ChemApproach: validation of a questionnaire to assess the learning approaches of chemistry students
Mika
Lastusaari
*a,
Eero
Laakkonen
b and
Mari
Murtonen
b
aUniversity of Turku, Department of Chemistry, Turku, Finland. E-mail: miklas@utu.fi
bUniversity of Turku, Department of Teacher Education, Turku, Finland
First published on 29th April 2016
Abstract
The theory of learning approaches has proven to be one of the most powerful theories explaining university students' learning. However, learning approaches are sensitive to the situation and the content of learning. Chemistry has its own specific features that should be considered when exploring chemistry students' learning habits, specifically the role of practicals (i.e. hands-on laboratory work), as they are crucial in chemistry education. Therefore, the aims of this study were to find and validate a questionnaire for measuring chemistry students' learning approaches. A 17-item questionnaire was tested with 561 Finnish chemistry students from four different universities. Students ranging from the first year bachelor level to the fifth year master level participated in the study. Statistical analyses showed that a four factor model fitted the data best and these factors were named submissive surface, technical surface, active deep, and practical deep. In order to establish validity, the model was further tested by analysis of the subgroups of the major subject and gender. The analyses show that the questionnaire is statistically valid and can be used for studying chemistry students' learning approaches.
Introduction
A number of teaching methods exist for varying educational levels. However, regardless of the educational level, it is clear that any one teaching method does not give the same kind of response for all students. Thus, discovering the most suitable methods may be a difficult task, especially given that teaching should ideally be such that no particular type of learner feels too much discomfort or comfort. This way most students will be somewhat stretched to grow in directions they would otherwise avoid (Felder and Brent, 2005). As a result of this complexity, it may become necessary to use a questionnaire to characterize objectively the learning habits of students. One way to approach this is through the surface-deep learning approach formalism introduced by Marton and Säljö, (1976). In sum, those who employ surface-level processing focus on the text to be studied and try to memorize as much as possible, whereas deep level processing involves the goal of grasping the underlying meaning of the text.The theory of learning approaches was developed in the 1970s (Marton and Säljö, 1976; see also Biggs, 1987). Since its introduction, it has proven to be one of the most powerful theories in explaining university students' learning and it has been studied extensively over the decades. Discoveries include students who report having different types of approaches, i.e. some express being more surface oriented, others mostly deep oriented, with some students approaching their studies more strategically (Entwistle and Ramsden, 1983; Biggs, 1987). Later studies have shown that the approaches are not very stable, i.e. approaches are not innate personal traits, but more like adopted ways of behaving in certain situations (Lindblom-Ylänne et al., 2014).
The stability of the approaches has been a central question in learning approach theories during the past few years primarily concerning personality, context and time. With regard to personality, current theories of learning and expertise development conceptualise personality as a very flexible feature that changes over time and due to the environment (Chamorro-Premuzic and Furnham, 2009). Accordingly, there is not a stable approach that would describe learning of one student during the entire period of their university studies. In addition, context has an impact on students' ways to approach learning. Students have been found to express different learning approaches in different contexts at the same period of time (Vermunt and Vermetten, 2004). Time also has an impact on approaches; students' approaches change during their education and these changes appear to be difficult to predict (Lindblom-Ylänne et al., 2014).
There is dissonant evidence regarding the connection between learning approaches and learning outcomes. In some studies, the deep approach has been found to be connected to higher learning outcomes, and vice versa, surface orientation to lower success in studies, whilst some studies have found a correlation between both deep and surface orientation and achievement, and some have not found any correlation (Vermunt, 2005; Chan and Bauer, 2016). In chemistry education, we discovered in our previous study (Lastusaari and Murtonen, 2013) that approaches are connected to willingness to change major; students who wanted to change their major scored higher in the surface approach than students who did not want to change major. Therefore research on students' approaches gives teachers important information for planning teaching and the curriculum. Some studies also show that the deep approach can be stimulated through instruction (Baeten et al., 2010; Dolmans et al., 2010), i.e. if we know that some students have an approach that does not support their learning these students could be stimulated towards a more appropriate approach.
