 in 24 hours (12 times at weekly or monthly intervals, or as a single annual dose) in mass treatment for treating W. bancrofti infection whereas at a dose of 3–6 mg kg−1 daily for 6–12 days (preferably in divided doses after meals) in an individual treatment and 3–6 mg kg−1 in 24 hours (6 times at weekly or monthly intervals) in a mass treatment for treating B. malayi and B. timori infections. Several trials that were conducted in India and China revealed that when a table salt medicated with diethylcarbamazine at a concentration of 0.1% when used consistently over a period of at least 6 months can eliminate W. bancrofti induced lymphatic filariasis. Also, concentration of about 0.3% may be necessary for 3–4 months where B. malayi is endemic. For the treatment of occult filariasis, DEC (1) at a dose of 8 mg kg−1 daily for 14 days is generally administered but if symptoms returns, the dose can be repeated.
Due to severe encephalitis and retinal hemorrhage (Mazzotti type reaction), DEC (1) is not recommended for the treatment of onchocerciasis or for areas in which both onchocerciasis and loiasis are endemic.65 Other side effects that are observed more frequently with an increasing dose include nausea, GIT upset, malaise, body aches, and anorexia (systemic reactions). However, abscess formation, lymphadenitis and transient lymphedema represent some of the localized reactions to DEC (1).66 Its exact mechanism is still unknown, but according to Maizels and Denham, alterations in arachidonic acid metabolism are a possibility in both host endothelial cells and microfilariae, resulting in blood vessel constriction and host granulocyte and platelet aggregation.67
Ivermectin
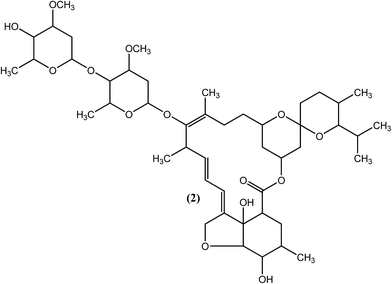
An effective microfilaricide, ivermectin (IVM), also known as mectizan (2), was the first commercially available macrocyclic lactone (introduced in 1981), with effects that persist for 12 months. Chemically, it is a 22,23-dihydro semisynthetic derivative of avermectin B1 (a fermentation product of actinomycetes S. avermitilis) developed by Merck in the mid-1970s, which has broad-spectrum activity against nematodes as well as arthropods.68–74 IVM (2), along with DEC (1) or individually, has shown efficacy in producing long-term suppression of microfilariae in bancroftian filariasis.60,75 It was also found to be effective for the treatment of brugian filariasis.76 It has been reported that IVM (2) irreversibly reduces the number of microfilariae of the adult parasite by at least 65% (single treatment at a dose of 400 μg kg−1).77,78 IVM (2) targets glutamate gated Cl− and K+ ion channels in nematodes, which leads to an increase in cell membrane permeability to chloride ions, resulting in a hyperpolarization that causes paralysis of the body wall muscle and pharynx. The drug also affects ligand-gated chloride ion channels gated by GABA (gamma aminobutyric acid).72,79–81 Genetic evidence of IVM (2) resistance has been demonstrated in various studies.82,83 It has also been observed that adult worms are not killed by the drug despite administration for six months at doses of 4800 μg kg−1.84,85 A single oral dose of IVM (2) effectively removes microfilariae of W. bancrofti, but the microfilariae gets rebound in the blood following post-treatment at a faster rate than that with DEC (1). Other side effects of IVM (2) are generally similar to those of DEC (1), and special care must be considered, such as avoiding its use in cases of pregnancy and in children younger than 5 years old.86–89 IVM (2) has also been found to compete efficiently with retinol for the retinol-binding site on retinol binding proteins (RBPs) in the parasite only, but no such binding was observed for the host RBP sites.90Suramin
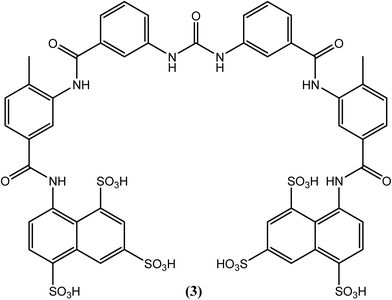
Suramin (3), which possesses a complex structure, is commercially available as a hexasodium salt (antrypol) and was initially used for the treatment of trypanosomiasis. Its activity against filarial worms was subsequently discovered serendipitously (for a long period, it is the only drug of choice used as a macrofilaricidal agent to treat onchocerciasis in humans). Chemically, it is an 8,8′-(carbonylbis[imino-3,1-phenylenecarbonylimino(4-methyl-3,1-phenylene)carbonylimino])bis-1,3,5-naphthalenetrisulfonic acid hexasodium salt, and it is the only currently available macrofilaricide that is effective against W. bancrofti and O. volvulus, among others.91–93 After entering the body, suramin (3) forms complexes with protein and then slowly dissociates, such that its effect can last for three months.94,95 It adversely affects enzymes associated with glucose catabolism and destabilizes DNA and protein kinase enzymes in filarial worms.96–98 However, it causes very severe side effects. Immediate withdrawal may be required due to serious adverse reactions such as fatal collapse, albuminuria, ulceration, and persistent high fever, among others. Thus, it should always be administered under medical supervision in the hospital. Since it is having nature to form complexes with protein and is consequently not absorbed from the gastrointestinal tract, suramin (3) is given slowly by intravenous route of administration (10% solution in water for injection). For adults, a total of 66.7 mg kg−1 dose should be administered in six incremental weekly doses (3.3, 6.7, 10.0, 13.3, 16.7, 16.7 mg kg−1 for 1st to 6th weeks respectively).99 It enters the extracellular space but does not penetrate into the cerebrospinal fluid. It dissociates slowly from plasma proteins and is detectable unchanged in the urine for up to 3 months after the last dose. Polyuria, tiredness, tenderness, anorexia, and increased thirst, among others, are some of the milder side effects associated with this drug. Due to its extreme toxicity, the use of suramin (3) is prohibited in geriatrics, pediatrics and in patients with renal or hepatic diseases.94,99–102Albendazole
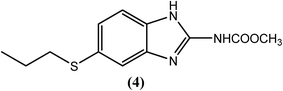
Albendazole (4), a benzimidazole derivative that is generally used to treat helminthiasis, has recently been assessed in a clinical trial for its antifilarial efficacy.103 It was found to be more effective when administered in combination with either DEC (1) or IVM (2). Albendazole (400 mg) together with ivermectin (150–200 μg kg−1) or with diethylcarbamazine (DEC) (6 mg kg−1) is generally given annually to an entire at-risk population.104–106 A macrofilaricidal effect of albendazole (4) had also been reported.107,108 Some studies showed that blockage of tubulin polymerization and thereby inhibiting microtubule formation by this drug further inhibits mitosis in the worm such that its embryogenesis and egg hatching is inhibited. It was also found to inhibit parasite intestinal cells, preventing glucose uptake and thereby leading to the death of the parasite due to starvation. However, like the other drugs, albendazole (4) also causes some side effects, such as embryotoxicity and teratogenicity, and therefore, it is prohibited during pregnancy.109–111Levamisole
Levamisole (5) is the drug of choice for the treatment of ascariasis and has been shown to be virtually free from side effects at the recommended doses. It has also been reported to exhibit significant activity against the microfilariae of Wuchereria bancrofti and Brugia malayi.112 According to one study, at an initial dose of 100 mg followed by the same dose twice daily for 10 days, levamisole (5) was found to be as effective as the total oral dosage of DEC at 126 mg per kg body weight.113 Some researchers have reported that levamisole (5) acts as nicotinic receptor agonist that causes prolonged activation of the excitatory nicotinic acetylcholine (nACh) receptors on the body wall muscle of parasites, leading to spastic muscle paralysis in the worm.114,115Antifilarials: from synthetic origins
Sincere efforts have been made in the past few decades in the search for new antifilarial agents and their development, resulting in different cores possessing antifilarial activity. Therefore, in the context of lymphatic filariasis, efforts are made herein to encompass the maximum reported antifilarials from synthetic as well as from natural origins (Fig. 5).Pyrimidine
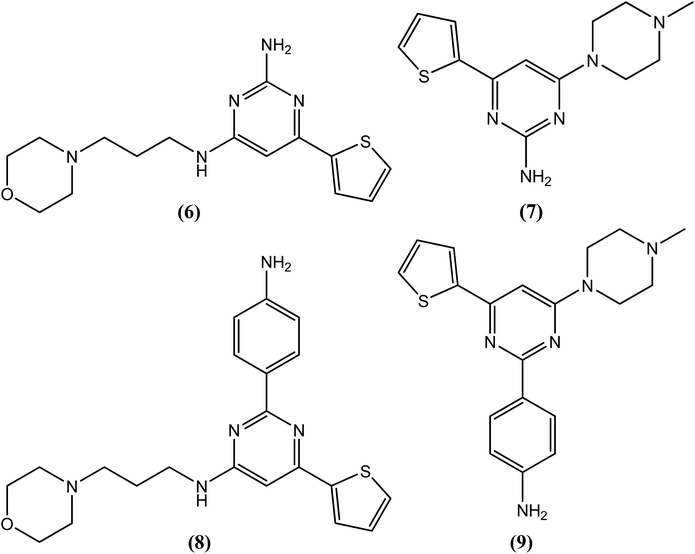
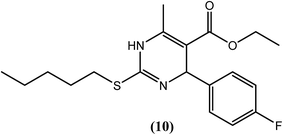
Chauhan et al. synthesized trisubstituted pyrimidine derivatives that were found to possess improved ATP-dependent DNA topoisomerase II inhibitory activity against the filarial parasite S. cervi, many of which were found to be superior to the available standard antifilarial drug (DEC) as well as the standard topoisomerase-II inhibitors (nalidixic acid and novobiocin). Among these derivatives, compounds (6), (7), (8) and (9) were found to be most effective. Compounds (6), (7) and (9) showed constant % inhibition (80%), whereas compound (8) showed 70% inhibition at tested concentrations of 40, 20 and 10 μg mL−1. Even at 5 μg mL−1, compounds (6) and (7) showed approximately 60% inhibition, while compounds (8) and (9) showed approximately 40% and 25% inhibition, respectively. However, standard antifilarial DEC showed very low inhibition, i.e., 45% and 10% at concentrations of 40 μg mL−1 and 20 μg mL−1, respectively, and was ineffective below these concentrations. Standard topoisomerase II inhibitors were also less effective, showing 10–80% inhibition at 10–40 μg mL−1 and no effect of them was observed at 5 μg mL−1 concentration. According to the authors, the amino group and 4-aminophenyl group at the second position plays an important role in exerting antifilarial activity by inhibiting the topoisomerase II enzyme of S. cervi.116The 2-sulfanyl-6-methyl-1,4-dihydropyrimidines were synthesized by Tripathi et al. After evaluation for their antifilarial activity against adult worms of B. malayi, compound (10) was found to be most promising at 25 and 50 μM. It also showed approximately 70% inhibition in the MTT reduction assay compared with standard DEC (ineffective even up to 200 μM). After in vivo testing against B. malayi in a Mastomys coucha infection model, compound (10) exhibited good antimacrofilarial action at a dose of 100 mg kg−1. When evaluated for its embryostatic activity, compound (10) caused approximately 68% sterilization of female worms, which was quite significant compared with the 20% sterilization that was observed in the untreated controls. It also showed no apparent signs of any toxicity in treated as well as untreated animals. When evaluated for in vivo activity in the B. malayi jirid model, compound (10) displayed approximately 46% macrofilaricidal activity. According to the authors, 4-aryl-1,4-dihydropyridine, which has a less bulky electron-withdrawing group at position 4 of the phenyl ring (compound 10), leads to a potent compound in the series.117
Indole
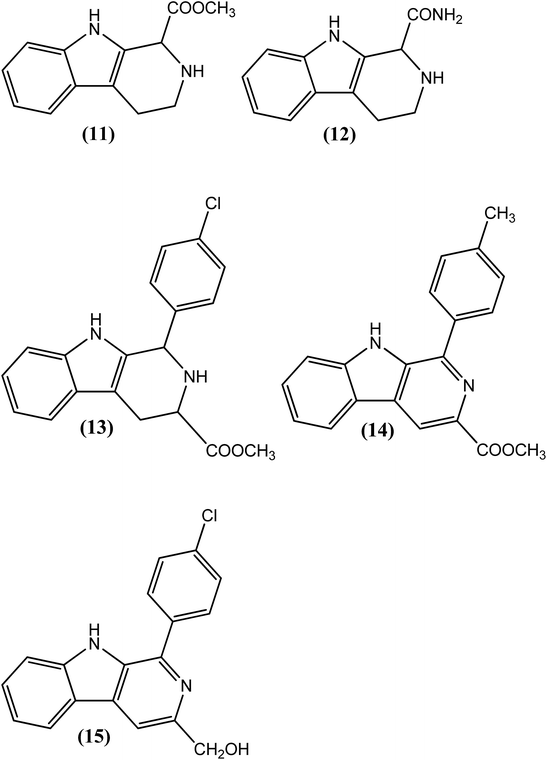
Agarwal et al. reported the synthesis and evaluation of substituted pyrido-indoles (β-carbolin). From the series, compound (11) administered at a dose of 30 mg kg−1 for 5 days exhibited 81% adulticidal activity against L. carinii in S. hispidus (cotton rats), whereas it showed 96% microfilaricidal activity when tested against A. viteae in M. natalensis at a dose of 50 mg kg−1 for 5 days. When evaluated against A. viteae in M. natalensi (at the same dose), another compound (12) obtained via aromatization of the pyridine ring containing the 1-carboxamido group, showed 100% micro and 50% macrofilaricidal activity, along with complete sterilization of surviving female worms. While discussing its SAR, researchers have explained that the substituent present at position 1 in tetrahydro or aromatized β-carbolines plays an important role in eliciting the antifilarial response in rodents.118 In another report, Chauhan et al. described the synthesis of β-carbolines (substituted 9H-pyrido[3,4-b]indoles) and evaluated their micro- and macrofilaricidal activities against 3 species of filariae viz. L. carinii (metabolically facultative filarial species maintained in cotton rats and also successfully tested in human filariasis) against A. viteae (metabolically similar to human filarial parasites, which are anaerobic in nature and recommended by the WHO for experimental chemotherapy of filariasis) and B. malayi in a M. coucha model. From these compounds, among the 4-halo substituents (at position 1 of β-carboline), compound (13) exhibited potent 94% microfilaricidal and 39% macrofilaricidal activities along with sterilization of all surviving female worms (when tested against A. viteae at a dose of 50 mg kg−1 for 5 days). The 4-chlorophenyl substituent was found to be important for the antifilarial response. In particular, indole containing the tetrahydropyridine ring along with the ester function at position 3 (13) was found to be beneficial. However, among the 4-substituted-phenyl substituents (at position 1 of β-carbolines), the highest, i.e., 100% macrofilaricidal activity (when tested against A. viteae at a dose of 50 mg kg−1 for 5 days), was observed for compound (14). The ester function at position 3 with a 4-methylphenyl substituent at position 1 was important for the adulticidal action. Another compound, compound (15), with a hydroxymethyl group at position 3 and 4-chlorophenyl group at position 1 of β-carboline was found to have good antifilarial activity against all three types of worms tested. Compound (15) showed 76% microfilaricidal and 56% macrofilaricidal activities, along with 75% inhibition of female worms against A. viteae. When its antifilarial activity was assessed against L. carinii, compound (15) showed 95% adulticidal and 29% microfilaricidal responses. Interestingly, it was the only compound that, when tested against human filarial parasite (B. malayi), was found to possess antifilarial activity. At an intraperitoneal dose of 50 mg kg−1 for 5 days, it showed 62% adulticidal activity and sterilized approximately 85% of the surviving female worms. The researchers further concluded that the β carboline skeleton and nature of the substituents, especially at positions 1 and 3, contribute to the macrofilaricidal activity of synthesized compounds.119–121Quinoline and related compounds
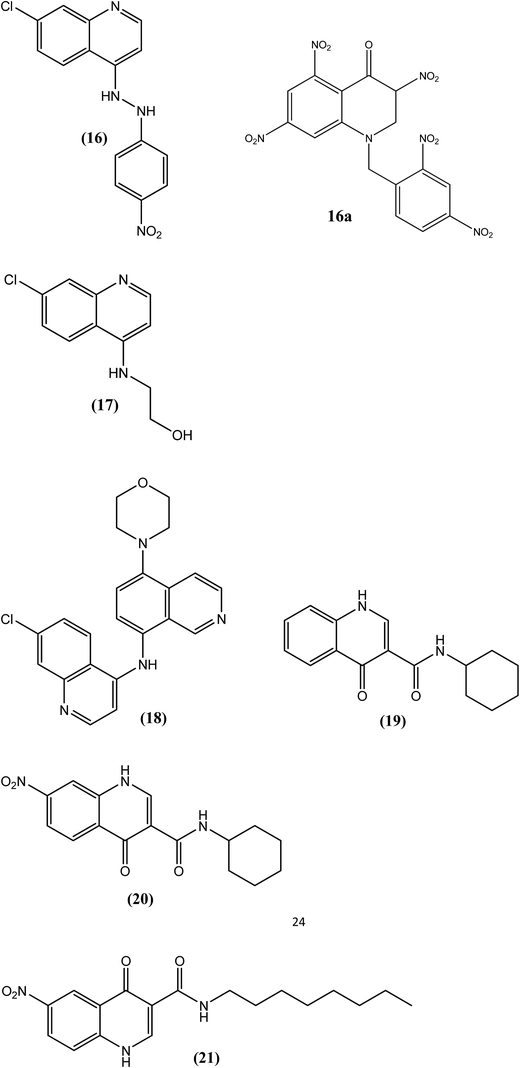
Chauhan et al. synthesized 7-chloro-4-(substituted amino)quinolines and evaluated their activities against filarial worms. Compound (16) with a nitrophenyl hydrazine function at position 4 of the quinoline ring was found to be most potent, exhibiting 41% microfilaricidal and 78% macrofilaricidal activities against A. viteae. A 50% sterilization of female worms was also observed with compound (16). Compound (17), which contains an ethanolamine function on the quinoline ring, exhibited 86% microfilaricidal activity, similar to the standard drug DEC (90%). However, where DEC was inactive for the adulticidal response, compound (17) showed 22% macrofilaricidal activity. The bulky substitution at position 4 of the quinoline ring was beneficial for the antifilarial activity, as observed for compound (16), which revealed a maximum antifilarial response after the study.122 Azad et al. synthesized 3-nitro-4-quinolones via ipso-nitration reaction. The synthesized compounds were evaluated for antifilarial activity and also for Brugia malayi thymidylate kinase inhibitory activity. Among all the synthesized compounds (16a) was the most active compound with IC50 2.9 μM.122Chauhan et al. also reported the synthesis of 5-amino and 5,8-diamino-substituted isoquinoline derivatives, and evaluated these compounds in vivo against A. viteae. Among the synthesized derivatives, compound (18) was the most potent, exhibiting 76% adulticidal and 60% microfilaricidal activities. After biochemical evaluation, compound (18) significantly reduced the ATP and tubulin contents of the worm (essential contents required for the functional integrity and survival of the parasite). The morpholine function at position 5 of the isoquinoline ring was found to be important for the biological response against A. viteae.123 Furthermore, Chauhan et al. reported that quinolones are a new class of antifilarial agents. Among the synthesized compounds that were screened in vivo against A. viteae at a dose of 200 mg kg−1 for 5 days, three compounds, viz. compounds (19), (20) and (21), displayed the most significant antifilarial activities, among which compound (19) showed 100% macrofilaricidal and 90% microfilaricidal action, whereas the standard drug DEC citrate showed only 90% microfilaricidal action with no effect on the macrofilarial count. Compound (20) exhibited 80% macrofilaricidal activity with 24% microfilaricidal activity, whereas compound (21) caused 100% sterilization of female worms with 40% microfilaricidal and 38% macrofilaricidal activities. After its evaluation against DNA topoisomerase II enzyme, compound (19) showed more than 80% enzyme inhibition. Regarding SAR, among the entire N-substituted quinol carboxamide derivatives, compound (19) with a cyclohexyl substituent on the nitrogen atom of carboxamide and with no substitution on the quinolone nucleus was found to be the most potent compound. Nitration at position 7 (compound 21) was also beneficial for antifilarial activity. Replacement of the cyclohexyl substituent with a bulkier group, such as a long-chain alkane at the nitrogen of carboxamide, resulted in a significant reduction in macrofilaricidal activity.124
