Selectivity engineering of O-methylation of hydroxybenzenes with dimethyl carbonate using ionic liquid as catalyst
Received
23rd January 2016
, Accepted 14th March 2016
First published on 20th April 2016
Abstract
Phenolic ethers are useful commercial entities which have been traditionally produced via polluting routes that could be replaced by benign catalytic processes. In the current work, O-methylation of mono-, di- and tri-hydroxy benzenes (phenolics), namely, phenol, catechol and pyrogallol, has been studied with dimethyl carbonate (DMC) as the etherification agent cum solvent in the presence of a phosphonium ionic liquid as catalyst. The co-products methanol and CO2 could be recycled to make DMC. The catalyst is recycled and thus the overall process is a green process. Ionic liquids possess many useful attributes and can be used as solvents and multi-functional catalysts, and some are amenable to recycling and reuse using clever strategies. Two different types of phosphonium-based ionic liquids were used – one group containing the trihexyl (tetradecyl) cation such as trihexyl (tetradecyl) phosphonium chloride (PC), trihexyl (tetradecyl) phosphonium bromide (PB), trihexyl (tetradecyl) phosphonium decanoate (PD), trihexyl (tetradecyl) phosphonium hexafluoro phosphate (HFP), and another symmetric reference containing the tetrabutyl phosphonium cation (tetrabutyl phosphonium bromide (TBPB)) – and were evaluated in the O-methylation of phenol, catechol and pyrogallol with DMC to the corresponding ethers. Depending on the number of hydroxyl groups on the benzene ring, different mono- and poly-ethers could be produced by using suitable process conditions such as molar ratio, catalyst, temperature and time. All of these intermediate and final ethers have different applications. Trihexyl (tetradecyl) phosphonium bromide (PB) was the best catalyst. Effects of various parameters on the rate of reaction, conversion and yield were studied including speed of agitation, catalyst concentration and reusability, reactant concentration and temperature. The best operating conditions were 200 °C, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 mole ratio of reactant to DMC, and trihexyl (tetradecyl) phosphonium bromide (PB) as catalyst. A reaction mechanism is proposed and discussed to deduce the kinetics.
6 mole ratio of reactant to DMC, and trihexyl (tetradecyl) phosphonium bromide (PB) as catalyst. A reaction mechanism is proposed and discussed to deduce the kinetics.
1. Introduction
Alkylation of aromatics and their derivatives is ubiquitous in the chemical and allied industries for the manufacture of intermediates, dyes, perfumes, flavours, agrochemicals, fine chemicals, pharmaceuticals and specialty chemicals. Aromatic moieties containing different functional groups or their combinations such as OH, SH, NH2 and CN present a formidable task of getting the desired selectivity for alkylation at the O, S, N or C centres.1 Besides, the nature of alkylating species, temperature, substrate and concentrations play a dominant role in product selectivity. The Williamson synthesis route for etherification is an age-old and polluting process.2 Apart from a number of heterogeneous solid acids3 and bases4 as catalysts, phase transfer catalysts are used to make ethers of different types and uses. Yadav and co-workers have reported the synthesis of several alkylated aromatics by using phase transfer catalysis (PTC)5 and ionic liquids (IL).6 They have also demonstrated the novelties of solid–liquid phase transfer catalysed synthesis and employed a promising class of ILs based on phosphonium ions which were used in O-alkylation.6
Methyl ethers of phenol, catechol and pyrogallol have several applications and could be produced using a number of synthetic protocols. Dimethyl sulphate and methyl halide have been used traditionally to make methyl ethers in the presence of alkalis; the processes are hazardous and highly polluting and need to be replaced by safer and cleaner reagents and process conditions. Dimethyl carbonate (DMC) is thus found as an attractive substitute in O-methylation or etherification reactions. DMC, with tuneable reactivity, non-toxicity and biodegradability, has been heralded as an attractive eco-friendly alternative to both methyl halides/dimethyl sulfate and phosgene for methylation processes. Depending on the temperature, either methylation or carbonylation takes place. At 90 °C, methoxy carbonylation occurs with methanol as a co-product, whereas at higher temperatures, methylation takes place with a variety of nucleophiles with methanol and carbon dioxide as co-products which can be recycled to make DMC.7 Combination of DMC as an O-methylating agent with ILs as catalysts was considered in this work as a novel approach to make methyl ethers of phenol and substituted phenols. ILs have received a lot of attention during the past two decades. There are a number of reviews1–3 on ILs describing their physical and chemical properties and applications