Since learning approaches have shown to be context specific, it is important to study them within a specific context. In our previous study on chemistry learning (Lastusaari and Murtonen, 2013) we discovered six distinct learning approaches which depicted different kinds of surface and deep learning approaches that chemistry students expressed. In chemistry, practicals (i.e. hands-on laboratory work) play a central role in learning. Traditional learning approach theories do not take into account different types of learning, i.e. those treat all learning and teaching methods as similar to each other. However, in disciplines like chemistry, theoretical learning, e.g. lectures, differs significantly from practicals in their learning methods. This may have an important effect on students' approaches to learning. According to our previous study (Lastusaari and Murtonen, 2013) some students emphasized the role of practicals to a greater extent than other students. We found that those students, who were classified as enthusiastic in their learning of chemistry, rated practicals more important and were more deeply oriented than the other students. Thus, a learning approach that emphasizes learning with the aid of practicals seems to be crucial in high quality learning in chemistry.
The role of practicals in chemistry learning attached to deep learning could be interpreted as an attempt to understand what one is about to learn. In some studies, a disposition to understanding has been found to be more stable than the deep approach and to support a student in maintaining a deep approach (Entwistle and McCune, 2009; Postareff et al., 2014). As a subject of study, chemistry can be considered to be comprised of three forms: macro, submicro and representational (Johnstone, 2000). The macro level can be understood as what people are accustomed to i.e. it is the tangible and descriptive form of chemistry. The atomic or submicro level is used to explain the phenomena behind that what is tangible. This level is essential for the understanding of chemistry and it must be recorded in a representational language and notation, e.g. symbols, formulas and graphs. All these three forms are equally important and complementing, but extending the knowledge from the macro level to what is not visible is a challenge for both the student and the teacher. This requires a student to have a disposition towards understanding, and practicals can assist understanding of the topic to be learnt. Thus, information on the learning approaches of the students becomes essential so that the teacher may adapt his/her teaching so as to best suit the particular group of students.
There is also evidence that the entire learning environment can play an important role in students' ways to approach their studies. According to McCune and Entwistle (2011), teaching–learning environments can be adapted to encourage a consistent tendency to want to understand deeply and the will to learn. Students' achievement goals also play an important role in studying (Richardson and Remedios, 2014). For example, Murtonen et al. (2008) found that university students who agreed that they will need research skills in their future work were more deeply oriented in research courses than those students who did not value these skills.
In our previous study on chemistry learning (Lastusaari and Murtonen, 2013), the combination of appreciating practicals and having a deep approach with high ratings on the scale measuring the use of study techniques was the most common among students at an advanced level of study (4th and 5th year Master students). Those were the same students who had not changed their major, i.e. they had remained in the bachelor program and following that, in the master of chemistry education programme. Changing a major is a considerable challenge in chemistry education programmes in Finland, thus it is important to know who the students are who choose to stay in the education programme.
The aim of this study is to validate the questionnaire used in our previous study (Lastusaari and Murtonen, 2013). This study showed the usability of the questionnaire, suggesting six distinct approaches to chemistry learning: submissive surface, memorizing surface, technical surface, active deep, processing deep and practical deep. In fact, the presence of the purely chemistry-related practical deep proved highly interesting. However, the dataset was not large enough for validating the questionnaire. For the present study, we modified the questionnaire based on our earlier experience and carried out a study with a larger dataset (N = 561). In this study, we aim to validate the questionnaire so that it can be used to study chemistry students' learning. Henceforth, the questionnaire will be referred to as ChemApproach.