Glycoside
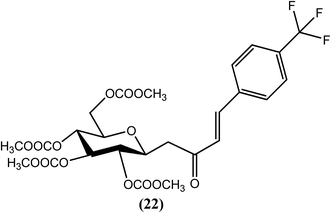
Jana, Sinha Babu and Misra et al. recently reported that C-cinnamoyl glycosides are a new class of antifilarial agents. They studied these compounds in terms of their antifilarial activity, mechanism of action (MOA) and 3D QSAR. From the series of synthesized compounds, compound (22) exhibited the most potent activity in terms of MIC (∼3.40 nM), IC50 (∼6.90 nM) and LC50 (∼25 nM) values, whereas the standard drug IVM showed MIC, IC50 and LC50 values of approximately ∼7 nM, ∼20 nM and ∼54 nM, respectively, when tested in vitro against the bovine filarial parasite S. cervi. Compound (22) also exhibited a CC50 value of approximately 103 nM (SI = ∼15) when evaluated against the normal kidney epithelial cell line in an MTT experiment. When evaluated against the microfilariae of the human filarial parasite W. bancrofti by RM assessment and MTT assay, the MIC and IC50 values of compound (22) were 4.4 nM mL−1 and 8.96 nM mL−1, respectively, together with a 40.6% reduction in worm viability at the highest tested concentration (18.4 nM mL−1). In contrast, the standard drug IVM showed a reduction in worm viability of only 17.6%. In the discussion of its MOA, they reported that after Hoechst and propidium iodide staining, compound (22) showed an induction of chromatin condensation and DNA fragmentation, which are characteristics of apoptosis. SEM studies further revealed that this compound also damaged the cuticular sheath of the microfilaria, as observed for DEC. The 3D QSAR studies showed the relative arrangements of substituents at the meta and para positions of aromatic groups that are responsible for two/three-fold differences in activities. The compounds, particularly those with a halide substitution meta to the aromatic ring, showed higher antifilarial activity.125Dioxocine
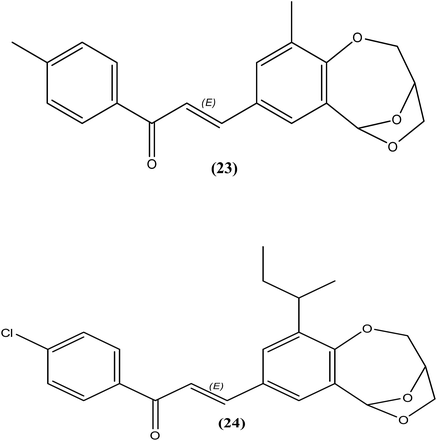
Sashidhara et al. reported the synthesis of 3,6-epoxy dioxocines. After antifilarial evaluation of these compounds against B. malayi in the M. coucha model, compound (23) showed good IC50 values (0.4 μg mL−1 and 1.8 μg mL−1, with selectivity indices (SI) of 100 and 22.2 with respect to macrofilariae and microfilariae, respectively). Another compound (24) was found to be potent in terms of both in vitro (IC50 = 1.6 μg mL−1 and 3.5 μg mL−1 for macrofilariae and microfilariae, respectively) as well as in vivo antifilarial activity. After in vivo evaluation of compound (24) in the B. malayi in jirid model, it showed no apparent signs of toxicity and minimal worm recovery compared with the control at a dose of 200 mg kg−1. It demonstrated 38% adulticidal activity and 63% sterilization of female worms, revealing its superior effect compared with DEC citrate (20% adulticidal activity and 11% sterilization of female worms). While discussing SAR of the synthesized compounds, authors stated that compounds containing naphthalene nucleus were found inactive while compounds that contains alkyl group at position number 10 were found to be more promising. Interestingly, the substitution on the phenyl ring of chalcone seems to have no effect, as the activity was conserved in both.126Alcohols
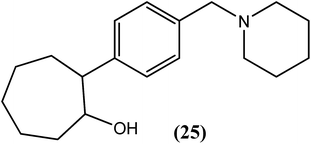
Murthy et al. reported the in vivo antifilarial activities of cyclic and acyclic alcohols. They further evaluated cyclohexanol, 2-substituted propanol and cyclooctanol derivatives against A. viteae and L. carinii in rodents. Among all of the tested compounds, compound (25), a cyclooctanol derivative containing 4-benzyl piperidine substituted at position 2, exhibited 100% macrofilaricidal activity (at a dose of 200 mg kg−1 for 5 days) with approximately 81% sterilization of female worms (at a dose of 100 mg kg−1 for 5 days) against A. viteae. The hydroxyl group along with the benzyl piperidine substituent was essential for the antifilarial activity, as observed by the loss of activity following acetylation of the hydroxyl group.127Triazine
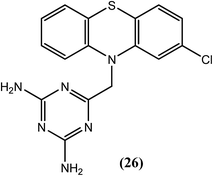
Goswami et al. explored DHFR (dihydrofolate reductase) inhibitors for their in vitro activity against B. malayi. Among the evaluated compounds, three were found to be most active, from which compound (26), caused almost 100% loss of motility of filarial worms at 20 μg mL−1 concentration. It also showed better activity (IC50 = 10.90 μM) when compared with standard antifolate (positive control) compounds, i.e., trimethoprim (IC50 = 12.92 μM) and pyrimethamine (IC50 = 20.10 μM). Furthermore, it exerted good inhibitory activity (approximately 74%) against PARP (polyadenosine diphosphate ribose polymerase) enzyme. The bulky tricyclic substituent and the basic DHFR pharmacophore (2,4-diamino pyrimidine converted to a 2,4-diamino triazine) were found to be essential for antifilarial activity.128,129Benzopyran (coumarin)
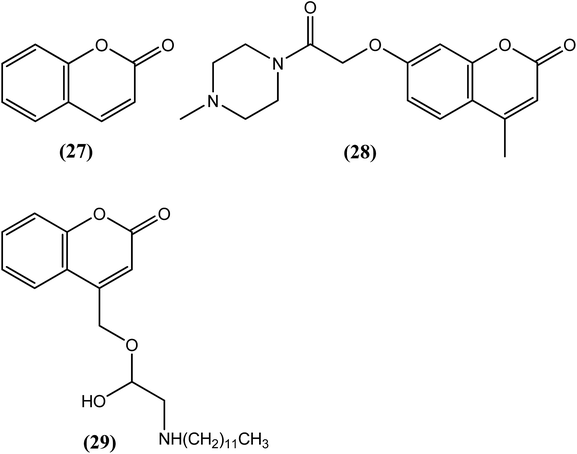
Murthy et al. synthesized benzopyran derivatives and evaluated them for antifilarial activity against L. carinii and A. viteae-induced infections in cotton rats and M. coucha models, respectively. A simple benzopyran compound (27) displayed approximately 56% macrofilaricidal and 74% microfilaricidal activity against A. viteae.130 In another work, the same research group synthesized a series of benzopyran derivatives, among which compound (28) was a promising antifilarial compound when evaluated against L. carinii (in cotton rats) and B. malayi (in the M. coucha and jirid model). When administered orally at a dose of 300 mg kg−1 for 5 days in the B. malayi–M. coucha model, it showed 53.6% macrofilaricidal and 46% microfilaricidal activity, along with approximately 46% sterilization of female worms. The said compound also interfered with infective L3 larva-induced infection to an extent of approximately 50% at the same dose. An inhibition of protease activity by 82% was also observed with 1 μM compound (28). Thus, not only the benzopyran moiety itself but also the benzopyran ring with a substitution at position 7 fused to benzopyran was found to be a promising lead for exerting antifilarial activity.131 Misra-Bhattacharya and Katiyar et al. synthesized and evaluated 4-oxycoumarin derivatives. After an in vitro evaluation against the human filarial parasite B. malayi, compound (29) with n-dodecyl substitution was found to exhibit the most potent antifilarial activity in the nanomolar range. The observed IC50 value for compound (29) was 14 nM (129 times lower than that of IVM) and 5.6 nM (714 times lower than that of IVM), whereas the standard drug IVM had IC50 values of 1800 nM and 4000 nM against adult worms and microfilariae of the parasite, respectively. The CC50 value for this compound was more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nM in the Vero cell line (monkey kidney cell), resulting in SI values of more than 7142 and 17
000 nM in the Vero cell line (monkey kidney cell), resulting in SI values of more than 7142 and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 857 for adult worms and microfilariae, respectively. When evaluated in vivo in the B. malayi–jirid model, compound (29) showed approximately 75% adulticidal along with moderate (∼50%) microfilaricidal activity at a dose of 100 mg kg−1 for 5 days, which was found to be better than the standard drug DEC with only approximately 33% adulticidal activity. Based on the activity data, it seems that the nature of the substituents at the amino nitrogen of 4-oxy coumarin derivatives is responsible for effective antifilarial activity. Also, a long-chain alkyl substitution at this amino nitrogen provides the best antifilarial response, as observed for compound (29).132
857 for adult worms and microfilariae, respectively. When evaluated in vivo in the B. malayi–jirid model, compound (29) showed approximately 75% adulticidal along with moderate (∼50%) microfilaricidal activity at a dose of 100 mg kg−1 for 5 days, which was found to be better than the standard drug DEC with only approximately 33% adulticidal activity. Based on the activity data, it seems that the nature of the substituents at the amino nitrogen of 4-oxy coumarin derivatives is responsible for effective antifilarial activity. Also, a long-chain alkyl substitution at this amino nitrogen provides the best antifilarial response, as observed for compound (29).132Naphthalene derivative
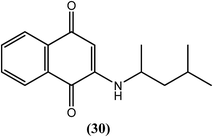
Mathew et al. synthesized 1,4-naphthoquinones and evaluated their antifilarial activity in vitro using the adult bovine filarial worm Setaria digitata. Compound (30) was the most potent, with an ED50 value of 2.6 μM after a 24 h incubation and 0.91 μM after a 48 h incubation (in the motility assay). Therefore, the researchers suggested that a 1,3-dimethyl substitution on the butylamino side chain favors an increased lipophilicity with potentially improved binding to the active site, which results in elevated macrofilaricidal activity.133Thiazolidine
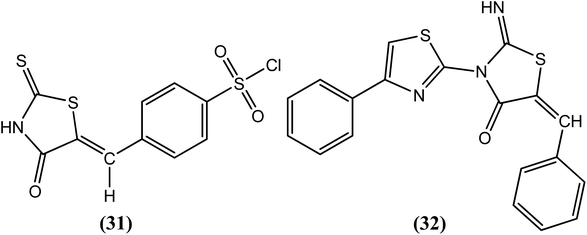
Goswami et al. recently synthesized heterocyclic thiazolidine compounds and evaluated them in vitro against the human lymphatic filarial parasite B. malayi. Among the compounds tested for antifilarial activity and cytotoxicity studies (using human peripheral blood mononuclear cells), two compounds, viz. compound (31) and compound (32), were found to be most potent, with IC50 values of 5.2 μM and 1.78 μM and LD50 values of 349 μM and 17.59 μM, respectively. After examining their effect on the motility of adult worms, compound (31)-treated worms were found to be less active, whereas compound (32)-treated worms were found to be completely immotile.134Butylated Hydroxy Anisole (BHA)
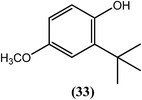
Rathaur et al. reported that after treatment with butylated hydroxyl anisole (BHA) (33), microscopic visualization revealed a ruptured body wall along with microfilarial stiffness in S. cervi. They further concluded that the BHA (33)-induced imbalance results from a major detoxification pathway in the filarial parasite, which leads to cytotoxicity and death of the parasite. When studied using proteomics and biochemical assays with the GSH–GST system and molecule signaling, BHA (33) at 100 μM was found to be a potent adulticide, and oxidative stress-induced apoptosis was found to be its major killing mechanism.135Piperazine
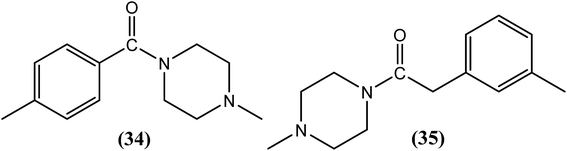
The use of piperazine as an antifilarial agent has been recently reported.136 Kalyanasundaram et al. synthesized benzoyl piperazine derivatives and evaluated them against adult S. cervi in terms of their antifilarial activity. From the series, two compounds, viz. compound (34) and compound (35) containing a 4-chloro (para) substituent and 3-methyl (meta) substituent on the aromatic ring, were found to be the most promising. Worms were immotile following treatment with these two compounds at a concentration of 8 mg mL−1. The authors further concluded that among the chloro-substituted compounds, substitution at the para position was more beneficial than substitution at the meta or ortho positions. However, in the case of methyl-substituted compounds, meta substituted compounds showed greater activity than para-substituted compounds.137Pyrrolidine
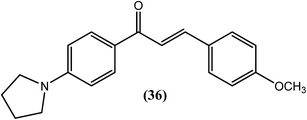
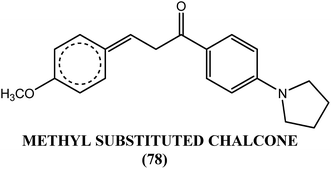
Awasthi et al. reported that the chalcone derivative (36) containing the pyrrolidine-methoxy group showed a significant suppression of glutathione-S-transferase (GST) activity in the macrofilariae of female S. cervi at a concentration of 3 μM in vitro. The activity of GST was completely lost (100% inhibition) following treatment with methoxy group derivatives (compound 36). This activity might be due to the formation of a β-carbonyl–glutathione complex, resulting in a reduction in free GSH for catalysis. Inhibition of viability by 96% was observed when this compound was tested against adult S. cervi using the MTT reduction assay. The authors proposed that small and medium-sized lipophilic or hydrophobic groups containing single or multiple nitrogen or oxygen atoms in an acetophenone ring of chalcone might be involved in the loss of GST activity, motility and viability in the worms. They also suggested that the decreased GST activity and GSH level observed in worms treated with compound (36) supported the possibility of a reduced chance of resistance to this compound.138Diaminoalkane
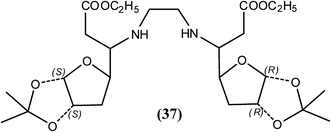
Tripathi et al. reported that among the synthesized N1,Nn-xylofuranosylated diaminoalkanes, compound (37) at 50 mg kg−1 provided approximately 38.7% recovery of macrofilariae and 63.80% sterilization of female parasites of B. malayi in the M. coucha model. The same compound also showed 33.5% adulticidal action along with 50% sterilization of female worms of B. malayi in the jirid model. According to researchers, lipophilic diamine spacer and 3-O-substituent along with 1,2-O-isopropylidene group were found to be important for effective antifilarial activity that probably help the compounds to bind with the active sites of enzymes or interfere with parasite metabolic machinery.139Secondary amines
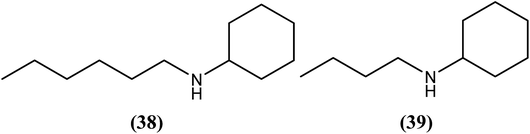
Chauhan et al. reported that from the synthesized secondary amines when tested against A. viteae, two compounds showed the most potent in vivo antifilarial activities at a dose of 200 mg kg−1 for 5 days. Compound (38) exhibited 100% macrofilaricidal activity, whereas compound (39) elicited a microfilaricidal response of approximately 93%. Concomitantly, both exhibited 50% sterilization of female worms. While discussing their SAR, they reported that aromatic secondary amines did not show any significant activity, but aliphatic amines such as secondary amines with longer alkyl chains (n > 5) were responsible for showing adulticidal activity. On the other hand, tertiary amines with a dialkyl chain (n = 3) may play an important role in exhibiting microfilaricidal response.140Glycyrrhetinic acid derivatives
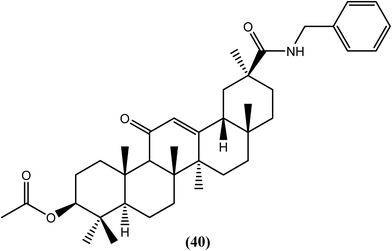
Murthy and Srivastava et al. isolated glycyrrhetinic acid from the roots of G. glabra, synthesized its analogs and evaluated them for antifilarial activity. Among the synthetic derivatives that were tested in vitro against B. malayi, compound (40), the benzyl amide analog, was found to be effective for killing microfilariae and macrofilariae at 50 and 25 μM, respectively, with an SI value of more than 60. The IC50 values were found to be 2.2 μM against microfilariae and 8.8 μM against macrofilariae of the worm. Moderate adulticidal activity (approximately 40%) was also observed at a dose of 100 mg kg−1 for 5 days when tested in vivo in the B. malayi–jirid model. It is quite interesting to note that the said compound showed macrofilaricidal activity along with microfilaricidal activity because its natural precursor, glycyrrhetinic acid, was only potent against microfilariae (IC50 = 1.20 μM). Acylation, esterification and amidation of the C-30 carboxylic group showed antifilarial activity, from which the presence of the amide group at C-30 (compound 40) showed remarkable activity against adult worms.141Nitazoxanide and tizoxanide
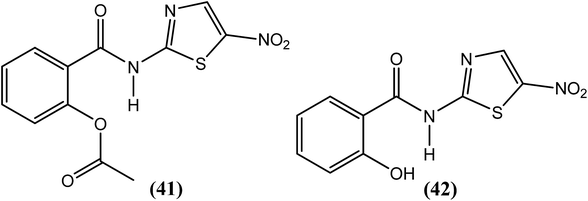
Rao et al. examined the in vitro as well as the in vivo effects of nitazoxanide (NTZ) (41) and tizoxanide (TZ) (42) on B. malayi nematodes. Macrofilariae were found completely immotile after 6 days when cultured with these two compounds at concentrations of 20 μg mL−1. On day 8 of culture at concentrations ≥2.5 μg mL−1, both drugs also caused a 50% decrease in worm viability. Microfilarial motility was also hampered by these compounds at concentrations exceeding 5 μg mL−1, and the worms were completely immotile following treatment with 20 μg mL−1 (after 48 h). The researchers further reported that both compounds reduced microfilarial production and impaired embryogenesis in female worms. They also suggested that mitochondria in the worms may be a possible target of NTZ (41) and TZ (42) because in addition to damaged worm tissues, they found alterations in the mitochondria.142Anthraquinone
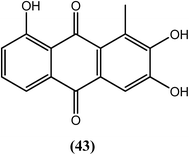
Nair et al. reported that among the synthesized series of anthraquinone derivatives (positional isomers of each other), compound (43) at 5 ppm (18–19 μM) showed 100% mortality within 1, 5, and 3 days against microfilariae and adult male and female worms of B. malayi, respectively. Compound (43) is basically a 3-methylcatechol with a substitution of acylium ions at the ortho position with respect to the methyl group which may be playing an important role for exerting antifilarial action. After histological analysis of the compounds, the researchers further suggested that most of the anthraquinones had marked effects on intrauterine embryos of parasite.143Sulfonamide
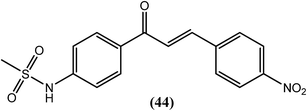