in chemical synthesis and catalysis;8 ILs are non-volatile and non-flammable and possess unique properties, finding use in different segments of the chemical and allied industries. They have several applications such as solvents, in purification of gases, homogenous and heterogeneous catalysis, as media for biological reactions and for removal of metal ions, electrolytes in batteries, lubricants, plasticizers, and matrices for mass spectroscopy.9 The O-methylation of phenolic compounds with methanol has been studied in the presence of a strong homogenous acid catalyst10 as well as heterogeneous catalysts using high temperatures and high pressures.11 A wide variety of protocols has been developed to prepare ethers such as PTC,7 ionic liquids8,12 and solid state reactions.13,14 Vapour-phase catalytic methylation of phenol using methanol has been reported over metal oxides or alkaline ion-exchanged zeolites, cerium, samarium and antimony phosphate catalysts promoted with cesium hydroxide.15O-Methylation of phenol using DMC over X-zeolites has been reported.16 The same reaction is studied using various base catalysts in continuous flow stirred tank reactors,17–19 as well as solid–liquid phase transfer catalysis (S–L PTC)20 and gas–liquid (G–L) PTC.21 Bautista et al.22 have reported a vapour phase catalytic alkylation of phenol using dimethyl carbonate over different AlPO4, Al2O3 and AlPO4–Al2O3 catalysts to produce anisole (O-alkylation) as the main product although O-cresol (C-alkylation) and methyl anisole (C,O-alkylation) were also formed. Calcined Mg–Al hydrotalcite has been reported as a catalyst in the reaction of catechol with DMC.23 Shen and co-workers24 have reported that ILs are also suitable catalysts for the O-alkylation reaction. Das and Das25 have reported on the O-methylation of phenols and benzyl alcohols using pyridinium-based ionic liquids and Nie et al.26 have reported using imidazolium-based ionic liquids. Although literature on imidazolium and pyridinium-based ionic liquids is available, studies on phosphonium-based ionic liquids are limited. The current work was undertaken to assess the efficacy of phosphonium-based ionic liquids as base catalysts along with DMC as the methylating agent to produce phenolic ethers of industrial utility. Thus, hydroxy substituted benzenes such as phenol, catechol and pyrogallol were chosen. The emphasis was on achieving high conversion, selectivity, yield and catalyst reusability. The mechanistic and kinetic aspects have also been covered which would be helpful for scale-up and fine-tuning of selectivity.
2. Experimental
2.1. Chemicals
All chemicals were procured from reputed firms and used without further purification; phenol, catechol, pyrogallol, dimethyl carbonate, and methanol of A.R. grade were procured from Sigma Aldrich. Two different types of phosphonium-based ILs (a) those containing the trihexyl (tetradecyl) cation such as trihexyl (tetradecyl) phosphonium chloride (PC), trihexyl (tetradecyl) phosphonium bromide (PB), trihexyl (tetradecyl) phosphonium decanoate (PD), and trihexyl (tetradecyl) phosphonium hexafluoro phosphate (HFP), and (b) tetrabutyl phosphonium bromide (TBPB) were supplied as gift samples by Cytec Industries Inc., Canada.
2.2. Reaction setup and methodology
For the methylation of phenol and catechol, the reaction was carried out in a 100 cm3 capacity stainless steel autoclave (Amar Equipments, Mumbai, India) equipped with temperature and speed controllers and a pressure indicator. A typical reaction mixture consisted of 0.05 mol of phenol/catechol in 0.4 mol of dimethyl carbonate and methanol (balance) to make a total volume of 50 cm3. The speed of agitation was maintained at 200 rpm. A known concentration of the catalyst was added and the mixture was heated to 180 °C. An initial sample was withdrawn before agitation was started. Then samples were withdrawn periodically and analysed by GC. The methylation of pyrogallol was carried out in a 50 cm3 capacity stainless steel (SS-316) autoclave (Autoclave Engineers, USA) mini reactor magnedrive III equipped with temperature and speed controllers, and a pressure indicator. A typical reaction mixture consisted of 0.025 mol of pyrogallol, 0.2 mol of DMC and methanol (balance) to make a total volume of 30 cm3. A known quantity of the catalyst was added and the mixture was heated to 180 °C. An initial sample was withdrawn before agitation. Then samples were withdrawn periodically and analyzed by GC.
2.3. Analysis
For methylation of phenol and catechol, the analysis was carried out by using a GC [Chemito Ceres 800+] equipped with a 0.22 mm I.D., 25 m long BP-1 Capillary column with an FID. The product confirmation was done by GC–MS.
For the methylation of pyrogallol, the analysis was carried out by using a GC (Agilent Technologies 6890 N) equipped with a BPX-50 capillary column (0.25 mm I.D., 30 m length) with an FID and an auto sampler 7683B series with a 10 μl syringe, and the product confirmation analysis was done by GC–MS.