Method
Samples
The population of this study consisted of Finnish chemistry students. Thus the data were collected from a number of universities in order to obtain a representative sample. The data were collected using the ChemApproach questionnaire in four Finnish universities educating chemistry bachelors and masters: the University of Turku, the University of Jyväskylä, the University of Oulu and the University of Eastern Finland (in Joensuu) from 2013 to 2015 (Table 1). In total, the number of students participating was 561 (53.2% chemistry majors, 46.8% students with chemistry as their minor subject). 49.7% of the students were female and 50.3% male. Students in all stages of bachelor and master's studies participated, however the bachelor students were clearly more (90.4%, N = 507) than the master ones (9.6%, N = 54). It is also noteworthy that 56.5% (N = 317) of all the participants were first year students. Comparing the present dataset with the total number of BSc and MSc students in Finland, one can state that the total amount of samples here (N = 561) corresponds to 22% of all chemistry majors in Finland and the number of BSc students (N = 507) corresponds to 31% of all chemistry BSc students in Finland and 94% of BSc students per year. If only the universities from which the data were collected are taken into account, the respective numbers are 35% (all BSc and MSc students), 56% (all BSc) and 150% (BSc per year) (Statistics Finland, 2015). The response rate to the questionnaire was 84% when all students enrolled in the courses from which the data were collected are considered. Such a high response rate indicates that the data can be considered as non-biased by self-selection. Moreover, all universities in Finland are funded by the Finnish government and participation in lectures is usually not compulsory, whereas laboratory courses require attendance. Because of these similarities, we expect that there are no important differences in the student material of different universities and the sample reflects very well the chemistry undergraduates in Finland.| University | N |
|---|---|
| University of Turku | 330 |
| University of Jyväskylä | 116 |
| University of Oulu | 74 |
| University of Eastern Finland | 41 |
Instrumentation
There are standardized general questionnaires, such as the Study Process Questionnaire (SPQ) by Biggs, the Approaches to Study Skills Inventory for Students (ASSIST) by Entwistle (see Lovatt et al., 2007), Learning and Study Strategies Inventory (LASSI), Motivated Strategies for Learning Questionnaire (MSLQ) (see Zeegers, 2001) and the Inventory of Learning Styles in Higher Education (ILS) (Vermunt, 1994), which probe the deep/surface approaches to studying. All of these are intended for general use in any discipline of studies and thus they have been used in a variety of fields including e.g. medicine (Dolmans et al., 2010), psychology (Lonka and Lindblom-Ylänne, 1996), accounting (Jackling 2005) and chemistry (Zeegers, 2001) as well as others such as physics, architecture, law, social sciences, philology, history and arts (Cano-Garcia and Justicia-Justicia, 1994; Richardson, 2005).The ChemApproach questionnaire is theoretically based on the above mentioned research tradition, as well as our earlier chemistry approach questionnaire (Lastusaari and Murtonen, 2013) to which only minimal changes were made based on the experiences with the earlier version. The goal was to improve the earlier version and develop a questionnaire that best suits the needs of chemistry instruction. The modified questionnaire contained six background questions (name, gender, major, subject, intention to change major subject, intended major subject and year of study). This was followed by 17 statements (items) about chemistry learning approaches, concerning preparation for a chemistry examination, chemistry lectures, ways of studying chemistry and chemistry practicals. The statements were chosen to reflect the learning approach in particular, but also included those covering regulation strategies, study preferences and opinions on practicals. One statement (I look for justifications and evidence to make my own conclusions about things to be learned) was adopted from the ILS by Vermunt (1994). The statements are shown in Table 2 and a five-point Likert scale was used, varying from complete disagreement (value: 1) to complete agreement (5) with the statement. Data were collected by the teachers during laboratory practicals and breaks between lectures. It took approximately five to ten minutes to complete the questionnaire. The questionnaire includes text explaining that only if the person signs the form, the answers will be used in the study, and that the answers will be handled anonymously. It was also made clear by the teachers collecting the data that it was not compulsory to participate in the study.
| Item | Statement | Component | |||
|---|---|---|---|---|---|