Chandak and Goswami et al. recently synthesized sulfonamide chalcones and evaluated them in vitro against B. malayi. Amongst them, compound (44) with a chloro substitution at the para position of the terminal phenyl ring demonstrated remarkable antifilarial activity and therapeutic efficacy. After testing for B. malayi motility, its IC50 value was found to be 4.4 μM, with an LD50 value of 188 μM when evaluated for cytotoxicity against human peripheral blood mononuclear cells. It also showed 100% loss of microfilarial motility of B. malayi at 500 μM concentration after 48 h of incubation.144Benzothiazole
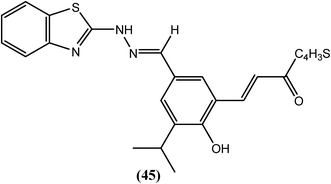
Sashidhara and Saxena et al. reported a series of novel chalcone–benzothiazole hybrids. Among all compounds that were tested in vitro against B. malayi, compound (45) had IC50 values of 2.12 μM and 1.63 μM, respectively for adult worms as well as microfilariae and a MIC value of 5 μM for both the forms. The CC50 value for compound (45) was 95.3 μM, with an SI value higher than 10. However, the standard drug IVM was highly active against adult worms (MIC = 0.625 μM and IC50 = 0.30 μM) but exhibited lower microfilaricidal activity (MIC = 10 μM and IC50 = 4 μM). A computational study revealed that compound (45) was selective towards the BmTMK enzyme. It showed higher binding interactions at the active site of BmTMK (B. malayi thymidylate kinase, an essential enzyme for nucleotide metabolism in B. malayi). It interacted with amino acids such as His-70, Thr-107, and Lys-110, and demonstrated a hydrogen bonding interaction with Arg-77 and Arg-98. It also displayed π–π stacking interactions and hydrophobic interactions with Phe-73, Phe-43, Tyr-57 and Tys-106. Furthermore, it showed reduced binding affinity towards HsTMK (human thymidylate kinase).145Thiazole
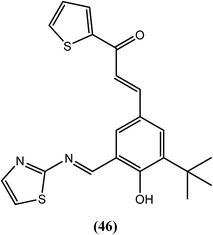
Shashidhara and Murthy et al. synthesized chalcone–thiazole derivatives and assessed their antifilarial activity against the human lymphatic filarial parasite B. malayi. Among all of the compounds, compound (46) showed 100% embryostatic activity when administered in vivo in the B. malayi–jirid model at a dose of 100 mg kg−1 for 5 days. When administered at the same dose in the B. malayi–M. coucha model, this compound exerted approximately 49% macrofilaricidal activity and 44% sterilization of female worms. It was also effective in vitro when compared with the standard drugs IVM and DEC (Table 1). The results indicated that the imine–thiazole moiety is required for antifilarial activity, and branching at position 3 of the phenyl ring along with the five-membered heterocycle as a chalcone counterpart further enhanced this activity.146| Compound | For adult worms (in motility assay) | Mean % inhibition in MTT reduction of adult worms | For microfilariae (in motility assay) | CC50 (μM) | ||||
|---|---|---|---|---|---|---|---|---|
| LC100 (μM) | IC50 (μM) | SI | LC100 (μM) | IC50 (μM) | SI | |||
| 46 | 2.5 | 0.9 | 44 | 63 | 5 | 1.8 | 22 | 40 |
| IVM | 5 | 3.05 | 75.41 | 5.80 | 2.5 | 1.57 | 146.49 | 230 |
| DEC | 1000 | 314.98 | 28.57 | 62.54 | 500 | 297.30 | 30.27 | 9000 |
Benzimidazole
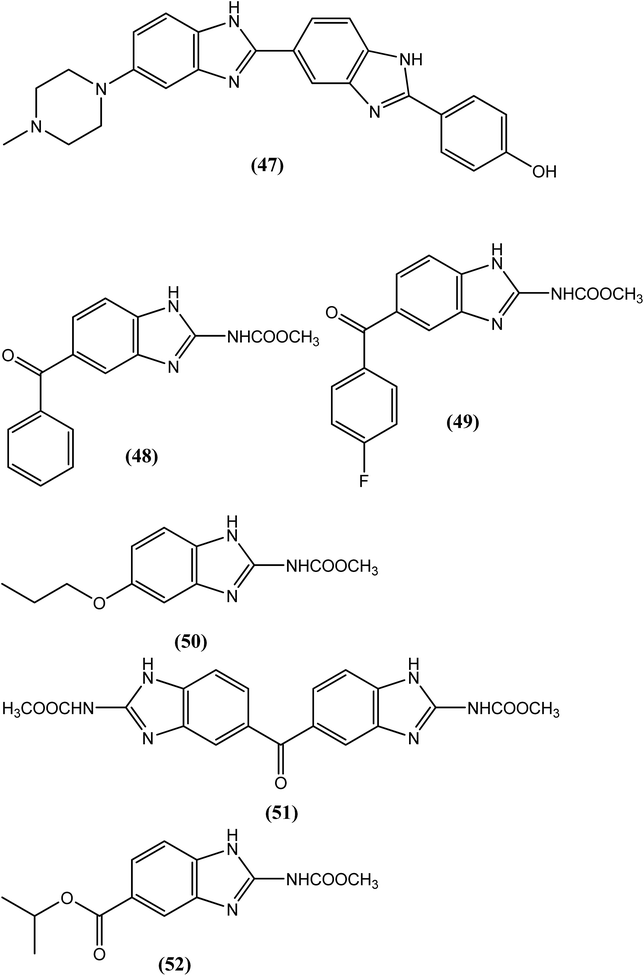
In 1971, compound (47), named HOE 33258, was the first benzimidazole derivative to be reported as an antifilarial agent with activity against the microfilariae of L. carinii and D. immitis.147 Some benzimidazole carbamates, such as mebendazole (48), flubendazole (49), albendazole (4) and oxibendazole (50), have demonstrated significant antifilarial activity.148–154 Chatterjee et al. evaluated compound (51), which is chemically defined as 2,2′-dicarbomethoxyamino-5,5′-dibenzimidazolyl ketone (82/437). Compound (51) eliminated almost 100% of adult worms and microfilariae of L. carinii in cotton rats. At a dose of 150 and 200 mg kg−1 for 5 days, it killed 100% of the macrofilariae and 97% of the microfilariae of D. viteae and B. malayi, respectively, in the M. natalensis model.155 Townsend et al. reported the synthesis of benzimidazole-2-carbamates and derivatives along with their evaluation of antifilarial agents. Among them, compound (52), with a methyl 2-carbamate and a 5-isopropoxycarbonyl group, showed curative action at a dose of 12.5 mg kg−1 for 5 days and 82% effectiveness at a dose of 6.25 mg kg−1 for 5 days.156 A recent study by Geary et al. showed that flubendazole (49) damages the tissues required for the reproduction and survival of parasite at pharmacologically relevant concentrations (per short-term in vitro culture experiments in adult filariae). In addition, a long-term study examining the effects of flubendazole (49) on adult Brugia malayi in jirids revealed prominent morphological damage in developing embryos accompanied by a decrease in microfilarial output at 4 weeks post-exposure of flubendazole (49); however, the exposed worms recovered and were found to be viable after 8 weeks of the treatment. In another study, Geary et al. also suggested that flubendazole (49) acts predominantly on rapidly dividing cells of the parasite which provides a basis for selecting molecular markers of drug induced damage that may be useful in predicting efficacious flubendazole regimens.157Silver
Dash et al. described the antifilarial activity of nanosilver against the filarial parasite B. malayi. Nanosilver showed an LD50 concentration (by trypan blue exclusion) of 101.2 μM and an IC50 value of 50.6 μM (complete microfilariae population found immotile). At 4.6 μM only, nanosilver caused a 50% decrease in the motility of the parasite. The researchers further reported that nanosilver induced approximately 70% attenuation of poly(adenosine diphosphate-ribose) polymerase (PARP) activity, leading to apoptosis. Furthermore, at a concentration of 50 μM, nanosilver induced ultrastructural changes in the microfilariae (marked loss in the sheath), as observed by electron microscopy. They also claimed that their report provides the first ever conclusive proof supporting apoptosis as novel strategy in the design of antifilarials, with nanosilver as the valid lead.158Antifilarials: from natural origins
Streptomyces sp. 17944
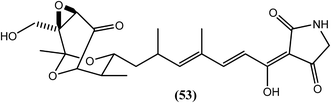
Of the three new tirandamycins from Streptomyces sp. 17944, compound (53) was found to selectively inhibit the B. malayi asparaginyl-tRNA-synthetase (BmAsnRS) enzyme at an IC50 value of 30 μM and to efficiently kill the B. malayi parasite. Compound (53) exhibited a minimum 10-fold selectivity for BmAsnRS over human AsnRS. The authors further reported that compound (53) dramatically induced filarial death after less than 24 h, whereas albendazole induced parasite death after more than 10 days, both at 100 μM. Additionally, the IC50 value to kill adult worms was calculated using the MTT reduction assay and found to be 1 μM (Fig. 6).159Streptomyces sp. 9078
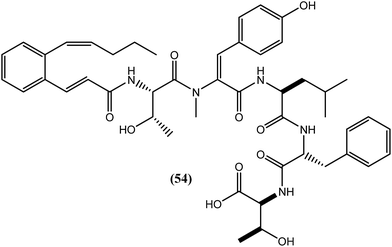
Depsipeptide was isolated for the first time as a tachykinin antagonist. A new congener of depsipeptide, WS9326D, i.e., compound (54), was isolated by bioassay-guided fractionation of Streptomyces sp. 9078 and found to specifically inhibit BmAsnRS (B. malayi asparaginyl-tRNA-synthetase) with an IC50 value of 50 μM. It also exerted filaricidal activity without showing any significant general toxicity toward human hepatic cells at 100 μM. Furthermore, it rapidly killed the macrofilariae of the same parasite within 24 h and displayed macrofilaricidal activity within 10 days at concentrations as low as 10 nM.160Streptomyces sp. 4875
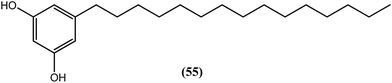
Four adipostatins (alkyl resorcinols) from the bioassay guided de-replication of the active strain of Streptomyces sp. 4875 selectively inhibited BmAsnRS and also markedly destroyed the adult B. malayi parasite. Compound (55) was the most potent among the compounds, killing the worms at concentrations as low as 1 μM. The assessment of general cytotoxicity using human HepG2 cells showed that compound (55) was non-toxic at concentrations as high as 100 μM.161Lantana camara (L. camara)
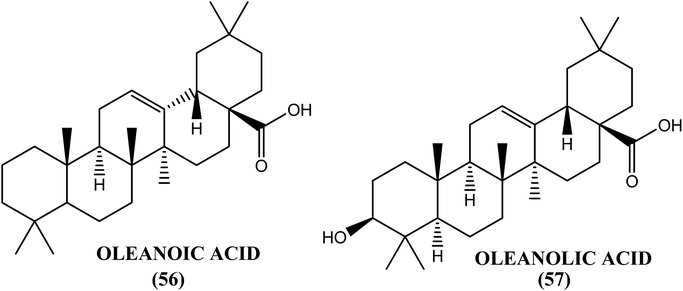
An Indian plant called L. camara has been found to have antifilarial properties. When evaluated against A. viteae, the crude extract was found to possess 95.04% microfilaricidal activity and sterilization of 60% of the female worms. After in vivo administration at a dose of 1 g kg−1 for 5 days, the L. camara crude extract showed 43.05% macrofilaricidal activity and sterilized 76% of the surviving female worms in the B. malayi–M. coucha rodent model. The chloroform fraction exhibited 34.5% macrofilaricidal activity and sterilized 66% of the female worms. However, a much higher (i.e., approximately 80%) macrofilaricidal activity with complete sterilization of the surviving females was observed in the adult B. malayi–jirid model. Two compounds, oleanoic acid (56) and oleanolic acid (57), isolated from the hexane and chloroform fractions of the crude extract were found to be active principles for the in vitro killing of adult B. malayi.162Taxodium distichum (T. distichum)
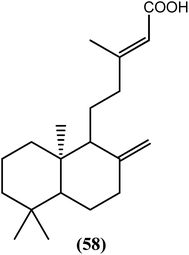
The ethanolic crude extract of the aerial parts of T. distichum displayed good antifilarial activity against B. malayi. When tested in vitro, the active component compound (58) (diterpenoid) killed microfilariae (IC50 = 18.58 μg mL−1) and macrofilariae (IC50 = 74.33 μg mL−1) of the worms together with more than 80% inhibition in the MTT reduction test of adult female worms. After an in vivo evaluation in the B. malayi–M. unguiculatus (jirid) model, the crude extract killed all adult worms in the maximum animal infection experiment, while compound (58) was approximately 95% embryostatic. In the B. malayi–M. coucha model, compound (58) killed approximately 54% of the adult worms and sterilized more than 30% of the female worms, while no further rise in microfilariae was observed after day 42 post-treatment.163Azadirachta indica (A. indica)
Aqueous and alcoholic extracts from flowers of A. indica have shown antifilarial activity against S. cervi. A lethal effect of both extracts was observed at very low concentrations, such that the alcoholic extract was found to show activity at an LC50 of 15 ng mL−1 (LC90 = 23 ng mL−1), and the aqueous extract exerted activity at an LC50 of 18 ng mL−1 (LC90 = 25 ng mL−1).164 The in vitro filaricidal activity of A. indica was potentially exerted by disturbing redox homeostasis via the down-regulation and alteration of the levels of some key antioxidants and regulatory enzymes in S. cervi, such as glutathione-S-transferase (GST), superoxide dismutase (SOD), catalase and glutathione peroxidase.165Eucalyptus tereticornis (E. tereticornis)
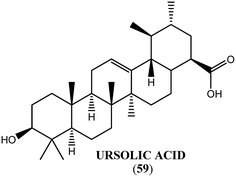
Ursolic acid (59) obtained from the leaves of E. tereticornis exerted antifilarial activity with LC100 and IC50 values of 50 μM and 8.84 μM, respectively, against microfilariae, and 100 μM and 35.36 μM, respectively, against adult worms of the human lymphatic filarial parasite B. malayi. It also inhibited the MTT reduction potential (86%) of adult parasites with a safe selectivity index.166Hibiscus sabdariffa (H. sabdariffa)
After extraction from the leaves of H. sabdariffa, when tested in vitro, the n-butanol insoluble fraction killed microfilariae at a concentration of 250 μg mL−1 and demonstrated a high selectivity index (approximately 20.8) with respect to microfilarial motility. At a dose of 500 mg kg−1 for 5 days, this leaf extract also showed 30% macrofilaricidal activity and sterilized 42% of the female worms in the B. malayi–jirid model. In the B. malayi–M. coucha model, the administration of 1 g kg−1 for 5 days resulted in sterilization of 64% of female worms and a lethal effect in 57% of adult worms.167Trachyspermum ammi (T. ammi)
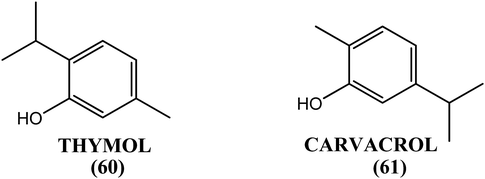
When investigated in vitro against the macrofilariae of bovine S. digitata worms, the methanolic extract obtained from the fruits of T. ammi exhibited significant activity (IC50 = 0.067 and 0.019 mg mL−1 after 24 h and 48 h incubation periods, respectively). The 2-isopropyl-5-methyl phenol (thymol, 60) was the active component, with IC50 values of 0.024 mg mL−1 and 0.002 mg mL−1 for the 24 h and 48 h incubation periods, respectively. Its positional isomer (i.e., 5-isopropyl-2-methyl phenol, carvacrol, 61) also showed promising macrofilaricidal results, with IC50 values of 0.025 mg mL−1 and 0.004 mg mL−1 for the 24 h and 48 h incubation periods, respectively. When tested in vivo in the B. malayi–M. coucha model, 2-isopropyl-5-methyl phenol (thymol, 60) resulted in a mean macrofilarial mortality of 58.93% (control: 19.05%) at a dose of 50 mg kg−1 for 5 days, and it sterilized 48.6% of female worms (control: 17.8%). Circulating microfilariae were not affected at the same dose by thymol (60).168Bauhinia racemosa (B. racemosa)
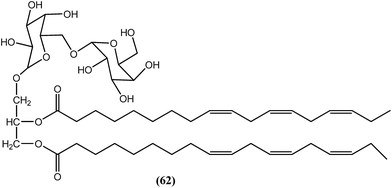
In the n-butanol fraction obtained after ethanolic extraction of the leaves of B. racemosa, compound (62), a galactolipid, was found to be most active toward both filarial forms of B. malayi. The MIC values for the compound (62) were 3.9 μg mL−1 and 15.6 μg mL−1 against adult worms and microfilariae of B. malayi respectively. The IC50 values were 1.25 μg mL−1 and 1.607 μg mL−1, respectively, against adult worms and microfilariae of the parasite. The CC50 value was more than 100 μg mL−1 when tested against Vero cells, together with SI values of >80 and >62.23 for adult worms and microfilariae of the parasite, respectively, which were quite good when compared with the standard IVM. The standard IVM provided MIC values of 7.8 μg mL−1 and 125 μg mL−1, IC50 values of 1.61 μg mL−1 and 3.62 μg mL−1, and SI values >62.11 and >27.62 against adult worms and microfilariae of the parasite, respectively. Compound (62) was also found to exert improved activity (approximately 58.3% adult worm mortality at a dose of 50 mg kg−1 for 5 days) compared with DEC (approximately 30.5% adult worm mortality at a dose of 100 mg kg−1 for 5 days) in terms of dose and efficacy.169Piper betel (P. betel)
After the in vivo evaluation in the B. malayi–M. coucha model, the crude methanolic extract of P. betel was found to suppress microfilaremia most effectively at a dose of 100 mg kg−1, and also showed somewhat reduced activity of approximately 26% against macrofilariae of the same species when compared to the untreated control groups.170Hibiscus mutabilis (H. mutabilis)
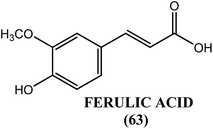
An active principle, ferulic acid (63), from the leaves of H. mutabilis showed antifilarial activity when evaluated in vitro against S. cervi. When assayed by the MTT method, reductions in viability of microfilariae and adult worms of approximately 97% and 90%, respectively, were observed in response to ferulic acid (63) at a concentration of 400 μg mL−1 after 48 h of incubation. The biochemical findings revealed that at a concentration of 200 μg mL−1, ferulic acid (63) reduced the level of GSH (glutathione) to approximately 40% but induced an increase by approximately 100% in the activity of GST (glutathione-S-transferase). Ferulic acid (63) was also found to increase SOD activity by 72.5% at the highest concentration tested (400 μg mL−1). Hence, ferulic acid (63) exerts its antifilarial effect by inducing apoptosis via the down-regulation and alteration of the level of some key antioxidants, such as GSH, GST and SOD. Ferulic acid (63) was also found to increase proapoptotic gene expression and simultaneously decrease the expression of antiapoptotic genes, with an elevation in the level of ROS and a gradual dose-dependent decline in the GSH level.171Caesalpinia bonducella (C. bonducella)
The crude extract from the seed kernel of C. bonducella exhibited 96% macrofilaricidal activity with complete sterilization of female worms. It also demonstrated a gradual decline in microfilariae up to 95%. The aqueous fraction showed complete sterilization of female worms with more than 90% microfilaricidal activity. Another hexane soluble fraction showed 95% macrofilaricidal activity along with a gradual decline in microfilariae with complete sterilization of female worms. Furthermore, when evaluated in the B. malayi–M. coucha model, F025 showed 80% sterilization of female worms with a gradual reduction of the microfilaremia.172Melaleuca cajuputi (M. cajuputi)
After in vitro testing against the filarial worm B. pahangi, the flower extract from M. cajuputi had completely halted the release of microfilariae and worm mobility after 6 days at 1000 μg mL−1. At the same concentration, this extract reduced the viability and microfilarial release more drastically than that observed using standard reference drugs such as IVM (25 μg mL−1) and tetracycline (40 μg mL−1). Additionally, the M. cajuputi flower extract exhibited in vivo antibacterial (anti-wolbachial) activity, especially at concentrations greater than 500 μg mL−1.173Xylocarpus granatum (X. granatum)
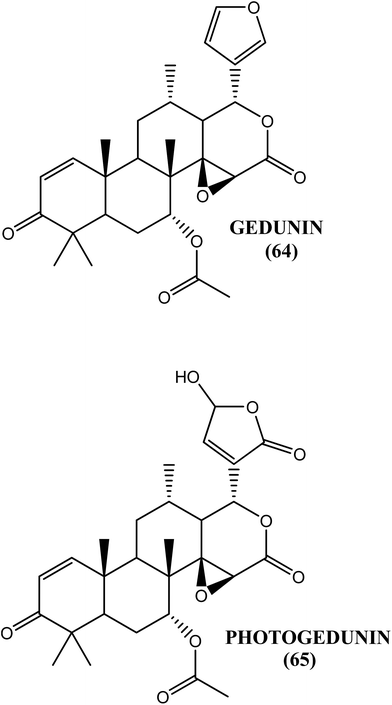