3. Results and discussion
O-Methylation of poly-hydroxy benzenes is a consecutive reaction which can lead to complete conversion and selectivity to ethers depending on the mole ratio of reactants, catalyst, process conditions and reaction time. The reaction mechanism for alkylation with DMC shows that only one of the two ‘–CH3’ groups reacts with the –OH group of the substrate to produce the relevant ether, methanol and CO2, of which both methanol and CO2 are recycled to produce DMC and hence are not wasted. Preliminary experiments suggested that the use of a stoichiometric mole ratio does not give a high rate of reaction and the reaction never goes to completion and to obtain high yields of the relevant ethers, excess DMC is required. Both the methanol generated in situ and the solvent after separation of CO2 from the reaction mass could be distilled and reused in the next set of experiments. Therefore we wanted to use the same strategy for mono-, di- and tri-hydroxyl benzenes for which one, two and three moles of DMC are required, respectively. The excess could always be recycled without any difficulty.
Besides, DMC serves as a solvent cum reactant and hence there was no need of using any additional solvent which would create separation and purification of products adding to the cost of processing.
3.1. Comparison of efficacies of various ionic liquids
The efficacies of various ILs as catalysts were studied under identical sets of conditions. Fig. 1 shows the conversions of phenol, catechol and pyrogallol. Trihexyl (tetradecyl) phosphonium hexafluorophosphate (HFP) and tetrabutyl phosphonium bromide (TBPB) showed the lowest activity, whereas the others had fairly good activity for all the three processes. It was found that trihexyl (tetradecyl) phosphonium bromide (TBPB) gives the best conversion and the highest selectivity for the methylation of phenol, catechol and pyrogallol compared to others. Phosphonium ILs are also stable at high temperatures and the ion-pairs govern the basicity and activity. Symmetric cations are less reactive than the asymmetric ones which is also related to their stability at high temperature. The chloride, bromide and decanoate anions with the same phosphonium cation, namely, trihexyl (tetradecyl) phosphonium, were found to be more active compared to the anion hexafluorophosphate. Amongst all anions, the bromide anion with the trihexyl (tetradecyl) phosphonium cation showed the highest catalytic activity, whereas the bromide anion with the symmetric tetrabutyl phosphonium cation had shown the least catalytic activity. The bromide anion from trihexyl (tetradecyl) phosphonium bromide was found to be much more active than from the tetrabutyl phosphonium bromide. Hence, trihexyl (tetradecyl) phosphonium bromide was selected for all other experiments.
 |
| | Fig. 1 Comparison of efficacies of various ionic liquids. Reaction conditions: mole ratio of reactants 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (phenol/catechol/pyrogallol 8 (phenol/catechol/pyrogallol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC), agitation speed 200 rpm, catalyst concentration 1.18 × 10−5 mol cm−3, temperature 180 °C, time duration 60 min. [( DMC), agitation speed 200 rpm, catalyst concentration 1.18 × 10−5 mol cm−3, temperature 180 °C, time duration 60 min. [( Green) phenol, ( Green) phenol, ( red) catechol, ( red) catechol, ( blue) pyrogallol]. blue) pyrogallol]. | |
3.2. Effect of speed of agitation
Initially the reaction mass was a single phase but as the reaction proceeded CO2 was evolved as the co-product along with methanol and it becomes a gas–liquid multi-phase system under autogenous pressure and 200 °C and could precipitate out if a lower temperature was used. The reactants as well as all products boil at temperatures higher than the operating temperature. Schemes 1–3 depict the reactions of DMC with phenol, catechol and pyrogallol, respectively. Phenol produces anisole (Scheme 1), catechol leads to guaiacol (2a) and veratrole (2b) (Scheme 2) whereas reactions of pyrogallol are given in Schemes 3a–c. Methanol and DMC also served as solvents. Fig. 2 shows the effect of the speed of agitation on the conversion of phenol, catechol and pyrogallol (substrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC mole ratio: 1
DMC mole ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) at 180 °C with the trihexyl (tetradecyl) phosphonium bromide catalyst with a concentration of 1.18 × 10−5 mol cm−3. It was observed that the speed of agitation had no effect on the conversion of any of the phenolics. It would mean that there was no external mass transfer resistance. There was natural circulation produced because of the high temperature and CO2 generated in situ. All experiments thereafter were conducted at 200 rpm so as to have well mixed reactants and uniform temperature throughout the reactor.