| 1 (subsurf) | 2 (tecsurf) | 3 (actdeep) | 4 (pradeep) | ||
| subsurf1 | Many things that I learn remain isolated and do not link as a part of a bigger picture. | 0.729 | |||
| subsurf2 | When reading the course material, I often do not understand how a new topic relates with any old one. | 0.827 | |||
| subsurf3 | I have to memorize things without having the opportunity to understand them. | 0.748 | |||
| subsurf4 | During a chemistry lecture, I often do not understand what a new thing is connected with. | 0.778 | |||
| tecsurf1 | I underline while reading for chemistry examination. | 0.645 | |||
| tecsurf2 | I divide the course material to parts, which I learn for the chemistry examination. | 0.503 | |||
| tecsurf3 | When reading for a chemistry examination, I try to make summaries of different unities with my own words | 0.658 | |||
| tecsurf4 | I make my own notes when studying for an examination. | 0.747 | |||
| tecsurf5 | I make mnemonics to learn things better. | 0.582 | |||
| actdeep1 | After a chemistry lecture, I often chew over the things taught. | 0.592 | |||
| actdeep2 | I usually search and read additional material concerning the course. | 0.679 | |||
| actdeep3 | I often chew over the thoughts awoken by scientific texts as well as connections between them. | 0.806 | |||
| actdeep4 | I look for justifications and evidence to make my own conclusions about things to be learned. | 0.745 | |||
| pradeep1 | I like to do practicals. | −0.302 | 0.634 | ||
| pradeep2 | I have often understood a chemical phenomenon only after doing practical work on it. | 0.813 | |||
| pradeep3 | When doing a practical, I usually try to understand what its different parts are based on. | −0.335 | 0.373 | 0.560 | |
| pradeep4 | One can learn a chemical phenomenon only by doing practical work on it. | 0.732 | |||
Analysis methods
Data analyses included descriptive statistics, principal component analysis (PCA), exploratory and confirmatory factor analyses (EFA and CFA) and analyses of the measurement invariance. After careful data screening and descriptive statistical analyses, the exploratory analyses were conducted to investigate the factor structure of the data. The number of principal components and factors was evaluated, goodness of fit of the solution was evaluated, loading structure was inspected and correlations among the factors were computed. In PCA, factor loadings were rotated using orthogonal varimax rotation and in EFA the oblique rotation method geomin was used.The hypothesized factor model based on the theory was fitted using confirmatory factor analysis (CFA, see Brown, 2006). The possible need for model modifications was investigated using information from the modification indices with substantive interpretations. All CFA models were estimated using a full information maximum likelihood method with robust standard errors (MLR), which can also handle missing at random data and departures from normal distribution. Model fit was evaluated using a chi-square test, comparative fit index (CFI), Tucker-Lewis index (TLI), root mean square error of approximation (RMSEA) and standardized root mean square residual (SRMR). A nonsignificant result of the chi square indicates a good fit. Because of the well-known problems concerning the chi-square test (Marsh et al., 1988), the model-fit evaluations were mostly based on the other fit indices listed above. The cutoff values used for accepting a model were for CFI and TLI above 0.90, and for RMSEA and SRMR below 0.08 (Hu and Bentler, 1999; Little, 2013).
Measurement invariance was assessed using multigroup confirmatory factor analysis. These analyses were done to ensure that the questionnaire was working similarly among different subgroups (see van de Schoot et al., 2012). If measurement invariance can be proved then the participants across the investigated groups interpret the individual questions of the questionnaire and the underlying latent factor in the same way. Two different groupings were used: gender groups (male, female) and major subject groups (chemistry, other). Configural, metric and scalar invariance was studied, in which equal structure, equal loadings and equal intercepts were fixed for the groups, respectively. Model comparisons were based on the Satorra–Bentler corrected chi-square difference test, in which the non-significant test result indicates support for the more restricted model (Satorra and Bentler, 2001). Another criterion for model selection was the difference in the CFI values between the nested models (Cheung and Rensvold, 2002). A small (less than or equal to 0.01) difference in CFI values indicates that the model with a higher degree of measurement invariance could be accepted.
Descriptive statistics and principal component analysis were carried out using IBM-SPSS 22.0. Exploratory and confirmatory factor analysis and the measurement invariance analyses were performed using the statistical program Mplus 7 (Muthén and Muthén, 1998–2015).