The fruit extract of the mangrove plant X. granatum was found to be an effective antifilarial agent when tested against the human lymphatic filarial parasite B. malayi. After an in vitro evaluation, the aqueous–ethanolic extract showed an IC50 value of 15.46 μg mL−1 and 13.17 μg mL−1 against macrofilariae and microfilariae, respectively, whereas approximately 53% macrofilaricidal and 63% embryostatic effects were observed in vivo in the B. malayi–M. coucha model at a dose of 50 mg kg−1 for 5 days. The ethyl acetate soluble fraction demonstrated an IC50 value of 8.5 and 6.9 μg mL−1 against macrofilariae and microfilariae, respectively. Gedunin (64) and photogedunin (65) were the active constituents in the fraction. The IC50, CC50 and SI values, respectively, for gedunin (64) against macrofilariae were 0.239 μg mL−1, 212.5 μg mL−1 and 889.1, while they were 0.213 μg mL−1, 262.3 μg mL−1 and 1231.4 for photogedunin (65). The IC50 and SI values, respectively, against microfilariae were 2.03 μg mL−1 and 435.58 for gedunin (64) and 2.23 μg mL−1 and 241.09 for photogedunin (65). When tested in vivo at a dose of 100 mg kg−1 for 5 days, gedunin (64) killed 80.0% of the transplanted adult worms, while photogedunin (65) caused approximately 70.0% adult worm mortality.174Vitex negundo (V. negundo) and Aegle marmelos (A. marmelos)
The root extract from V. negundo and the leaf extract from A. marmelos possessed antifilarial activity against microfilariae of B. malayi. Both extracts at a concentration of 100 ng mL−1 caused a complete loss of microfilarial motility after 48 h of incubation. Saponin, flavonoids and alkaloids were found to be present in the root extract of V. nigundo, whereas coumarin was observed in the leaf extract of A. marmelos.175Botryocladia leptopoda (B. leptopoda)
The crude ethanolic extract from the marine red alga B. leptopoda showed good in vitro lethal activity against three species of filaria, with LC100 of 62.5 μg mL−1 against A. viteae, 31.25 μg mL−1 against L. sigmodontis and 125 μg mL−1 against Brugia malayi. When tested at a dose of 200 mg kg−1 for 5 days in L. sigmodonti–cotton rats and the A. viteae–M. coucha and B. malayi–M. coucha models, it exhibited 71.6%, 63.2% (ethanolic extract) and 45% (hexane fraction) macrofilaricidal activity, respectively.176Haliclona oculata (H. oculata)
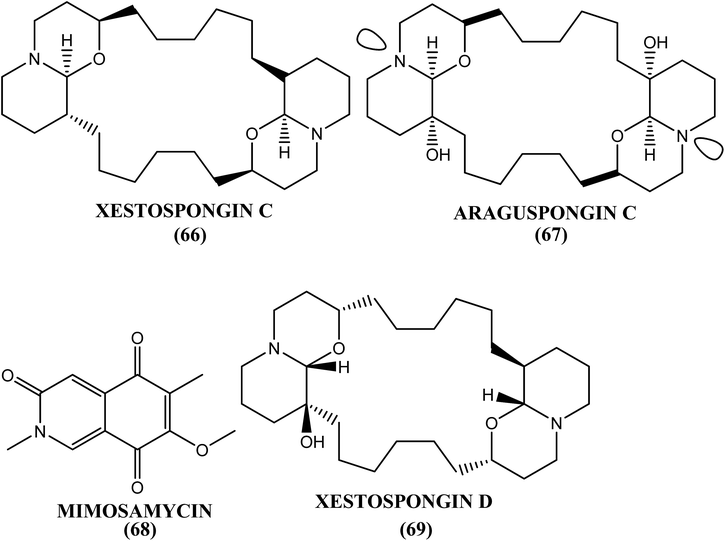
The methanolic extract of the marine sponge H. oculata displayed good antifilarial activity in vitro and in vivo against the human lymphatic filarial parasite B. malayi. The IC50 value for this extract was 5 μg mL−1 and 1.88 μg mL−1 toward macrofilariae and microfilariae, respectively. The chloroform fraction and its one chromatographic fraction from this extract showed antimacrofilarial activity (IC50) at 1.80 μg mL−1 and 1.62 μg mL−1, respectively, whereas concentrations of 1.72 μg mL−1 and 1.19 μg mL−1 were effective against microfilariae of the same species. The CC50 values were >300 μg mL−1, and the SI values were >100 after testing these three extracts against Vero cell lines. The in vivo evaluation in the primary jirid model at a dose of 100 mg kg−1 for 5 days revealed 51.3%, 64% and 70.7% macrofilaricidal activities in the methanol extract, chloroform fraction and chromatographic fraction, respectively. The most active chromatographic fraction was found to contain four major alkaloids: xestospongin-C (66), araguspongin-C (67), mimosamycin (68) and xestospongin-D (69).177Haliclona exigua (H. exigua)
After extraction from the marine sponge H. exigua, three extracts, viz. methanol extract, the n-butanol soluble fraction and the chloroform fraction, were found to possess antifilarial activities. When evaluated with respect to both motility as well as the MTT reduction assay, the methanol extract and n-butanol soluble fraction showed activity at approximately 31.25 μg mL−1 (LC100), whereas the chloroform fraction exhibited antifilarial activity at 15.6 μg mL−1 (LC100). All of these compounds also demonstrated macrofilaricidal activity, which was more pronounced in the chloroform fraction (approximately 50%). This activity could be associated with a single molecule, araguspongin C, which resulted in worm mortality at 15.6 μg mL−1. The chloroform fraction also showed approximately 58.6% adverse effects on the reproductive potential of female worms.178Eucalyptus globulus (E. globulus)
The leaf extract from E. globulus was active in vitro, with IC50 values of 62.5 μg mL−1 and 31.2 μg mL−1, respectively, against adult worms and microfilariae of B. malayi. In the B. malayi–M. coucha model and transplanted B. malayi jirid model at a dose of 100 mg kg−1 for 5 days, the extract exhibited 66.7% adulticidal activity and an embryostatic effect.179Moringa oleifera (M. oleifera)
The gum extract obtained from M. oleifera showed 100% inhibition (LC100) of microfilarial and adult worm motility at 1000 μg mL−1 and 125 μg mL−1, respectively, together with more than 56% inhibition of the MTT reduction potential of adult female worms of B. malayi. The IC50 values obtained for adult worms and microfilariae were >1000 μg mL−1 and 74.33 μg mL−1, respectively. The extract was also safe when its cytotoxicity was examined using the Vero cell line (CC50 = approximately 5694 μg mL−1), with an SI value of 76.61 (with respect to microfilarial motility). At a dose of 500 mg kg−1 for 5 days (primary screen), the extract showed 69% adulticidal activity and sterilized 83% of the female worms in the B. malayi–jirid model. In contrast, at a dose of 1000 mg kg−1 for 5 days, it showed 44% adulticidal activity in the B. malayi–M. coucha model (second screen).180Butea monosperma (B. monosperma)
The leaf and root extract of B. monosperma (Butea monosperma) showed significant inhibition of B. malayi microfilarial motility in a dose-dependent manner.181 When tested in vitro against macrofilariae of S. cervi, the methanol and hexane–ethanol fraction of the leaf extract showed IC50 values of 1.25 and 3.6 mg mL−1, respectively, while the antibiotic ciprofloxacin provided an IC50 value of 7.5 mg mL−1, supporting the potency of these fractions over ciprofloxacin.182Others
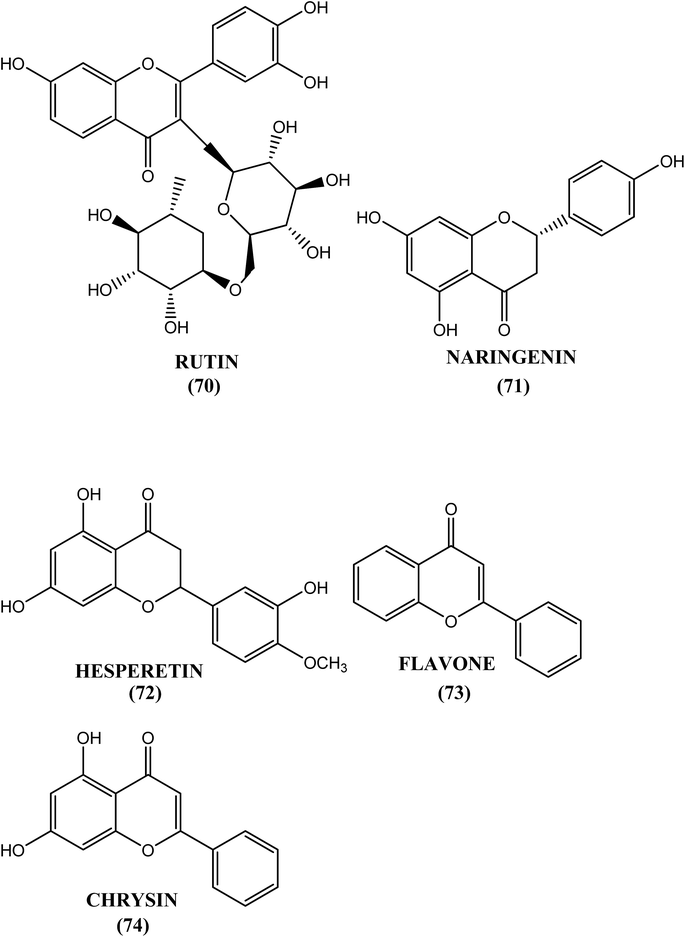
The methanolic extract from the seeds of R. communis (Ricinus communis) induced approximately 40%, 70% and 90% death in the developmental stages of the metazoan filarial parasite B. malayi treated with 10 μg mL−1, 50 μg mL−1 and 100 μg mL−1 concentrations, respectively.183 Rutin (70), naringenin (71) and hesperetin (72) showed effective microfilaricidal activity in nature at concentrations below 500 μg mL−1, and they were also found to inhibit the reduction of MTT by 60% at concentrations of 500 μg mL−1, 7.8 μg mL−1 and 31.2 μg mL−1. Naringenin (71) demonstrated in vitro macrofilaricidal activity at 125 μg mL−1 and inhibited female worm motility at 2.5 μg mL−1 (IC50). In contrast, when tested in vivo at 50 mg kg−1, it eliminated 73% and 31% of the macrofilariae in the B. malayi–Meriones and B. malayi–M. coucha models, respectively. Flavone (73) and chrysin (74) were other flavonoids with microfilaricidal activity at 62.5 μg mL−1 and 2.50 μg mL−1, respectively. At a concentration of 31.2 μg mL−1, flavone (73) effectively inhibited the motility of adult female worms. Rutin (70), a flavanol, can be obtained from onions, buckwheat, black tea, and citrus fruits, among others.184 Flavones (73) and chyrysin (74) can be obtained from grains, passion flower, and leafy vegetables, among others, while flavanones such as naringin and hesperetin can be obtained from citrus fruits, tomatoes, and grape fruit, among others.185Antifilarial targets
Wolbachia bacteria
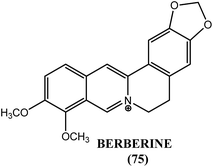
Wolbachia are α-proteobacteria inherited maternally in various arthropods also found in filarial worms. It has been suggested that Wolbachia may contribute to the nucleotide pool of nematodes. According to Slatko et al., Wolbachia have been a target for drug discovery initiatives due to their obligate nature in filarial parasites. It can be done using several approaches including diversity and focused library screening and genomic sequence analysis. Treatment with tetracycline was resulted in the depletion of these Wolbachia resulting in upregulation of phosphate permease gene, required for nucleotide synthesis.186 Another study with doxycycline showed that Wolbachia depletion was associated with a reduction in the levels of vascular endothelial growth factors (VEGFs) that are essential for lymphangiogenesis.187 One study explained essential proteins and possible therapeutic targets of Wolbachia endosymbiont of B. malayi in lymphatic filariasis and developed drug target database for them. Investigation by the researchers revealed 61 potential drug targets (outer membrane proteins, ribosomal proteins, DNA polymerases, mutases, ligases, isomerases, cell division proteins, transferases, synthetases, reductases etc.) and 4 potential vaccine extracellular targets such as putative peptidoglycan lipid II flippase, deoxycytidine triphosphate deaminase, GTP cyclohydrolase II and RNA pyrophosphohydrolase. All this information was stored by them in a single web based database called FiloBase.188 In another study, Wolbachia cell division protein FtsZ was explained as a new approach for drug targeting. Researchers had investigated the cell division machinery of Wolbachia endosymbiont of B. malayi and determined that it possessed the essential cell division gene known as FtsZ, a GTPase bacteria specific filaminting temperature sensitive protein (important in bacterial cytokinesis) that was expressed in all developmental stages of B. malayi. According to the authors, fourth stage larvae and adult female worms contained Wolbachia most abundantly while it was found least abundant in infective third stage larvae indicating a massive multiplication of Wolbachia soon after infection of the mammalian host. E. coli FtsZ inhibitor berberine, a natural alkaloid was examined by researchers against GTPase activity of FtsZ in B. malayi and it was observed that at 10–40 μM concentration, berberine (75) had adversely affected production of microfilariae as well as motility of adult females of B. malayi (Fig. 7).189N-Myristoyltransferase
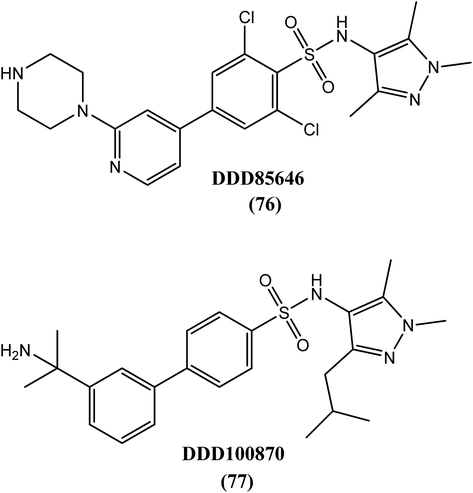
The addition of myristic acid, a 14-carbon unsaturated fatty acid, to the N-terminus of glycine in a subset of proteins via myristoyl-CoA:protein N-myristoyltransferase (NMT) promotes their binding to cell membrane. This process is called myristoylation. A homology model indicated that B. malayi genomes contained a single copy of the NMT gene encoding N-myristoyltransferasee, which possessed a canonical NMT structure with a typical NMT fold (characteristic of the acyl-CoA superfamily) and a large saddle-like β sheet flanked by several helices. A known NMT enzyme inhibitor in tripanosomatids, DDD85646 (76), and its analogue DDD100870 (77) were tested against B. malayi NMT proteins and provided IC50 values of ∼10 nM and 2.5 nM, respectively. Researchers have also reported that microfilariae were more sensitive towards both compounds than adult worms at concentrations (IC50) as low as 12.5 μM.190Proteins and amino acids
Free amino acids are required for intracellular osmoregulation and protein synthesis. The total enzymes that are required for the methionine-to-cysteine pathway has been identified in B. pahangi. The enzymes such as S-adenosylmethionine methyltransferase, methionine adenosyltransferase, and S-adenosylhomocysteine hydrolase are required for the conversion of methionine to homocysteine in the methionine cycle; however, B. pahangi lacks the final enzymes in the pathway, such as methionine synthetase (required for the formation of methionine from homocysteine) and is thus unable to resynthesize this methionine from homocysteine. Consequently, the parasite depends completely on the mosquito host for methionine uptake.191–194 The external surface of the parasitic nematode consists of cuticle, which in turn is composed of a collagen network held together by intermolecular disulfide bonds. The enzyme prolyl-4-hydroxylase has been reported to play a vital role in the biosynthesis of this collagen.195,196 Macrofilariae of B. malayi and B. pahangi contain high levels of transglutaminase activity. It has been reported that the reactions catalyzed by transaminoglutamase (a 22 kDa protein) play a significant role in the growth, development and maturation of the nematode. According to one report, unlike mammalian transglutaminase, nematodal transglutaminase does not require Ca2+ ions for its catalytic activity. A pseudosubstrate monodansylcadaverine (MDC) and the active site inhibitors cystamine or iodoacetamide were found to inhibit L3-stage parasite mobility in a dose-dependent manner that was associated with irreversible biochemical lesions, resulting in the death of the parasite. When evaluated in vitro against B. malayi, 100% inhibition was observed after a 4 h incubation with MDC at a dose of 0.5 mM. After 24 h of incubation with a 0.1 mM dose of iodoacetamide, complete immobility of L3-stage larvae was observed. At relatively higher concentrations (M.T. 0.3 mM), MDC and cystamine showed complete inhibitory effects on female worms of B. malayi. MDC also exhibited macrofilaricidal as well as embryostatic effects when tested in vitro against A. viteae adult worms.197–202 Following administration to B. malayi, retinoic acid was taken up and localized in early and late embryonic forms at high concentrations, suggesting that they may play a role in its growth and development.203 The comparative amounts of retinoic acid-binding proteins (RABPs) were between 2.7 and 3.1 pmol of retinoic acid bound/mg of extractable protein in filarids (D. viteae, B. pahangi).204 Parasitic nematodes require lipophilic retinol for various biological processes, such as embryogenesis, differentiation, and growth. For inter-as well as intracellular movement through aqueous environments, retinol requires specific retinol-binding proteins (RBPs).205–208 Ivermectin(II) was found to compete efficiently with retinol for the retinol-binding sites on RBP of the parasite but not for the host RBP sites.90Biogenic amine and polyamines
Biogenic amines play a role in neuromuscular activity and behavioral coordination in nematodes.209,210 Norepinephrine (NE), histamine, 5-hydroxytryptamine (5-HT) and dopamine are some of the biogenic amines that have been identified in L. carinii.211 Monoamine oxidase (i.e., MAO), acetylcholinesterase and dopamine-β-hydroxylase were found in macro-as well as microfilariae of S. cervi, and DEC, levamisole and centperazine were found to inactivate these enzymes. Dopamine-β-hydroxylase has also been observed in L. carinii and D. viteae.212–214 Octapamine, an amine identified in B. pahangi, was found to regulate various important processes in nematodes. This biogenic amine was also the only single amine without an important role in mammalian biological processes.215,216 Putrescine, spermine and spermidine are polyamines that are required for growth, differentiation and macromolecular synthesis in all living organisms as constituents of the polyamine salvage pathway. S. cervi and L. carinii were found to possess high concentrations of spermine and spermidine but low levels of putrescine. According to one report by Wittich et al., filarial worms possess a complete reverse pathway in which putrescine is generated from spermine and spermidine with the aid of the enzyme polyamine oxidase.217–222 Berenil and aromatic methylglyoxal bis(guanylhydrazone) analogues are inhibitors of an important regulatory enzyme, S-adenosyl-methionine decarboxylase (SAMDC), which is required for polyamine biosynthesis.223Carbohydrates
Carbohydrates are important sources of energy in all living things. Various carbohydrate biosynthesis pathways have been reported in mammals as well as in parasites. In contrast to mammalian systems, which have an active TCA (tricarboxylic acid) cycle and electron transport system (ETS), filarial parasites were found to possess active glycogenic and glycolytic pathways with a less active TCA cycle. Most microfilariae require aerobic pathways for motility but not for their survival, whereas most macrofilariae use glycolysis as a preferred route for their energy requirements.224–228 Fructose 1,6-diphosphate aldolase, phosphoenol pyruvate carboxykinase, fumarate reductase, succinate dehydrogenase, and phosphofructokinase, among others, are enzymes, and some of the cycles described above, such as glucose uptake, have been reported to be possible targets by various researchers (Table 2). Chitin metabolism is present in parasites but not in the mammalian hosts. There is evidence for the presence of chitinases in three different stages of the filarial life cycle. Allosamidin can competitively inhibit chitinase. Two isoforms of chitinase were purified from the microfilariae of B. malayi, whereas a chitinase-like antigen was expressed in L3 larvae of W. bancrofti.229–233 Similarly to chitin, sugar is known as a trehalose that is synthesized from glucose and is absent in mammals but present in parasitic nematodes such as L. carinii and B. pahangi, functioning as a reserve source of carbohydrates for energy production.234–237| Enzymes and other targets in carbohydrate biosynthesis | Description |