Results and discussion
The dataset was first subjected to principal component analysis (PCA) factoring. According to the Scree test, an eigenvalue exceeding 1 can be obtained with the number of factors being four or less. Using four factors, the PCA resulted in a clearly structured solution (Table 2). According to our previous study (Lastusaari and Murtonen, 2013), these factors were named submissive surface (subsurf), active deep (actdeep), technical surface (tecsurf) and practical deep (pradeep). In comparison with our results from the earlier version of the questionnaire which was used for a clearly smaller sample with N = 118, the present solution lacks the processing deep and memorizing surface factors. The former has merged with actdeep and the latter with techsurf making the present solution more coherent than the previous one. Specifically, because the differences between the active and processing deep as well as memorizing and active surface were rather small for some of the items. It is noteworthy that the items pradeep1 (I like to do practicals) and pradeep3 (When doing a practical, I usually try to understand what its different parts are based on) are also negatively correlated with subsurf, as expected. The pradeep3 item is also naturally positively correlated with actdeep. However, the factor loadings for all of these three remain below 0.4 in absolute value, which is clearly less than what they have for pradeep. Thus, they satisfactorily link both subsurf and actdeep features with those of pradeep, while still remaining as non-dominating elements. The dataset was then subjected to exploratory factor analysis (EFA), to further test the model obtained with the PCA. This was done in order to obtain the statistical significances and correlations that cannot be obtained with PCA. As an initial analysis, the BIC (Bayesian information criterion) value was calculated for different numbers of factors. In agreement with the Scree test discussed above, the BIC values have their minimum with the number of factors being four. This indicates that with four factors, the balance between the complexity of the model and the fit obtained is at the optimal value for the present dataset. The factor analysis was then carried out with four components using Geomin as the method of rotation. Unlike the Varimax used in the PCA, the Geomin allows for correlations between the factors. The obtained factor loadings (Table 3) are very similar to those from the PCA (Table 2) and also the role of pradeep1 and pradeep3 is similar to what was obtained with the PCA. The results indicate some significant correlations between subsurf and actdeep (negative correlation), tecsurf and actdeep, actdeep and pradeep as well as tecsurf and pradeep (positive correlation). However, these correlations (Table 4) are weak (less than 0.3, Cohen 1988) indicating evidence of discriminant validity of the constructs.| Item | Factor | |||
|---|---|---|---|---|
| 1 (subsurf) | 2 (tecsurf) | 3 (actdeep) | 4 (pradeep) | |
| subsurf1 | 0.636 | |||
| subsurf2 | 0.818 | 0.079 | ||
| subsurf3 | 0.657 | |||
| subsurf4 | 0.731 | |||
| tecsurf1 | 0.113 | 0.522 | ||
| tecsurf2 | 0.336 | |||
| tecsurf3 | 0.483 | 0.182 | ||
| tecsurf4 | 0.724 | |||
| tecsurf5 | 0.458 | −0.104 | 0.102 | |
| actdeep1 | 0.470 | |||
| actdeep2 | 0.489 | |||
| actdeep3 | −0.090 | 0.799 | ||
| actdeep4 | 0.651 | |||
| pradeep1 | −0.301 | 0.467 | ||
| pradeep2 | 0.844 | |||
| pradeep3 | −0.338 | 0.264 | 0.436 | |
| pradeep4 | 0.598 | |||
| Factor | subsurf | tecsurf | actdeep | pradeep |
|---|---|---|---|---|
| subsurf | 1.000 | |||
| tecsurf | 0.032 | 1.000 | ||
| actdeep | −0.261 | 0.196 | 1.000 | |
| pradeep | 0.078 | 0.253 | 0.211 | 1.000 |
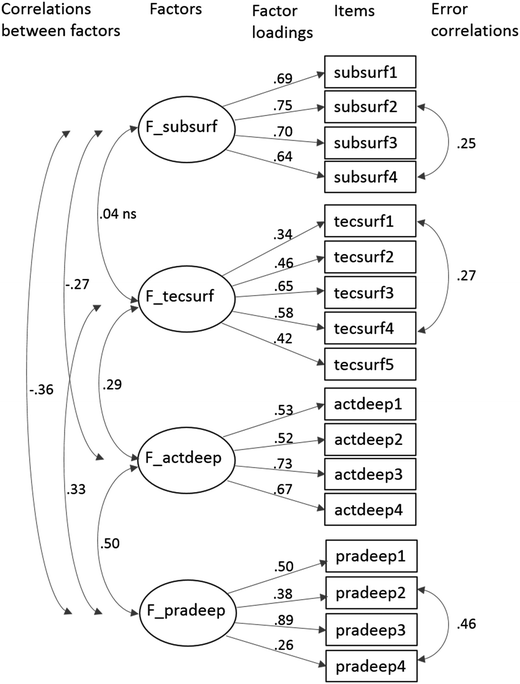
In the third analysis, the dataset was subjected to confirmatory factor analysis (CFA). In CFA, a hypothesized factor model is fitted to the data and the model fit is evaluated. The starting model, model 1, contained 17 items and 4 factors, also suggested by the PCA and EFA above. In model 1, each item loads on only one factor, the factors are correlated and the residuals associated with each item are uncorrelated. The model results in a relatively acceptable fit, but the very low values for CFI and TLI (should be ≥0.9) indicate that the model needs improvement even if the RMSEA and SRMR values fall within the conventional limit (should be <0.08) (Table 5). Thus, model 2 was modified from model 1 by including three additional parameters suggested by the modification index values of model 1. These residual covariances were added into the model one by one, and they indicate an additional association between some items that the common latent variable (factor) only is not able to capture. The fit for model 2 is shown in Fig. 1. Model 2 results in a good fit according to all criteria (Table 5 and Fig. 1).
| Model 1 | Model 2 | |
|---|---|---|
| CFI = comparative fit index. TLI = Tucker–Lewis index. RMSEA = root mean square error of approximation. SRMR = standardized root mean square residual. | ||
| χ 2 test: value | 382.42 | 237.57 |
| Degrees of freedom | 113 | 110 |
| P value | 0.000 | 0.000 |
| CFI | 0.851 | 0.929 |
| TLI | 0.821 | 0.913 |
| RMSEA estimate (with 95% CI) | 0.065 (0.058, 0.072) | 0.045 (0.038, 0.053) |
| SRMR value | 0.070 | 0.059 |
It also shows that the CFA model 2 contains three correlations between residual terms, i.e. subsurf2–subsurf4, tecsurf1–tecsurf4 and pradeep2–pradeep4. This indicates that subsurf2 (When reading the course material, I often do not understand how a new topic relates with any old one) and subsurf4 (During a chemistry lecture, I often do not understand what a new thing is connected with) would be partly describing a similar thing. Comparing their statements, it is clear that they refer to the same thing but in a different environment. In addition, tecsurf1 (I underline while reading for chemistry examination) and tecsurf4 (I make my own notes when studying for an examination) have an understandable link between each other as do pradeep2 (I have often understood a chemical phenomenon only after doing practical work on it) and pradeep4 (One can learn a chemical phenomenon only by doing practical work on it). Moreover, these similarities serve as a control for the internal coherence of a student's set of answers.
Considering that the ChemApproach questionnaire is aimed at all students of chemistry, it was important to ensure that the measurement properties functioned similarly for different subgroups within the whole population. Thus, model 2 was tested in two sets of subgroups, i.e. gender: female (N = 277) – male (N = 280) or major subject: chemistry (N = 284) – other (N = 250) for configural (model i1), metric (i2) and scalar invariance (i3). For the major subject subgroups, model 2 was supported for all tests of invariance, but full scalar invariance was not obtained for gender (Table 6). Partial scalar invariance was reached, when intercept unequality was allowed for techsurf1, 2 and 3 (model i4). This means that there was gender dependence in these three items. However, because there are still two more items in the techsurf factor that pass the scalar invariance test, it can be stated that the invariance of model 2 was supported by the results.
| Model | χ 2 | Df | CFI | TLI | RMSEA | χ 2-diff(df); pa | CFI-diffb | Result |
|---|---|---|---|---|---|---|---|---|
| a The χ2-difference-test for model comparison with Satorra–Bentler scaling correction. b A more restricted model is supported if the difference in CFI values is less than 0.01. c The same as i3 except three unequal intercepts (items techsurf1, techsurf2, and techsurf3). | ||||||||
| Gender: female (N = 277) vs. male (N = 280) | ||||||||
| i1: configural invariance | 325.63 | 220 | 0.939 | 0.925 | 0.042 | i1 supported | ||
| i2: metric invariance (m 1 + equal loadings) | 339.71 | 233 | 0.938 | 0.928 | 0.041 | 14.22(13); p = 0.359 | 0.001 | i2 supported |
| i3: scalar invariance (m 2 + equal intercepts) | 416.75 | 246 | 0.901 | 0.891 | 0.050 | 77.04(13); p < 0.001 | 0.037 | i3 not supported |
| i4: partial scalar invariancec | 366.56 | 243 | 0.929 | 0.920 | 0.043 | 28.82(9); p < 0.001 | 0.009 | i4 supported |
| Major subject: chemistry (N = 284) vs. other (N = 250) | ||||||||
| i1: configural invariance | 375.74 | 220 | 0.912 | 0.891 | 0.051 | i1 supported | ||
| i2: metric invariance (m 1 + equal loadings) | 397.71 | 233 | 0.907 | 0.891 | 0.051 | 22.00(13); p = 0.055 | 0.005 | i2 supported |
| i3: scalar invariance (m 2 + equal intercepts) | 406.30 | 246 | 0.909 | 0.900 | 0.049 | 7.67(13); p = 0.865 | 0.002 | i3 supported |
Conclusions and implications
In order to enhance the quality of university-level chemistry instruction, furthering our understanding of our students' approaches to learning is vital. In the study by Lastusaari and Murtonen (2013), it was found that chemistry students' deep learning approach predicted their willingness to continue their chemistry studies as a major student. In addition, appreciating chemistry practicals emerged as one of the most important differences between the students who wanted to change major and those who did not want to change. This could be used to aid the planning of education, for example, by increasing chemistry students' deep motivation by emphasising the role of practicals in education.Previous studies have shown that students' approaches are not stable, but sensitive to time, situation and content of learning (Lindblom-Ylänne et al., 2014). Thus, designing a specific method for examining chemistry students' learning is needed. In the present study, we introduced a questionnaire for measuring chemistry students' approaches to learning and demonstrated its validity. The ChemApproach questionnaire is based on the more general questionnaires on learning approaches (see Lovatt et al., 2007; Vermunt, 1994) and on our previous questionnaire on chemistry students' learning approaches (Lastusaari and Murtonen, 2013). Our earlier questionnaire was specifically focused on chemistry learning processes and also on the role of practicals in studying. However, our earlier sample was not large enough to validate the questionnaire. Therefore the current study validated and tested the functionality of the questionnaire.