|---|---|
| Fructose 1,6-diphosphate aldolase | Its immunogenic component in filarial worms is distinguishable from that of mammals thus, identifying it as possible vaccine target238 |
| Phosphoenol pyruvate carboxykinase | Inhibited by DEC239 |
| Fumarate reductase | Inhibited by DEC and benzimidazoles239,240 |
| Succinate dehydrogenase | Inhibited by DEC239 |
| Phosphofructokinase | Blocked by antimonial stibophen in B. pahangi and L. carinii when compared to isofunctional mammalian enzyme241 |
| Glucose uptake | Altered by DEC, amoscanate and arsenicals239,242 |
| Utilization of glucose | Decreased by levamisole228 |
Lipid metabolism
Free fatty acids, hydrocarbon fractions, sterols, triacylglycerols and all of the major phospholipid classes were found to be present in the filarial parasite B. pahangi. The sterol ring cannot be synthesized by nematodes, but it is detected during early stages when the sterol precursor (polyisoprenoid) is required in filarial nematodes. Quinones (play a role in filarial electron transport), geranyl geraniol (unknown role), juvenile hormones (regulators of larval development), dolichols (required for glycoprotein synthesis) and isopentyl pyrophosphate (IPP constituent of filarial tRNA) are synthesized via the filarial isoprenoid biosynthetic pathway. HMG-CoA reductase is a rate-limiting enzyme and is involved in the isoprenoid pathway of filaria. In one study, the biosynthesis of genanyl geraniol and dolichols was inhibited by mevinolin, a known inhibitor of HMG-CoA reductase.243–248Folate metabolism
Various enzymes, such as reductases, transferases, synthases, dehydrogenases, hydrolases, mutases, ligases and deaminases, are involved in the interconversation of folate analogues observed in the synthesis of different tetrahydrofolate cofactors by macrofilariae. Specifically, dihydrofolate reductase activity, which is commonly observed in macrofilariae, was found to be absent in the microfilariae of B. pahangi. In contrast to all vertebrates in which N5-methylene FH4 forms irreversibly from N5–N10-methylene FH4 with the aid of the enzyme 5,10-methylene FH4 reductase, a complete reverse pathway was observed in filarial parasite (i.e., N5–N10-methylene FH4 formed instead of N5-methylene FH4). Similarly, the 10-formyl FH4 dehydrogenase enzyme, which was found to play a vital role in the regulation of the endogenous FH4 cofactor concentrations, was more active in B. pahangi than in mammals.249,250 DEC and suramin were found to inhibit some enzymes involved in folate metabolism.25,192,249Metabolism of glutathione
Glutathione has been proposed to constitute the antioxidant system (γ-glutamyl cycle) that extends the survival of filarial parasites in mammalian hosts, thereby protecting them from host-mediated membrane lipid peroxidation.252,253 Among the six enzymes involved in the γ-glutamyl cycle, two are well-characterized filarial parasites: glutamate-cysteine ligase (rate-limiting enzyme) and γ-glutamyl transpeptidase. Because filarial parasites lack the cytochrome-P-450 system (present in mammals as a major detoxification system), glutathione-S-transferases (GSTs) are one of the major detoxifying systems in filarial parasites and can detoxify cytotoxic products of lipid peroxidation via the conjugation of glutathione (GSH) to various endogenous xenobiotic electrophiles.254–258 A bovine filarial parasite called S. digitata resembles human parasites in terms of its nocturnal periodicity and antigenic pattern, and it has been found to possess GST activity in its adult female form.259 One study has confirmed that non-mammalian GSTs are expressed in parasites, and thus, the remarkable variations between host enzymes and the tertiary structures of helminth GSTs make them promising antifilarial targets.255,260,261 Arsenicals used for the treatment of filariasis have been proposed to exert their activity via the depletion of filarial glutathione.262–264 Phytocompounds such as plumbagin, curcumin and a phenoxyacetic acid derivative, i.e., ethacrynic acid (with a ketone and a methylene group), were found to inhibit filarial GST in a dose-dependent manner and have IC50 values of 51.41, 114.86 and 19.42 μM, respectively.185 In a report of a homology modeling approach via in silico analysis of the filarial GST of B. malayi, albendazole and a methyl-substituted chalcone showed the lowest binding energy towards filarial GST and also occupied the binding pocket near the enzyme substrate binding site because the side chain of these compounds interacted with the hydrophilic residues of the B. malayi GST enzyme. Studies further revealed that the O (12) atom in the carbamate moiety of albendazole formed 4 H-bonds and exhibited the lowest interaction energy and was therefore the most favorable interacting site, while electronegative residues such as Asp159, Glu162, His179 and Glu183 anchored the thiol and benzoimidazole moiety of albendazole within the site. The researchers further suggested that Arg11 was situated on the opposite side and close to both Tyr7 (original binding site of GSH) as well as Leu13 (another GSH binding site), which can lead to conformational changes in the protein or cause a steric hindrance that might interfere in GSH binding. The binding location for methyl-substituted chalcone (78) was present in a crevice between the α1, α6, and α8 helices. It was finally concluded that Arg11, Ile163, His167 and Cys168 were the most favorable common sites for the binding of antifilarials and chalcones. The displacement of GSH from its original binding site due to ligand binding suggested a non-competitive type of inhibition of GST activity.265Receptors, hormones and the microtubular system
Levamisole exerts its agonistic action at the neuromuscular junction of nicotinic acetylcholine receptors.266 B. malayi and B. pahangi have been reported to possess a microtubular system along with some β-tubulin isoforms. Benzimidazole carbamates (microtubule depolymerizing drugs) act by disrupting the assembly of tubulin dimers of microtubules.267–271Patents
Patents on the antifilarials are summerized in Table 3| Name | Description |
|---|---|
| Compounds for use in the treatment of filariasis | In the present patent, compound with following general formula (79) and its various forms are reported by Pfarr et al. as antifilarial agents |
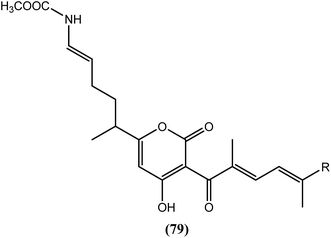 |
|
| According to inventors, when tested in vitro, more than 50% depletion of the Wolbachia was observed with Corallopyronin A (80) at concentration of 0.1 μg mL−1 whereas it depleted Wolbachia to levels equivalent to 4 μg mL−1 of the doxycycline (a tetracycline) at 1 μg mL−1 concentration. Also, when given in vivo at a dose of 35 mg per kg per day to infected BALB/c mice for 28 days, it had depleted more than 99% of the Wolbachia when compared to the control272 | |
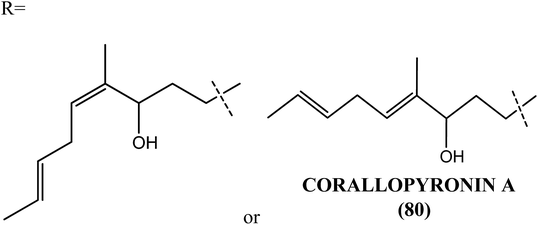 |
|
| Multivalent vaccine for filariasis | This invention by Kalyanasundaram presents multivalent vaccine for treating an animal against filariasis. This vaccine composed of two or more isolated antigens from one or more filarial species such as W. bancrofti, B. malayi, B. timori, O. volvulus and Loa loa. Antigens isolated are covalently attached protein based, DNA based or combination of them. Antigens also include small heat shock protein (HSP), Vespid venom allergen homologue like protein (VAL-1), abundant larval transcript (ALT-2) etc. according to some embodiments, vaccine also comprises of known antigens (from W. bancrofti, B. malayi, B. timori, O. volvulus and Loa loa) such as glutathione peroxidase, recombinant antigen, heat shock cognate 70 and paramyosin. When vaccine composed of nucleic acid it is called as genetic vaccine. When viruses such as pox virus, alpha virus, herpes virus etc. are used as carrier which carries recombinant molecule of the invention, packaged in its viral coat that can be expressed in animal after its administration, it is said as recombinant virus vaccine. Recombinant virus vaccine following its administration infects cells of immunized animal and directs production of protective protein capable of protecting animal from filarial nematodes273 |
| Pyrroloindole | According to Alasia et al. compound (81) and compound (82) were found to be most effective with IC50 concentration below 1 μM against Hsp82 protein of filarial parasites. Brugia and Bancroft's parasites were reported to contain another similar protein known as Hsp90 protein. B. pahangi and human sequences, were found to be 87% similar and 80% identical and was found to be sensitive towards geldanamycin (83).274,275 |
 |
|
| Herbal composition | In a patent by Das, it is reported that herbal extract composition obtained from Typhonium trilobatum at a single dose of 100–200 mg daily most showed antifilarial action without any toxicity. When it was tested in vivo in rat, LD50 value of the extract was found to be >2000 mg kg−1 with no toxic symptoms, signs or mortality of the treated animals. After clinical evaluation of this extract, it was observed that elimination of disability in patients suffering from acute filariasis have completed in 30 days treatment. On the other hand, following serological study, a gradual decline in antigen units was found in 90 days indicating that reduction was occurred in microfilariae production276 |
| Anthraquinones | In this patent by Nair et al. hydroxyl substituted anthraquinones are reported as antifilarials. They reported that the compounds of the invention are used for inhibiting parasite which comprises of exposing the parasite to an inhibitory concentration of hydroxyl substituted anthraquinone. It is found that B. malayi can be inhibited both in vitro as well as in vivo by these compounds. Hydroxyl substitution can also be a methoxy substitution. Anthraquinone (84), a phytoconstituent obtained from the roots of day lilly was found effective against B. malayi showing 100% filaricidal activity in 2 days against adult female worms at concentration of 5 ppm. Against adult female of same filarial species and at same concentrations, compounds (85) and (86) showed 100% filaricidal activity within 24 h only.277 |
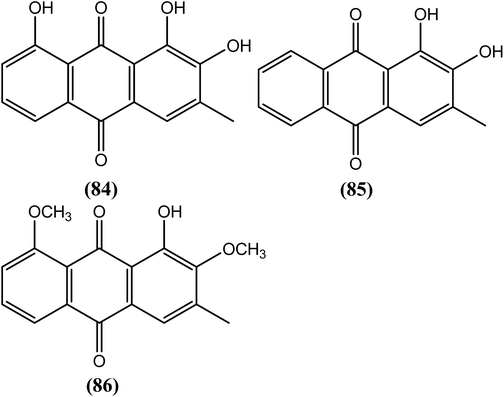 |
Antifilarials in clinical trials
Molecules that are/were in clinical trials are mostly equivalent to existing drugs, but the studies are ongoing to determine their efficacy in combination (multidrug) treatments.Moxidectin
In a study examining the treatment of onchocerciasis, moxidectin was found to be more effective than ivermectin in most animal models, demonstrating potent effects on microfilariae along with long-term sterilization of adult female worms. It is currently in the early stages of clinical trials for the treatment of onchocerciasis and can be studied for its effectiveness for the treatment of filariasis.278,279Albendazole combinations
A recent observational study titled, “Optimization of mass drug administration with existing drug regimens for lymphatic filariasis and onchocerciasis foe Liberia,” included annual vs. semiannual albendazole plus IVM mass drug administration (MDA). It is estimated that approximately 5200 people will participate every year. This investigation will test the hypothesis that semiannual MDA for LF is superior to annual MDA. The primary outcomes will explain microfilarial prevalence, whereas the secondary outcomes will measure the prevalence of filarial antigenemia in blood and the intensity of filarial worm infections. The study was initiated in 2012 and estimated to be completed in 2017.280 Through the involvement of its clinical researchers, TDR is supporting studies to evaluate albendazole co-administration with ivermectin (IVM) or diethylcarbamazine (DEC) for the treatment of LF patients. These studies were performed according to the WHO/GlaxoSmithKline/Merck recommendations for the co-administration of albendazole with IVM or DEC for the elimination of LF. When tested in adult worms, preliminary results for the use of IVM with albendazole, albendazole with levamisole or IVM with levamisole suggested that in addition to the safety of these combinations, none was found to be more effective than IVM; however, three studies are still in progress. A randomized double-blind study was undertaken in Tanzania, which consisted of 1000 subjects to compare single-dose IVM + albendazole with single-dose IVM alone.281 To evaluate the pharmacokinetic profiles of albendazole and DEC when administered as a single drug or as combination drugs, a study is currently in progress in the Alleppey (India) to compare LF infected vs. non-infected individuals. This is also the first study to determine the presence of any toxic pharmacokinetic drug interactions. A third study is also underway in Wardha, India, and it includes 1347 subjects to evaluate the safety, efficacy, tolerability and population pharmacokinetics of DEC co-administered with albendazole compared with DEC alone. Another clinical trial has suggested that single-dose DEC–albendazole dramatically reduces blood microfilariae, but most of the treated subjects failed to completely clear the microfilariae after a single-dose administration. It was also found that although both regimens provided similar activity against macrofilariae, a multidose regimen of DEC–albendazole was more effective for clearing and reducing microfilariae of the worm compared with single-dose therapy.282Eradication programs
In one report by a WHO team headed by Dr Kazuyo Ichimori, it was mentioned that preventive chemotherapy, vector control and morbidity management can work most efficiently as one package at the global, national and local levels. To eliminate LF by 2020, the WHO established the Global Programme to Eliminate Lymphatic Filariasis (GPELF) in 2000. GPELF has been one of the most rapidly expanding global health programs in the history of public health. Fifty-three of the 81 endemic countries have started implementing MDA to halt the transmission of LF. During the decade from 2000–2009, more than 2.8 billion doses of medicine were delivered to 845 million people (cumulative targeted population).To achieve the goal set by GPELF, two strategies have been developed. In the first strategy, single annual doses of diethylcarbamazine (DEC) or ivermectin plus albendazole will be delivered to the entire endemic area to prevent transmission of the LF parasite. MDA also provides significant health benefits, such as reduced morbidity from intestinal worms and ectoparasites (e.g., lice). The second strategy is to alleviate suffering and disability by introducing basic measures, such as improved hygiene and skin care, to those with lymphedema and performing surgery in men with hydrocele. The investment target for preventive chemotherapy during 2015–2020 is approximately US$ 154 million (US$ 105–208 million) per year. Two objectives have been determined by the WHO, which include “70% of endemic countries requiring MDA will have enter post-intervention surveillance by 2016” and “all endemic countries will have to complete post-intervention surveillance by 2020”.1,283
Future perspectives and conclusions
Filariasis is one of the oldest, most disabling and disfiguring neglected tropical diseases with various clinical manifestations and a high morbidity rate. Very few drugs are available for treatment of filariasis but their repetitive use may give rise to drug resistance. Further they have beneficial effect in elimination of the larval stage of the worms only. They are found to be fairly ineffective towards eliminating adult worms. Filarial nematodes are unable to survive in in vitro culture for long-term, which greatly limits research into the basic biology of these parasites and thus affects in vitro screening of novel anti-filarial agents. Therefore much efforts are needed to improve the stability of these nematodes in the culture. Numbers of antifilarial targets have been explored in last two decades, however their evaluation with reference to assay feasibility, target validation, drugabillity, toxicity, resistance potential and structural information needs to be highlighted in future.Considering the limitations of the existing antifilarial drugs more focused efforts are needed by the researchers to explore the mechanism of development of drug resistance to detect it at an early stage. Though large number of chemical classes have been explored till date, very few of them enters in clinical development due to toxicity and pharmacokinetics problem. Researchers working in the field are therefore needed to consider these issues during antifilarial drug development. Some of the initial hints about genome sequences of B. malayi, Wolbachia, mosquito vector may be helpful for the researchers in development of some new agents that overcomes the limitations of existing antifilarial agents. With some of the positive results, vaccination though at very primary stage now, may turn fruitful approach in future to combat filariasis.