The results of this study showed that the instrument was valid and can thus be used to explore chemistry students' learning approaches. The results obtainable with this instrument can be utilized to optimize the teaching conditions so as to best suit the particular group of students. In addition to chemistry, we also suggest that the instrument can be tested in other fields of study where practical work is included. In those cases, the term “chemistry” should be changed to the target discipline. In the case of chemistry, the role of practicals may appear to be an especially important factor in students continuing their studies and succeeding in them. According to previous research, students' deep approach can be stimulated through instruction (Baeten et al., 2010; Dolmans et al., 2010) and the whole learning environment may have an effect on students' learning (McCune and Entwistle, 2011). In chemistry education, planning a learning environment that relies heavily on the practicals may be the key to deeply motivated and successful studies.
Acknowledgements
The authors gratefully thank the following people for their invaluable help with the data collection: Harri Lönnberg, Helmi Neuvonen, Henri Kivelä, Tuomas Lönnberg (University of Turku), Leena Mattila, Tanja Lahtinen, Saara Kaski, Piia Valto, Rose B. Matilainen (University of Jyväskylä), Leila Alvila, Tapani Pakkanen (University of Eastern Finland, Joensuu), Johanna Kärkkäinen (University of Oulu). Finally, Pirjo Vahviala and Heidi Ponsiluoma are gratefully thanked for data handling.References
- Baeten M., Kyndt E., Struyven K. and Dochy F., (2010), Using student-centred learning environment to stimulate deep approaches to learning: factors encouraging or discouraging their effectiveness, Educ. Res. Rev., 5, 243–260.
- Biggs J., (1987), Student approaches to learning and studying, Victoria, Australia: Australian Council for Educational Research.
- Brown T. A., (2006), Confirmatory Factor Analysis for Applied Research, New York, USA: Guilford Press.
- Cano-Garcia F. and Justicia-Justicia F., (1994), Learning strategies and approaches: an analysis of their interrelationships, High. Educ., 27, 239–260.
- Chamorro-Premuzic T. and Furnham A., (2009), Mainly openness: the relationship between the Big Five personality traits and learning approaches, Learn. Individ. Differ., 19, 524–529.
- Chan J. Y. K. and Bauer C. F., (2016), Learning and studying strategies used by general chemistry students with different affective characteristics, Chem. Educ. Res. Pract., DOI: 10.1039/c5rp00205b.
- Cheung G. W. and Rensvold R. B., (2002), Evaluating goodness-of-fit indexes for testing measurement invariance, Struct. Equ. Modeling, 9, 233–255.
- Cohen J., (1988), Statistical Power Analysis for the Behavioral Sciences, USA: LEA.
- Dolmans D. H. J. M., Wolfhagen I. H. A. P. and Ginns P., (2010), Measuring approaches to learning in a problem based learning context, Int. J. Med. Educ., 1, 55–60.
- Entwistle N. and McCune V., (2009), The disposition to understand for oneself at university and beyond: learning processes, the will to learn and sensitivity to context, in Zang L.-F. and Sternberg R. J. (ed.) Perspectives on the nature of intellectual styles, New York, USA: Springer, pp. 29–62.