Acknowledgements
Authors JNS and AAK are thankful to Dr Zahid Zaheer, Principal Y. B. Chavan College of Pharmacy, Aurangabad India for their constant support and encouragement.References
- World Health Organization, Sustaining the drive to overcome the global impact of neglected tropical diseases, second WHO report on neglected tropical diseases, Geneva, 2013, pp. 1–137 Search PubMed.
- World Health Organization global programme to eliminate lymphatic filariasis, Lymphatic filariasis, Progress report 2000–2009 and strategic plan 2010–2020, Geneva, 2010, pp. 1–78 Search PubMed.
- Lymphatic Filariasis, research and control in eastern and southern Africa, ed. P. E. Simonsen, M. N. Malecela, E. Michael and C. D. Mackenzie, DBL-Center for health and research development, Denmark, 2008, pp. 1–155 Search PubMed.
- P. K. Singh, A. Ajay, S. Kushwaha, R. P. Tripathi and S. Misra-Bhattacharya, Future Med. Chem., 2010, 2, 251–283 CrossRef CAS PubMed.
- (a) World Health Organization, Global programme to eliminate lymphatic filariasis, Lymphatic filariasis: practical entomology, Geneva, 2013, pp. 1–90 Search PubMed; (b) Vector control-Methods for use by individuals and communities, ed. J. A. Rozendaal, World Health Organization, Geneva, 1997, pp. 29–34 Search PubMed; (c) Parasites-Lymphatic filariasis. Biology-Life cycle of Brugia malayi. Centers for disease control and prevention, 2015, https://www.cdc.gov/parasites/lymphaticfilariasis/biology_b_malayi.html.
- G. J. Weil, R. M. Ramzy, R. Chandrashekar, A. M. Gad, R. C. Lowrie Jr and R. Faris, Am. J. Trop. Med. Hyg., 1996, 55, 333–337 CAS.
- S. K. Dunyo, F. K. Nkrumah, C. K. Ahorlu and P. E. Simonsen, Trans. R. Soc. Trop. Med. Hyg., 1998, 92, 539–540 CrossRef CAS PubMed.
- G. Dreyer, Z. Medeiros, M. J. Netto, N. C. Leal, L. G. De Castro and W. F. Piessens, Trans. R. Soc. Trop. Med. Hyg., 1999, 93, 413–417 CrossRef CAS PubMed.
- D. H. Connor, J. R. Palmieri and D. W. Gibson, Z. Parasitenkd., 1986, 72, 13–28 CrossRef CAS PubMed.
- (a) Lymphatic Filariasis-USAIDs neglected tropical disease program, https://www.neglecteddiseases.gov/target_diseases/lymphatic_filariasis/; (b) World Health Organization, Lymphatic pathology and immunopathology in filariasis: report of the twelfth meeting of the scientific working group on filariasis, Geneva, mimeographed document, TDR/FIL-SWG (12)/85.3, 1985 Search PubMed; (c) World Health Organization, Lymphatic filariasis: Disease and its control. Fifth report of the WHO expert comitee on filariasis, Geneva, 1992, Technical report series, no. 821 Search PubMed; (d) S. P. Pani and A. Sividya, Indian J. Med. Res., 1995, 102, 114–118 CAS.
- (a) J. E. McMahon, S. A. Magayuka, N. Kolstrup, F. W. Mosha, F. M. Bushrod, D. E. Abaru and J. H. Bryan, Ann. Trop. Med. Parasitol., 1981, 75, 415–431 CrossRef CAS PubMed; (b) D. W. Meyrowitsch, P. E. Simonsen and W. H. Makunde, Ann. Trop. Med. Parasitol., 1995, 89, 653–663 CrossRef CAS PubMed.
- G. Dreyer, J. Noroes, J. Figueredo-Silva and W. F. Piessens, Parasitol. Today, 2000, 16, 544–548 CrossRef CAS PubMed.
- H. W. Acto and S. S. Rao, Indian Med. Gaz., 1930, 65, 541–546 Search PubMed.
- E. A. Ottesen, Curr. Opin. Infect. Dis., 1994, 7, 550–558 CrossRef.
- D. O. Freedman, M. J. de Almeida Filo, S. Besh, M. C. de Silva, C. Braga, A. Maciel and A. F. Furtado, J. Infect. Dis., 1995, 171, 997–1001 CrossRef CAS PubMed.
- M. Philipp, T. B. Davis, N. Storey and C. K. S. Carlow, Annu. Rev. Microbiol., 1988, 42, 685–716 CrossRef CAS PubMed.
- (a) K. P. Day, Parasitol. Today, 1991, 7, 341–343 CrossRef CAS PubMed; (b) E. A. Ottesen, Springer Semin. Immunopathol., 1980, 2, 373–385 CrossRef.
- World Health Organisation, Global Programme to Eliminate Lymphatic Filariasis: Progress Report on Mass Drug Administration, 2010, Weekly Epidemiological Record, 2011, vol. 86, pp. 377–388 Search PubMed.
- (a) G. Weil, P. J. Lammie and N. Weiss, Parasitol. Today, 1997, 13, 401–404 CrossRef CAS PubMed; (b) L. A. Andrade, Z. Medeiros, M. L. Pires, A. Pimental, A. Rocha, J. Figurado-Silva, A. Coutinho and G. Dreyer, Trans. R. Soc. Trop. Med. Hyg., 1995, 89, 319–321 CrossRef CAS PubMed.
- C. B. Harinath and M. V. R. Reddy, Journal of International Medical Sciences Academy, 2000, 13, 8–12 Search PubMed.
- J. P. Narain, A. P. Dash, B. Parnell, S. K. Bhattacharya, S. Barua, R. Bhatia and L. Savioli, Bull. W. H. O., 2010, 88, 206–210 CrossRef PubMed.
- J. N. Demarquay, Gaz. Med., 1863, 18, 665–667 Search PubMed.
- T. R. Lewis, Lancet, 1872, 100, 889–890 CrossRef.
- T. S. Cobbold, Lancet, 1877, 2, 70–71 Search PubMed.
- I. A. McGregor, Trans. R. Soc. Trop. Med. Hyg., 1995, 89, 1–8 CrossRef CAS PubMed.
- D. I. Grove, A History of Human Helminthology, CAB International, Wallingford UK, 1990, p. 597 Search PubMed.
- D. Molyneux, Filaria J., 2003, 2, 13 CrossRef PubMed.
- E. A. Ottesen, Am. J. Trop. Med. Hyg., 1989, 41, S9–S17 Search PubMed.
- E. Michael and D. A. P. Bundy, Parasitol. Today, 1997, 13, 472–476 CrossRef CAS PubMed.
- World Health Organisation, Fact Sheet, http://www.who.int/mediacentre/factsheets/fs102/en.
- World Health Organisation, Reducing Risks, Promoting Healthy Life, WHO report, Geneva, 2002, pp. 1–248 Search PubMed.
- A. M. Khan and J. Mahanta, Curr. Sci., 2005, 88, 1718–1719 Search PubMed.
- World Health Organisation, Epidemiology, http://www.who.int/lymphatic_filariasis/epidemiology/en/.
- World Health Organisation, Global Programme to Eliminate Lymphatic Filariasis: Progress Report, 2013, Weekly Epidemiological Record, 2014, vol. 89, pp. 409–418 Search PubMed.
- K. D. Ramaiah, P. K. Das, E. Michael and H. Guyatt, Parasitol. Today, 2000, 16, 251–253 CrossRef CAS PubMed.
- T. G. Ton, C. Mackenzie and D. H. Molyneux, Infectious Diseases of Poverty, 2015, 4, 1–8 CrossRef PubMed.
- D. W. Meyrowitsch, P. E. Simonsen and S. M. Magesa, Ann. Trop. Med. Parasitol., 2004, 98, 155–169 CrossRef CAS PubMed.
- D. H. Molyneux and N. Zagaria, Ann. Trop. Med. Parasitol., 2002, 96, S15–S40 CrossRef PubMed.
- L. X. Liu and P. F. Weller, N. Engl. J. Med., 1996, 334, 1178–1184 CrossRef CAS PubMed.
- DNDi, Drugs for Neglected Diseases Initiative, http://www.dndi.org/diseases-projects/filarial-diseases/filaria-disease-background/.
- M. Pinder, Parasitol. Today, 1988, 4, 279–284 CrossRef CAS PubMed.
- J. O. Gyapong, D. Kyelem, I. Kleinschmidt, K. Agbo, F. Ahouandogbo, J. Gaba, G. Owusu-Banahene, S. Sanou, Y. K. Sodahlon, G. Biswas, O. O. Kale, D. H. Molyneux, J. B. Roungou, M. C. Thomson and J. Remme, Ann. Trop. Med. Parasitol., 2002, 96, 695–705 CrossRef CAS PubMed.
- H. A. Farid, Z. S. Morsy, H. Helmy, R. M. Ramzy, M. El Setouhy and G. J. Weil, Am. J. Trop. Med. Hyg., 2007, 77, 593–600 Search PubMed.
- J. M. Mladonicky, J. D. King, J. L. Liang, E. Chambers, M. Paau, M. A. Schmaedick, T. R. Burkot, M. Bradley and P. J. Lammie, Am. J. Trop. Med. Hyg., 2009, 80, 769–773 Search PubMed.
- D. O. Freedman, P. J. de Almeoda Filho and S. Besh, J. Infect. Dis., 1994, 170, 927–933 CrossRef CAS PubMed.
- J. McCarthy, Diagnosis of Lymphatic Filarial Infection, Lymphatic Filariasis, ed. T. B. Nutman, Imperial College Press, London, 2000, pp. 127–141 Search PubMed.
- F. Amaral, G. Dreyer and J. Figueredo-Silva, Am. J. Trop. Med. Hyg., 1994, 50, 735–757 Search PubMed.
- K. Anitha and R. K. Shenoy, Indian J. Dermatol. Venereol., 2001, 67, 60–65 CAS.
- B. de Meillon and N. G. Hairston, Bull. W. H. O., 1968, 38, 935–941 Search PubMed.
- (a) R. Pink, A. Hudson, M.-A. Mouries and M. Bendig, Nat. Rev. Drug Discovery, 2005, 4, 727–740 CrossRef CAS PubMed; (b) R. I. Hewitt, S. Kushner, H. Stewart, E. White, W. Wallace and Y. Subbarow, J. Lab. Clin. Med., 1947, 32, 1314–1329 CAS; (c) S. Babu and T. B. Nutman, Parasite Immunol., 2014, 36, 338–346 CrossRef CAS PubMed.
- R. I. Hewitt, E. White, W. S. Wallace, H. Stewart, S. Kushner and Y. Subbarow, J. Lab. Clin. Med., 1947, 32, 1304–1313 CAS.
- D. Santiago-stevenson, J. Oliver-gonzalez and R. I. Hewitt, JAMA, J. Am. Med. Assoc., 1947, 135, 708–712 CrossRef CAS.
- F. Hawking, Diethylcarbamazine: A Review of Literature with Special Reference to its Pharmacodynamics, Toxicity, and Use in the Therapy of Onchocerciasis and other filarial infections. WHO/ONCHO/78, 1978, vol. 142, pp. 1–82 Search PubMed.
- F. Hawking and W. Laurie, Lancet, 1949, 2, 146–147 CrossRef CAS.
- R. M. Maizels and D. A. Denham, Parasitology, 1992, 105, S49–S60 CrossRef PubMed.
- S. Misra, D. P. Singh and R. K. Chatterjee, Trop. Med., 1988, 30, 1–11 Search PubMed.
- W. A. Stolk, G. J. V. Oortmarssen, S. P. Pani, S. J. DeVlas, S. Subramanian, P. K. Das and J. D. Habbema, Am. J. Trop. Med. Hyg., 2005, 73, 881–887 CAS.
- W. Melrose, Lymphatic Filariasis: A Review 1862-2002, Warwick Educational Publishing Inc., Australia, 2004, pp. 1–80 Search PubMed.
- G. J. Weil, P. J. Lammie, F. O. Richards and M. L. Eberhard, J. Infect. Dis., 1991, 164, 814–816 CrossRef CAS PubMed.
- M. M. Ismail, G. J. Weil, K. S. A. Jayasinghe, U. N. Premaratne, W. Abeyewickreme, H. N. Rajaratnam, H. R. Sheriff, C. S. Perera and A. S. Dissanaike, Trans. R. Soc. Trop. Med. Hyg., 1996, 90, 684–688 CrossRef CAS PubMed.
- J. S. McCarthy, A. Guinea, G. J. Weil and E. A. Ottesen, J. Infect. Dis., 1995, 172, 521–526 CrossRef CAS PubMed.
- C. A. Peixoto, A. Rocha, A. Aguiar-Santos and M. S. Florencio, Parasitol. Res., 2004, 92, 513–517 CrossRef CAS PubMed.
- S. Subramanian, P. Vanamail, P. K. Das, S. P. Pani and R. Ravi, Randomized Controlled Clinical Trials of Antifilarial Drugs for Lymphatic Filariasis: Endpoints of Outcome Measures Influence Drug Efficacy. Presented at: Proceedings of the 23rd Conference of the Indian Society for Medical Statistics, Jawaharlal Nehru Medical College, Belgaum, India, January 2005, pp. 19–21 Search PubMed.
- O. Hussein, M. E. Setouhy, E. S. Ahmed, A. M. Kandil, R. M. Ramzy, H. Helmy and G. J. Weil, Am. J. Trop. Med. Hyg., 2004, 71, 471–477 Search PubMed.
- (a) Essential medicines and health products information portal-A World Health Organization resource, http://apps.who.int/medicinedocs/en/d/Jh2922e/3.5.1.html; (b) J. P. Chippaux, M. Boussinesq, J. Gardon, N. Gardon-Wendel and J. C. Ernould, Parasitol. Today, 1996, 12, 448–450 CrossRef CAS PubMed.
- G. Dreyer, M. L. Pires, L. D. Andrade, E. Lopes, Z. Medeiros, J. Tenorio, A. Coutinho, J. Noroes and J. Figueredo-Silva, Trans. R. Soc. Trop. Med. Hyg., 1994, 88, 232–236 CrossRef CAS PubMed.
- R. M. Maizels and D. A. Denham, Parasitology, 1992, 105, S49–S60 CrossRef PubMed.
- J. W. Mak, Trop. Biomed., 2004, 21, 27–38 CAS.
- H. B. Woodruff and R. W. Burg, The Antibiotics Explosion. Discoveries in Pharmacology Volume 3, in Pharmacological Methods, Receptors and Chemotherapy, ed. M. J. Parnham and J. Bruinvels, Elsevier, Amsterdam, The Netherlands, 1986, pp. 338–341 Search PubMed.
- B. Thylefors, Trop. Med. Int. Health, 2004, 9, A1–A3 CrossRef CAS PubMed.
- G. Schares, B. Hofmann and H. Zahner, Trop. Med. Parasitol., 1994, 45, 97–106 CAS.
- W. C. Campbell, Annu. Rev. Microbiol., 1991, 45, 445–474 CrossRef CAS PubMed.
- E. A. Ottesen and W. C. J. Campbell, J. Antimicrob. Chemother., 1994, 34, 195–203 CrossRef CAS PubMed.
- S. Townson, S. K. Tagboto, J. Castro, A. Lujan, K. Awadzi and V. P. K. Titanji, Trans. R. Soc. Trop. Med. Hyg., 1994, 88, 101–106 CrossRef CAS PubMed.
- G. Dreyer, J. Noroes, F. Amaral, A. Nen, Z. Medeiros, A. Coutinho and D. Addiss, Trans. R. Soc. Trop. Med. Hyg., 1995, 89, 441–443 CrossRef CAS PubMed.
- R. K. Shenoy, V. Kumaraswami, K. Rajan, S. Thankom and A. Jalajakumari, Ann. Trop. Med. Parasitol., 1993, 87, 459–467 CrossRef CAS PubMed.
- N. L. Nguyen, J. P. Moulia-Pelat and J. L. Cartel, Trans. R. Soc. Trop. Med. Hyg., 1996, 90, 689–691 CrossRef CAS PubMed.
- A. P. Plaisier, W. C. Cao, G. J. Van Oortmarssen and J. D. Habbema, Parasitology, 1999, 119, 385–394 CrossRef CAS PubMed.
- S. Omura, Mode of Action of Avermectin, in Macrolide Antibiotics: Chemistry, Biology and Practice, ed. S. Omur, Academic Press, NY, USA, 2002, pp. 571–576 Search PubMed.
- J. P. Arena, K. K. Liu, P. S. Paress, J. M. Schaeffer and D. F. Cully, Brain Res., 1992, 15, 339–348 CrossRef CAS PubMed.
- D. F. Cully, D. K. Vassilatis, K. K. Liu, P. S. Paress, L. H. Van der Ploeg, J. M. Schaeffer and J. P. Arena, Nature, 1994, 371, 707–711 CrossRef CAS PubMed.
- S. Lustigman and J. P. McCarter, PLoS Neglected Trop. Dis., 2007, 1, e76 Search PubMed.
- J. K. Eng, W. J. Blackhall, M. Y. Osei-Atweneboana, C. Bourguinat, D. Galazzo and R. N. Beech, Mol. Biochem. Parasitol., 2006, 150, 229–235 CrossRef CAS PubMed.
- G. J. Weil, K. V. P. Sethumadhavan, S. Santhanam, D. C. Jain and T. K. Ghosh, Am. J. Trop. Med. Hyg., 1988, 38, 589–595 CAS.
- G. Dreyer, D. Addiss, J. Noroes, F. Amaral, A. Rocha and A. Coutinho, Trop. Med. Int. Health, 1996, 1, 427–432 CrossRef CAS PubMed.
- W. Cao, C. P. Ploeg, A. P. Plaisier, I. J. van der Sluijs and J. D. Habbema, Trop. Med. Int. Health, 1997, 2, 393–403 CrossRef CAS PubMed.
- N. D. E. Alexander and B. T. Grenfell, Parasitology, 1999, 119, 151–156 CrossRef PubMed.
- P. J. Lammie, W. L. Hitch, E. M. W. Allen, W. Hightower and M. L. Eberhard, Lancet, 1991, 337, 1005–1006 CrossRef CAS.
- P. K. Das, A. Sirvidya, P. Vanamail, K. D. Ramaiah, S. P. Pani, E. Michael and D. A. Bundy, Trans. R. Soc. Trop. Med. Hyg., 1997, 91, 677–679 CrossRef CAS PubMed.
- B. P. Sani and A. Vaid, Biochem. J., 1988, 249, 929–932 CrossRef CAS PubMed.
- D. Subrahmanyam, Ciba Found. Symp., 1987, 127, 246–264 CAS.
- A. Harder, Parasitol. Res., 2002, 88, 395–397 CrossRef CAS PubMed.
- P. Nickel, H. J. Haack and H. Widjaja, Drug Res., 1986, 36, 1153–1157 CAS.
- World Health Organization, Model prescribing information: Drugs used in parasitic diseases, Geneva, 2nd edn, 1995, pp. 1–146 Search PubMed.
- C. P. Chijioke, R. E. Umeh, A. U. Mbah, P. Nwonu, L. L. Fleckenstein and O. O. Okonkwo, Eur. J. Clin. Pharmacol., 1998, 54, 249–251 CrossRef CAS PubMed.
- U. Pandaya and O. P. Shukla, Med. Sci. Res., 1994, 22, 833–834 Search PubMed.
- R. D. Walter and H. Schulz-Kay, Tropenmed. Parasitol., 1980, 31, 55–58 CAS.
- R. D. Walter and E. J. Albiez, Mol. Biochem. Parasitol., 1981, 4, 53–600 CrossRef CAS PubMed.
- (a) Essential medicines and health products information portal-A World Health Organization resource, http://apps.who.int/medicinedocs/en/d/Jh2922e/3.6.2.html; (b) L. Gonzalezguerra, E. Rasi and A. Rivas, Rev. Venez. Sani. Asist. Soc. Caracas., 1964, 29, 90–97 CAS.
- C. P. Chijioke, R. E. Umeh, A. U. Mbah, P. Nwonu, L. L. Fleckenstein and O. O. Okonkwo, Eur. J. Clin. Pharmacol., 1998, 54, 249–251 CrossRef CAS PubMed.
- B. Thylefors and A. Rolland, Bull. W. H. O., 1979, 57, 479–480 CAS.
- F. Hawking, Adv. Pharmacol. Chemother., 1978, 15, 289–322 CAS.
- J. McCarthy, Am. J. Trop. Med. Hyg., 2005, 73, 232–233 Search PubMed.
- D. G. Addiss and J. Louis-Charles, Am. J. Trop. Med. Hyg., 1997, 57, S215 Search PubMed.
- M. E. Selkirk, V. P. Smith, G. R. Thomas and K. Gounaris, Int. J. Parasitol., 1998, 28, 1315–1332 CrossRef CAS PubMed.
- (a) D. G. Addiss, M. J. Beach, T. G. Streit, S. Lutwick, F. H. LeConte, J. G. Lafontant, A. W. Hightower and P. J. Lammie, Lancet, 1997, 350, 480–484 CrossRef CAS PubMed; (b) WHO Media Centre, Lymphatic filariasis, Fact sheet no. 102, Updated March, 2017 Search PubMed.
- E. A. Ottesen, M. M. Ismail and J. Horton, Parasitol. Today, 1999, 15, 382–386 CrossRef CAS PubMed.
- A. Harder, Parasitol. Res., 2002, 88, 272–277 CrossRef CAS PubMed.
- J. F. Rossignol and H. Maisonneuve, Gastroenterol. Clin. Biol., 1984, 8, 569–576 CAS.
- R. S. Esworthy, F. F. Chu, P. Geiger, A. W. Girotti and J. H. Doroshow, Arch. Biochem. Biophys., 1993, 307, 29–34 CrossRef CAS PubMed.
- M. E. Oxberry, T. G. Geary and R. K. Prichard, Parasitology, 2001, 122, 683–687 CrossRef CAS PubMed.
- M. J. Miller, Drugs, 1980, 20, 122–130 CrossRef CAS PubMed.
- J. W. Mak and V. Zaman, Trans. R. Soc. Trop. Med. Hyg., 1980, 74, 285–291 CrossRef CAS PubMed.
- J. Aceves, D. Erlij and R. Martinez-Maranon, Br. J. Pharmacol., 1970, 38, 602–607 CrossRef CAS PubMed.
- I. D. Harrow and K. A. F. Gration, Ascaris suum. Pest. Sci., 1985, 16, 662–672 CrossRef CAS.
- S. B. Katiyar, I. Bansal, J. K. Saxena and P. M. S. Chauhan, Bioorg. Med. Chem. Lett., 2005, 15, 47–50 CrossRef CAS PubMed.
- B. K. Singh, M. Mishra, N. Saxena, G. P. Yadav, P. R. Maulik, M. K. Sahoo, R. L. Gaur, P. K. Murthy and R. P. Tripathi, Eur. J. Med. Chem., 2008, 43, 2717–2723 CrossRef CAS PubMed.
- A. Agarwal, S. K. Agarwal, S. N. Singh, N. Fatma and R. K. Chatterjee, Bioorg. Med. Chem. Lett., 1996, 6, 225–228 CrossRef CAS.
- S. K. Srivastava, A. Agarwal, P. M. S. Chauhan, S. K. Agarwal, A. P. Bhaduri, S. N. Singh, N. Fatima and R. K. Chatterjee, J. Med. Chem., 1999, 42, 1667–1672 CrossRef CAS PubMed.
- R. I. Hewitt, S. Kushner, H. Stewart, E. White, W. S. Wallace and Y. Subbarow, J. Lab. Clin. Med., 1947, 32, 1314–1329 CAS.
- World Health Organisation, Report of the seventh meeting of the scientific working group on filariasis: Filaricide screeners, TDR/Fil/SWG(7)82,3, World Health Organisation, Geneva, 1982 Search PubMed.
- (a) S. Tewari, P. M. S. Chauhan, A. P. Bhaduri, N. Fatima and R. K. Chatterjee, Bioorg. Med. Chem. Lett., 2000, 10, 1409–1412 CrossRef CAS PubMed; (b) S. C. Azad, V. M. Balaramnavar, I. A. Khan, P. K. Doharey, J. K. Saxena and A. K. Saxena, RSC Adv., 2015, 5, 82208–82214 RSC.
- S. K. Srivastava, P. M. S. Chauhan, S. K. Agarwal, A. P. Bhaduri, S. N. Singh, N. Fatma, R. K. Chatterjee, C. Bose and V. M. L. Srivastava, Bioorg. Med. Chem. Lett., 1996, 6, 2623–2628 CrossRef CAS.
- S. K. Srivastava, P. M. S. Chauhan, A. P. Bhaduri, N. Fatima and R. K. Chatterjee, J. Med. Chem., 2000, 43, 2275–2279 CrossRef CAS PubMed.
- P. Roy, D. Dhara, P. K. Parida, R. J. Kar, A. Bhunia, K. Jana, S. P. Sinha Babu and A. K. Misra, Eur. J. Med. Chem., 2016, 114, 308–317 CrossRef CAS PubMed.
- K. V. Sashidhara, A. Kumar, K. B. Rao, V. Kushwaha, K. Saxena and P. K. Murthy, Bioorg. Med. Chem. Lett., 2012, 22, 1527–1532 CrossRef CAS PubMed.
- A. Agarwal, S. K. Awasthi and P. K. Murthy, Med. Chem. Res., 2011, 20, 430–434 CrossRef CAS.
- R. D. Sharma, S. Bag, N. R. Tawari, M. S. Degani, K. Goswami and M. V. R. Reddy, Parasitology, 2013, 140, 959–965 CrossRef CAS PubMed.
- S. Bag, N. R. Tawari, R. Sharma, K. Goswami, M. V. Reddy and M. S. Degani, Acta Trop., 2010, 113, 48–51 CrossRef CAS PubMed.
- R. P. Tripathi, R. Tripathi, A. P. Bhaduri, S. N. Singh, R. K. Chatterjee and P. K. Murthy, Acta Trop., 2000, 76, 101–106 CrossRef CAS PubMed.
- R. P. Tripathi, V. K. Tiwari, S. Misra-Bhattacharya, K. Tyagi, V. M. L. Srivastava and P. K. Murthy, Acta Trop., 2003, 87, 215–224 CrossRef CAS PubMed.
- S. Misra, L. K. Singh, P. J. Gupta, S. Misra-Bhattacharya and D. Katiyar, Eur. J. Med. Chem., 2015, 94, 211–217 CrossRef CAS PubMed.
- N. Mathew, T. Karunan, L. Srinivasan and K. Muthuswamy, Drug Dev. Res., 2010, 71, 188–196 CAS.
- A. Mandvikar, S. V. Hande, P. Yeole, K. Goswami and M. V. R. Reddy, Int. J. Pharma Sci. Res., 2016, 7, 1480–1492 CAS.
- S. Rathaur, M. Yadav, N. Singh and A. Singh, J. Proteomics, 2011, 74, 1595–1606 CrossRef CAS PubMed.
- R. Saxena, S. Sharma, R. N. Iyer and N. Anand, J. Med. Chem., 1971, 14, 929–931 CrossRef CAS PubMed.
- M. Kalyanasundaram, N. Mathew, K. P. Paily and G. Prabhakaran, Acta Trop., 2009, 111, 168–171 CrossRef CAS PubMed.
- S. K. Awasthi, N. Mishra, S. K. Dixit, A. Singh, M. Yadav, S. S. Yadav and S. Rathaur, Am. J. Trop. Med. Hyg., 2009, 80, 764–768 CAS.
- V. K. Tiwari, N. Tewari, D. Katiyar, R. P. Tripathi, K. Arora, S. Gupta, R. Ahmad, A. K. Srivastava, M. A. Khan, P. K. Murthy and R. D. Walter, Bioorg. Med. Chem., 2003, 11, 1789–1800 CrossRef CAS PubMed.
- S. K. Srivastava, P. M. S. Chauhan, A. P. Bhaduri, P. K. Murthy and R. K. Chatterjee, Bioorg. Med. Chem. Lett., 2000, 10, 313–314 CrossRef CAS PubMed.
- K. Kalani, V. Kushwaha, R. Verma, K. P. Murthy and S. K. Srivastava, Bioorg. Med. Chem. Lett., 2013, 23, 2566–2570 CrossRef CAS PubMed.
- R. U. Rao, Y. Huang, K. Fischer, P. U. Fischer and G. J. Weil, Exp. Parasitol., 2008, 121, 38–45 CrossRef PubMed.
- M. R. Dhananjeyan, Y. P. Milev, M. A. Kron and M. G. Nair, J. Med. Chem., 2005, 48, 2822–2830 CrossRef CAS PubMed.
- S. P. Bahekar, S. V. Hande, N. R. Agarwal, H. S. Chandak, P. S. Bhoj, K. Goswami and M. V. R. Reddy, Eur. J. Med. Chem., 2016, 124, 262–269 CrossRef CAS PubMed.
- K. V. Sashidhara, S. R. Avula, P. K. Doharey, L. R. Singh, V. M. Balaramnavar, J. Gupta, S. Misra-Bhattacharya, S. Rathaur, A. K. Saxena and J. K. Saxena, Eur. J. Med. Chem., 2015, 103, 418–428 CrossRef CAS PubMed.
- K. V. Sashidhara, K. B. Rao, V. Kushwaha, R. K. Modukuri, R. Verma and P. K. Murthy, Eur. J. Med. Chem., 2014, 81, 473–480 CrossRef CAS PubMed.
- W. Raether and G. Lammler, Ann. Trop. Med. Parasitol., 1971, 65, 107–115 CrossRef CAS PubMed.
- D. A. Denham, R. R. Suswillo and R. Rogers, Trans. R. Soc. Trop. Med. Hyg., 1978, 72, 546–547 CrossRef CAS PubMed.
- D. A. Denham, R. Samad, S.-Y. Cho, R. R. Suswillo and S. C. Skippins, Trans. R. Soc. Trop. Med. Hyg., 1979, 73, 673–676 CrossRef CAS PubMed.
- A. Dominguez-Vazquez, H. R. Taylor, A. M. Ruvalcaba-Macias, R. P. Murphy, B. M. Greene, A. R. Rivas-Alcala and F. Beltran-Hernandez, Lancet, 1983, 1, 139–143 CrossRef CAS.
- D. A. Denham, Trans. R. Soc. Trop. Med. Hyg., 1980, 74, 829 CrossRef CAS PubMed.
- A. B. Reddy, U. R. Rao, R. Chandrashekar, R. Shrivastava and D. Subrahmanyam, Tropenmed. Parasitol., 1983, 34, 259–262 CAS.
- K. Maertens and M. Wery, Trans. R. Soc. Trop. Med. Hyg., 1975, 69, 359–360 CrossRef CAS PubMed.
- World Health Organization, Technical Report Series 702. Lymphatic Filariasis, 1984 Search PubMed.
- N. Fatma, S. Sharma and R. K. Chatterjee, Acta Trop., 1989, 46, 311–321 CrossRef CAS PubMed.
- S. Ram, D. S. Wise, L. L. Wotring, J. W. McCall and L. B. Townsend, J. Med. Chem., 1992, 35, 539–547 CrossRef CAS PubMed.
- (a) M. O'Neill, A. Mansour, U. DiCosty, J. Geary, M. Dzimianski, S. D. McCall, J. W. McCall, C. D. Mackenzie and T. G. Geary, PLoS Neglected Trop. Dis., 2016, 10, e0004698 Search PubMed , 1–13; (b) M. O'Neill, C. Ballesteros, L. Tritten, E. Burkman, W. I. Zaky, J. Xia, A. Moorhead, S. A. Williams and T. G. Geary, Int. J. Parasitol.: Drugs Drug Resist., 2016, 6, 288–296 CrossRef PubMed.
- S. K. Singh, K. Goswami, R. D. Sharma, M. V. R. Reddy and D. Dash, Int. J. Nanomed., 2012, 7, 1023–1030 CAS.
- Z. Yu, S. Vodanovic-Jankovic, N. Ledeboer, S.-X. Huang, S. R. Rajski, M. Kron and B. Shen, Org. Lett., 2011, 13, 2034–2037 CrossRef CAS PubMed.
- Z. Yu, S. Vodanovic-Jankovic, M. Kron and B. Shen, Org. Lett., 2012, 14, 4946–4949 CrossRef CAS PubMed.
- M. E. Rateb, D. Yang, S. Vodanovic-Jankovic, Z. Yu, M. A. Kron and B. Shen, J. Antibiot., 2015, 68, 540–542 CrossRef CAS PubMed.
- N. Misra, M. Sharma, K. Raj, A. Dangi, S. Srivastava and S. Misra-Bhattacharya, Parasitol. Res., 2007, 100, 439–448 CrossRef PubMed.
- V. Kushwaha, K. Saxena, R. Verma, D. Katoch, N. Kumar, B. Lal, P. K. Murthy and B. Singh, Parasites Vectors, 2016, 9, 1–11 CrossRef PubMed.
- V. Mishra, N. Parveen, K. C. Singhal and N. U. Khan, Fitoterapia, 2005, 76, 54–61 CrossRef PubMed.
- N. Mukherjee, P. Saini, S. Mukherjee, P. Roy and S. P. Sinhababu, Asian Pac. J. Trop. Med., 2014, 7, 841–848 CrossRef CAS PubMed.
- K. Kalani, V. Kushwaha, P. Sharma, R. Verma, M. Srivastava, F. Khan, P. K. Murthy and S. K. Srivastava, PLoS One, 2014, 9, 1–13 Search PubMed.
- K. Saxena, V. Dube, V. Kushwaha, V. Gupta, M. Lakshmi, S. Mishra, S. Gupta, A. Arora, V. Lakshmi, R. K. Sharma, G. K. Jain and P. K. Murthy, Med. Chem. Res., 2011, 20, 1594–1602 CrossRef CAS.
- N. Mathew, S. Misra-Bhattacharya, V. Perumal and K. Muthuswamy, Molecules, 2008, 13, 2156–2168 CrossRef CAS PubMed.
- K. V. Sashidhara, S. P. Singh, S. Misra, J. Gupta and S. Misra-Bhattacharya, Eur. J. Med. Chem., 2012, 50, 230–235 CrossRef CAS PubMed.