- Entwistle N. and Ramsden P., (1983), Understanding student learning, London, UK: Croom Helm.
- Felder R. M. and Brent R., (2005), Understanding student differences, J. Eng. Educ., 94, 57–72.
- Hu L. and Bentler P. M., (1999), Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives, Struct. Equ. Modeling, 6, 1–55.
- Jackling B., (2005), An analysis of the learning context, perceptions of the learning environment and approaches to study: a longitudinal study, Accounting and Finance, 45, 597–612.
- Johnstone A. H., (2000), Teaching of chemistry – Logical or psychological? Chem. Educ.: Res. Pract. Eur., 1, 9–15.
- Lastusaari M. and Murtonen M., (2013), University chemistry students' learning approaches and willingness to change major, Chem. Educ. Res. Pract., 14, 496–506.
- Lindblom-Ylänne S., Parpala A. and Postareff L., (2014), Challenges in analysing change in students' approaches to learning, in Gijbels D., Donche V., Richardson J. T. E. and Vermunt J. D. (ed.) Learning patterns in higher education, Dimensions and research perspectives, London, UK: Routledge, pp. 232–248.
- Little T., (2013). Longitudinal Structural Equation Modeling, New York, USA: Guilford Press.
- Lonka K. and Lindblom-Ylänne S., (1996), Epistemologies, conceptions of learning, and study practices in medicine and psychology, High. Educ., 31, 5–24.
- Lovatt J., Finlayson O. E. and James P., (2007), Evaluation of student engagement with two learning supports in the teaching of 1st year undergraduate chemistry, Chem. Educ. Res. Pract., 8, 390–402.
- Marsh H. W., Balla J. R. and McDonald R. P., (1988), Goodness of fit indexes in confirmatory factor analysis: the effect of sample size, Psychol. Bull., 103, 391–410.
- Marton F. and Säljö, R., (1976), On qualitative differences in learning – I: outcomes and processes, Br. J. Educ. Psychol., 46, 4–11.
- McCune V. and Entwistle N., (2011), Cultivating the disposition to understand in 21st century university education, Learn. Individ. Differ., 21, 303–310.
- Murtonen M., Olkinuora E., Tynjälä, P. and Lehtinen E., (2008). Do I need research skills in working life? – University students' motivation and difficulties in quantitative methods courses, High. Educ., 56, 599–612.
- Muthén L. K. and Muthén B. O., (1998–2015), Mplus User's Guide, Seventh edn, Los Angeles, CA, USA: Muthén & Muthén.
- Postareff L., Lindblom-Ylänne S. and Parpala A., (2014), Explaining university students' strong commitment to understand through individual and contextual elements, Frontline Learning Research, 3, 31–49.
- Richardson J. T. E., (2005), Students' perceptions of academic quality and approaches to studying in distance education, Br. Educ. Res. J., 31, 7.
- Richardson J. T. E. and Remedios R., (2014), Achievement goals, approaches to studying and academic attainment, in Gijbels D., Donche V., Richardson J. T. E. and Vermunt J. D. (ed.) Learning patterns in higher education, Dimensions and research perspectives, London, UK: Routledge, pp. 125–140.
- Satorra A. and Bentler P. M., (2001), A scaled difference chi-square test statistic for moment structure analysis, Psychometrika, 66, 507–514.
- Statistics Finland, (2015), (http://www.stat.fi) accessed on July 2015.
- van de Schoot R., Lugtig P. and Hox J., (2012), A checklist for testing measurement invariance, Eur. J. Dev. Psychol., 9, 486–492.
- Vermunt J. D., (1994), Inventory of learning styles (ILS) in higher education, Tilburg, the Netherlands: Tilburg University.
- Vermunt J. D., (2005), Relations between student learning patterns and personal and contextual factors and academic performance, High. Educ., 49, 205–234.
- Vermunt J. D. and Vermetten Y. J., (2004), Patterns in student learning: relationships between learning strategies, conceptions of learning, and learning orientations, Educ. Psychol. Rev., 16, 359–384.
- Zeegers P., (2001), Approaches to learning in science: a longitudinal study, Br. J. Educ. Psychol., 71, 115–132.
| This journal is © The Royal Society of Chemistry 2016 |