- M. Singha, S. Shakya, V. K. Soni, A. Dangi, N. Kumar and S. Misra-Bhattacharya, Int. Immunopharmacol., 2009, 9, 716–728 CrossRef PubMed.
- P. Saini, P. Gayen, A. Nayak, D. Kumar, N. Mukherjee, B. C. Pal and S. P. Sinhababu, Parasitol. Int., 2012, 61, 520–531 CrossRef CAS PubMed.
- R. L. Gaur, M. K. Sahoo, S. Dixit, N. Fatma, S. Rastogi, D. K. Kulshreshtha, R. K. Chatterjee and P. K. Murthy, Indian J. Med. Res., 2008, 128, 65–70 CAS.
- N. M. Al-Abd, Z. M. Nor, M. Mansor, M. S. Hasan and M. Kassim, Korean J. Parasitol., 2016, 54, 273–280 CrossRef PubMed.
- S. Misra, M. Verma, S. K. Mishra, S. Srivastava, V. Lakshmi and S. Misra-Bhattacharya, Parasitol. Res., 2011, 109, 1351–1360 CrossRef PubMed.
- K. N. Sahare, V. Anandhraman, V. G. Meshram, S. U. Meshram, M. V. Reddy, P. M. Tumane and K. Goswami, Indian J. Exp. Biol., 2008, 46, 128–131 CAS.
- V. Lakshmi, R. Kumar, P. Gupta, V. Varshney, M. N. Srivastava, M. Dikshit, P. K. Murthy and S. Misra-Bhattacharya, Parasitol. Res., 2004, 93, 468–474 CAS.
- J. Gupta, S. Misra, S. K. Mishra, S. Srivastava, M. N. Srivastava, V. Lakshmi and S. Misra-Bhattacharya, Exp. Parasitol., 2012, 130, 449–455 CrossRef PubMed.
- V. Lakshmi, S. Srivastava, S. K. Mishra, S. Misra, M. Verma and S. Misra-Bhattacharya, Parasitol. Res., 2009, 105, 1295–1301 CrossRef PubMed.
- V. Lakshmi and S. Misra-Bhattacharya, Bangladesh Pharm. J., 2016, 19, 44–47 CrossRef.
- V. Kushwaha, K. Saxena, S. K. Verma, V. Lakshmi, R. K. Sharma and P. K. Murthy, Chronicals of Young Scientists, 2016, 2, 201–206 Search PubMed.
- K. N. Sahare, V. Anandharaman, V. G. Meshram, S. U. Meshram, D. Gajalakshmi, K. Goswami and M. V. Reddy, Indian J. Med. Res., 2008, 127, 467–471 CAS.
- M. Deshmukh, K. N. Sahare, R. K. Patidar, B. Mahajan and V. Singh, Trends in Vector Research and Parasitology, 2014, 1, 1–5 CrossRef.
- R. Shanmugapriya and T. Ramnathan, J. Pharm. Res., 2012, 5, 1448–1450 Search PubMed.
- V. Lakshmi, S. K. Joseph, S. Srivastava, S. K. Verma, M. K. Sahoo, V. Dube, S. K. Mishra and P. K. Murthy, Acta Trop., 2010, 116, 127–133 CrossRef CAS PubMed.
- L. Srinivasan, N. Mathew and K. Muthuswamy, Parasitol. Res., 2009, 105, 1179–1182 CrossRef PubMed.
- (a) B. E. Slatko, A. N. Luck, S. L. Dobson and J. M. Foster, Mol. Biochem. Parasitol., 2014, 195, 88–95 CrossRef CAS PubMed; (b) U. Heider, M. Blaxter, A. Hoerauf and K. M. Pfarr, Int. J. Med. Microbiol., 2006, 296, 287–299 CrossRef CAS PubMed.
- A. Y. Debrah, S. Mand, S. Specht, Y. Marfo-Debrekyei, L. Batsa, K. Pfarr, J. Larbi, B. Lawson, M. Taylor, O. Adjei and A. Hoerauf, PLoS Pathog., 2006, 2, e92 CrossRef PubMed.
- O. P. Sharma and M. S. Kumar, Sci. Rep., 2016, 6, 1–11 CrossRef PubMed.
- Z. Li, A. L. Garner, C. Gloeckner, K. D. Janda and C. K. Carlow, PLoS Neglected Trop. Dis., 2011, 5, e1411 CAS.
- B. D. Galvin, Z. Li, E. Villemaine, C. B. Poole, M. S. Champman, M. P. Pollastri, P. G. Wyatt and C. K. S. Carlow, PLoS Neglected Trop. Dis., 2014, 8, e3145 Search PubMed.
- B. Kurelec and M. Rijavec, Comp. Biochem. Physiol., 1966, 19, 525–531 CAS.
- J. J. Jaffe, Folate Related Metabolism, in Report of the Vth Meeting of the Scientific Working Group on Filariasis, WHO, Geneva, 1988, pp. 19–20 Search PubMed.
- J. J. Jaffe and L. R. Chrin, J. Parasitol., 1979, 65, 226–232 CrossRef CAS PubMed.
- J. J. Jaffe and L. R. Chrin, J. Parasitol., 1979, 65, 550–554 CrossRef CAS PubMed.
- G. N. Cox, J. Parasitol., 1992, 78, 1–15 CrossRef CAS PubMed.
- K. I. Kivirikko, R. Mallyla and T. Pihlajaniemi, Fed. Am. Soc. Exp. Biol. J., 1989, 3, 1609–1617 CAS.
- R. Chandrashekar and K. Mehta, Parasitol. Today, 2000, 16, 11–17 CrossRef CAS PubMed.
- K. Mehta, U. R. Rao, A. C. Vickery and P. J. Birckbichler, Biochem. Biophys. Res. Commun., 1990, 173, 1051–1057 CrossRef CAS PubMed.
- K. Mehta, U. R. Rao, A. C. Vickery and L. Fesus, Mol. Biochem. Parasitol., 1992, 53, 1–15 CrossRef CAS PubMed.
- S. Lustigman, B. Brotman, T. Huima, A. L. Castelhano, R. N. Singh, K. Mehta and A. M. Prince, Antimicrob. Agents Chemother., 1995, 39, 1913–1919 CrossRef CAS PubMed.
- H. Ando, M. Adachi, K. Umeda, A. Matsuura, M. Nonaka, R. Uchio, H. Tanaka and M. Motoki, Agric. Biol. Chem., 1989, 53, 2613–2617 CAS.
- U. R. Rao, K. Mehta, D. Subrahmanyam and A. C. Vickery, Antimicrob. Agents Chemother., 1991, 35, 2219–2224 CrossRef CAS PubMed.
- K. M. Wolf and A. L. Scott, Exp. Parasitol., 1995, 80, 282–290 CrossRef PubMed.
- B. P. Sani and J. C. W. Comley, Trop. Med. Parasitol., 1985, 36, 20–23 Search PubMed.
- B. P. Sani, Methods Enzymol., 1990, 189, 348–356 CAS.
- A. C. Ross, Fed. Am. Soc. Exp. Biol. J., 1993, 7, 317–327 CAS.
- A. C. Ross, J. Nutr., 1993, 123, 346–350 CAS.
- G. Wolf, Nutr. Rev., 1991, 49, 1–12 CrossRef CAS PubMed.
- N. A. Croll, Can. J. Zool., 1975, 53, 894–903 CrossRef CAS PubMed.
- G. Gainutsos and J. L. Bennett, Comp. Biochem. Physiol., 1977, 58, 157–159 CrossRef.
- J. K. Saxena, S. K. Bose, R. Sen, R. K. Chatterjee, A. B. Sen and S. Ghatak, Exp. Parasitol., 1977, 43, 239–243 CrossRef CAS PubMed.
- A. Agarwal, S. K. Mishra, S. Rathaur, R. K. Chatterjee, J. C. Katiyar and S. Ghatak, Indian J. Parasitol., 1982, 6, 223–225 Search PubMed.
- S. Rathaur, N. Anwar and S. Ghatak, Z. Parasitenkd., 1980, 62, 85–93 CrossRef CAS PubMed.
- S. Rathaur, N. A. Kaushal and S. Ghatak, Indian J. Parasitol., 1985, 9, 203–205 CAS.
- D. A. Brownlee, L. Holden-Dye and R. Walker, Adv. Parasitol., 2000, 45, 109–180 CAS.
- R. E. Isaac, R. Muimo and A. N. MacGregor, Science, 1990, 216, 1012–1014 Search PubMed.
- C. W. Tabor and H. Tabor, Annu. Rev. Biochem., 1984, 53, 749–790 CrossRef CAS PubMed.
- F. Svensson, I. Kockum and L. Persson, Mol. Cell. Biochem., 1993, 124, 141–147 CrossRef CAS PubMed.
- D. K. Srivastava, T. K. Roy and O. P. Shukla, Indian J. Parasitol., 1980, 4, 187–189 CAS.
- R. M. Wittich, H. D. Kilian and R. D. Walter, Mol. Biochem. Parasitol., 1987, 24, 155–162 CrossRef CAS PubMed.
- R. D. Walter, Parasitol. Today, 1988, 4, 18–20 CrossRef CAS PubMed.
- V. Sharma, B. L. Tekwani, J. K. Saxena, S. Gupta, J. C. Katiyar, R. K. Chatterjee, S. Ghatak and O. P. Shukla, Exp. Parasitol., 1991, 72, 15–23 CrossRef CAS PubMed.
- A. A. Da’dara, H. Mett and R. D. Walter, Mol. Biochem. Parasitol., 1998, 97, 13–19 CrossRef.
- H. J. Saz, Trends Biochem. Sci., 1981, 6, 117–119 CrossRef CAS.
- J. Barrett, J. Helminthol., 1983, 52, 1–18 Search PubMed.
- J. Barrett, A. H. Mendis and P. E. Butterworth, Int. J. Parasitol., 1986, 16, 465–469 CrossRef CAS PubMed.
- P. Kohler, Parasitol. Today, 1991, 7, 21–25 CrossRef CAS PubMed.
- R. S. Rew and H. J. Saz, J. Parasitol., 1977, 63, 123–129 CrossRef CAS PubMed.
- M. Shahabuddin, T. Toyoshima, M. Aikawa and D. C. Kaslow, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 4266–4270 CrossRef CAS.
- G. W. Gooday, L. J. Brydon and L. H. Chappell, Mol. Biochem. Parasitol., 1988, 29, 223–225 CrossRef CAS PubMed.
- J. A. Fuhrman, W. S. Lane, R. F. Smith, W. F. Piessens and F. B. Perler, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 1548–1552 CrossRef CAS.
- N. Raghavan, D. O. Freedman, P. C. Fitzgerald, T. R. Unnasch, E. A. Ottesen and T. B. Nutman, Infect. Immun., 1994, 62, 1901–1908 CAS.
- S. Sakuda, A. Isogai, S. Matsumoto and A. Suzuki, J. Antibiot., 1987, 40, 296–300 CrossRef CAS PubMed.
- C. A. Behm, Int. J. Parasitol., 1997, 27, 215–229 CrossRef CAS PubMed.
- D. Fairbairn, Can. J. Zool., 1958, 36, 787–795 CrossRef CAS.
- J. W. Powell, J. N. Stables and R. A. Watt, Mol. Biochem. Parasitol., 1986, 18, 171–182 CrossRef CAS PubMed.
- J. W. Powell, J. N. Stables and R. A. Watt, Mol. Biochem. Parasitol., 1986, 19, 265–271 CrossRef CAS PubMed.
- J. S. McCarthy, M. Wieseman, J. Tropea, D. Kaslow, D. Abraham, S. Lustigman, R. Tuan, R. H. Guderian and T. B. Nutman, Infect. Immun., 2002, 70, 851–858 CrossRef CAS PubMed.
- D. Subrahmanyam, Ciba Found. Symp., 1987, 127, 246–264 CAS.
- R. K. Prichard, Int. J. Parasitol., 1973, 3, 409–417 CrossRef CAS PubMed.
- H. J. Saz and G. A. Dunbar, J. Parasitol., 1975, 61, 794–801 CrossRef CAS PubMed.
- N. F. Nelson and H. J. Saz, J. Parasitol., 1984, 70, 194–196 CrossRef CAS PubMed.
- J. C. W. Comley and J. J. Jaffe, J. Parasitol., 1981, 67, 609–616 CrossRef CAS PubMed.
- J. C. W. Comley, J. J. Jaffe and L. R. Chrin, Mol. Biochem. Parasitol., 1982, 5, 19–31 CrossRef CAS PubMed.
- J. C. W. Comley, Trop. Med. Parasitol., 1985, 36, 10–14 Search PubMed.
- R. D. Walter, E. Ossikovski and E. J. Albiez, Mol. Biochem. Parasitol., 1985, 14, 211–217 CrossRef CAS PubMed.
- A. H. Mendis and S. Townson, Mol. Biochem. Parasitol., 1985, 14, 337–354 CrossRef CAS PubMed.
- M. S. Brown and J. L. Goldstein, J. Lipid Res., 1980, 21, 505–517 CAS.
- J. J. Jaffe, Filarial Folate-Related Metabolism as a Potential Target for Selective Inhibitors, in Host-Invader Interplay, ed. H. Van den Bossche, Elsevier/North-Holland, Amsterdam, 1980, pp. 605–614 Search PubMed.
- J. C. W. Comley, J. J. Jaffe, L. R. Chrin and R. B. Smith, Mol. Biochem. Parasitol., 1981, 2, 271–283 CrossRef CAS PubMed.
- J. J. Jaffe and L. R. Chrin, J. Parasitol., 1980, 66, 53–58 CrossRef CAS PubMed.
- H. L. Callahan, R. K. Crouch and E. R. James, Parasitol. Today, 1988, 4, 218–225 CrossRef CAS PubMed.
- P. M. Brophy and D. I. Pritchard, Parasitol. Today, 1992, 8, 419–422 CrossRef CAS PubMed.
- P. M. Brophy and J. Barrett, Biochem. Cell Biol., 1990, 68, 1288–1291 CrossRef CAS PubMed.
- P. M. Brophy and D. I. Pritchard, Exp. Parasitol., 1994, 79, 89–96 CrossRef CAS PubMed.
- P. M. Brophy and J. Barrett, Parasitology, 1990, 100, 345–349 CrossRef CAS PubMed.
- W. Y. Precious and J. Barrett, Parasitol. Today, 1989, 5, 156–160 CrossRef CAS PubMed.
- A. M. Michele, R. W. DeWight and A. T. John, J. Mol. Biol., 1995, 246, 21–27 CrossRef.
- N. A. Kaushal, D. C. Kaushal and S. Ghatac, Immunol. Invest., 1987, 16, 139–149 CrossRef CAS PubMed.
- A. M. Campbell, A. J. van Eldik, E. Liebau, J. Barrett, P. M. Brophy, P. H. Teesdale-Spittle and M. F. Wang, Chem.-Biol. Interact., 2001, 133, 240–243 CAS.
- J. Lazdins and M. Kron, Parasitol. Today, 1999, 15, 305–306 CrossRef CAS PubMed.
- D. J. Worthington and M. A. Rosemeyer, Eur. J. Biochem., 1974, 48, 167–177 CrossRef CAS PubMed.
- G. Krohne-Ehrich, R. H. Schirmer and R. Untucht-Grau, Eur. J. Biochem., 1977, 80, 65–71 CrossRef CAS PubMed.
- K. K. Bhargava, N. Le Trang, A. Cerami and J. W. Eaton, Mol. Biochem. Parasitol., 1983, 9, 29–35 CrossRef CAS PubMed.
- M. Yadav, A. Singh, S. Rathaur and E. Liebau, J. Mol. Graphics Modell., 2010, 28, 435–445 CrossRef CAS PubMed.
- J. Lewis, C. Wu, J. Levine and H. Berg, Neuroscience, 1980, 5, 967–989 CrossRef CAS PubMed.
- R. Helm, M. E. Selkirk, J. E. Bradley, R. G. Burns, A. J. Hamilton, J. Croft and R. M. Maizels, Parasite Immunol., 1989, 11, 479–502 CrossRef CAS PubMed.
- S. Guenette, R. K. Prichard, R. D. Klein and G. Matlashewski, Mol. Biochem. Parasitol., 1991, 44, 153–164 CrossRef CAS PubMed.
- L. Tang and R. K. Prichard, Mol. Biochem. Parasitol., 1989, 32, 145–152 CrossRef CAS PubMed.
- E. Lacey, Int. J. Parasitol., 1988, 18, 885–936 CrossRef CAS PubMed.
- E. Lacey and R. K. Pritchard, Mol. Biochem. Parasitol., 1986, 19, 171–181 CrossRef CAS PubMed.
- K. M. Pfarr, A. Hoerauf, G. M. Koenig, S. Specht, A. Schiefer, T. F. Schaeberle, A. Schmitz and S. Kehraus, US Pat., US 2015/0182497 A1, 2015.
- R. Kalyanasundaram, US Pat., US 2013/0236490 A1, 2013.
- M. Alasia, H. Minoux and J.-M. Ruxer, US Pat., US 2011/0184015 A1, 2011.
- E. Devaney, K. O'neill, W. Harnett, L. Whitesell and J. H. Kinnaird, Int. J. Parasitol., 2005, 35, 627–636 CrossRef CAS PubMed.
- P. Das, WO Pat., WO 2011/145105 A2, 2011.
- M. G. Nair, M. R. Dhanjeyan, M. A. Kron and Y. P. Milev, US Pat., US 7,658,937 B2, 2010.
- S. K. Tagboto and S. Townson, Ann. Trop. Med. Parasitol., 1996, 90, 497–505 CrossRef CAS PubMed.
- M. H. Bradley and V. Kumaraswamy, Am. J. Trop. Med. Hyg., 2004, 71, 7–11 Search PubMed.
- G. Weil, Mass Drug Administration for Lymphatic Filariasis and Onchocerciasis for Liberia (DOLF-LIBERIA), Washington University School of Medicine, 2015, https://clinicaltrials.gov/ct2/show/NCT01905436 Search PubMed.
- Drug development: Onchocerciasis and Lymphatic Filariasis. Progress Report 15-New and Improved Tools, http://www.who.int/tdr/research/progress/9900/drug_dev_oncho_filariasis/en/.
- M. E. Setouhy, R. M. R. Ramzy, E. S. Ahmed, A. M. Kandil, O. Hussain, H. A. Farid, H. Helmy and G. J. Weil, Am. J. Trop. Med. Hyg., 2004, 70, 191–196 Search PubMed.
- Third WHO report on neglected tropical diseases, Investing to Overcome the Global Impact of Neglected Tropical Diseases. 2015, WHO Geneva.
| This journal is © The Royal Society of Chemistry 2017 |