8) at 180 °C with the trihexyl (tetradecyl) phosphonium bromide catalyst with a concentration of 1.18 × 10−5 mol cm−3. It was observed that the speed of agitation had no effect on the conversion of any of the phenolics. It would mean that there was no external mass transfer resistance. There was natural circulation produced because of the high temperature and CO2 generated in situ. All experiments thereafter were conducted at 200 rpm so as to have well mixed reactants and uniform temperature throughout the reactor.
 |
| | Scheme 1 Methylation of phenol with DMC. | |
 |
| | Scheme 2 Methylation of catechol with DMC. | |
 |
| | Scheme 3 Methylation of pyrogallol with DMC. | |
 |
| | Fig. 2 Effect of speed of agitation. Reaction conditions: mole ratio of reactants 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (phenol/catechol/pyrogallol 8 (phenol/catechol/pyrogallol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC), catalyst concentration (trihexyl (tetradecyl) phosphonium bromide) 1.18 × 10−5 mol cm−3, temperature 180 °C, time duration 60 min. [( DMC), catalyst concentration (trihexyl (tetradecyl) phosphonium bromide) 1.18 × 10−5 mol cm−3, temperature 180 °C, time duration 60 min. [( Green) phenol, ( Green) phenol, ( red) catechol, ( red) catechol, ( blue) pyrogallol]. blue) pyrogallol]. | |
3.3. Effect of catalyst concentration
The trihexyl (tetradecyl) phosphonium bromide concentration was varied over the range of 2.95 × 10−6–1.18 × 10−5 mol cm−3 for all three phenolics. Fig. 3 shows that with the increase in catalyst concentration, the conversion increases. As an illustrative example, the initial rate of the O-methylation of pyrogallol is plotted against the catalyst concentration, and the rate is observed to increase linearly (Fig. 4). The catalyst forms a complex with the substrate which actually participates in the reaction. It will be further discussed in the reaction mechanism and kinetics section. The rate of formation of di-ether and tri-ether increases as the catalyst concentration was increased for catechol and pyrogallol, respectively, since these are consecutive reactions.
 |
| | Fig. 3 Effect of catalyst concentration. Reaction conditions: temperature 180 °C, mole ratio of reactants 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (phenol/catechol/pyrogallol 8 (phenol/catechol/pyrogallol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC), catalyst trihexyl (tetradecyl) phosphonium bromide, agitation speed 200 rpm and time 60 min. [( DMC), catalyst trihexyl (tetradecyl) phosphonium bromide, agitation speed 200 rpm and time 60 min. [( Green) phenol, ( Green) phenol, ( red) catechol, ( red) catechol, ( blue) pyrogallol]. blue) pyrogallol]. | |
 |
| | Fig. 4 Initial rate vs. catalyst concentration for O-methylation of pyrogallol. | |
In the case of phenol, the formation of anisole increased with the catalyst concentration and the reaction was 100% selective. The selectivity to mono-ethers was more at a lower concentration of 0.88 × 10−5 mol cm−3 catechol and pyrogallol. The standard experiments were limited to 1 h for all three substrates to find out the selectivity to mono-O-methylation. Since the reactivities of the phenolics were different, experiments were conducted for an extended time to compare polyether formation and mono-ether formation (phenol 1.5 h, catechol 3 h and pyrogallol 6 h). It was observed that the conversion to mono-, di- and tri-ethers increased substantially for phenol (97%), catechol (90%) and pyrogallol (84%), for the respective times.
3.4. Effect of mole ratio of reactants
The effect of the mole ratio of phenol/catechol/pyrogallol to DMC was studied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (Fig. 5). It was found that with an increase in the number of moles of DMC, the conversion of phenol/catechol/pyrogallol also increased. The current studies included three phenolics having 1, 2 and 3 hydroxyl groups in the benzene ring. The stoichiometry requires 2 moles of DMC per mole of substrate for 100% conversion. Thus, a 1
8 (Fig. 5). It was found that with an increase in the number of moles of DMC, the conversion of phenol/catechol/pyrogallol also increased. The current studies included three phenolics having 1, 2 and 3 hydroxyl groups in the benzene ring. The stoichiometry requires 2 moles of DMC per mole of substrate for 100% conversion. Thus, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 mol ratio was required with reference to pyrogallol and all substrates were reacted for 1 h for production of mono-ether as the main product. Methanol was also used as a solvent in this reaction. Both methanol and DMC could be distilled and reused. Considering a large scale operation at an industrial level with higher concentrations of DMC, a large amount of DMC will need to be separated and recycled. In order to avoid this problem, the lowest possible mole ratio of reactants was considered for further study. It was observed that at the mole ratio of 1
6 mol ratio was required with reference to pyrogallol and all substrates were reacted for 1 h for production of mono-ether as the main product. Methanol was also used as a solvent in this reaction. Both methanol and DMC could be distilled and reused. Considering a large scale operation at an industrial level with higher concentrations of DMC, a large amount of DMC will need to be separated and recycled. In order to avoid this problem, the lowest possible mole ratio of reactants was considered for further study. It was observed that at the mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, the reaction gives good conversion and maximum selectivity towards the mono O-methylated product.
6, the reaction gives good conversion and maximum selectivity towards the mono O-methylated product.
 |
| | Fig. 5 Effect of mole ratio of reactants. Reaction conditions: temperature 180 °C, agitation speed 200 rpm, catalyst concentration (trihexyl (tetradecyl) phosphonium bromide) 0.88 × 10−5 mol cm−3, time duration 60 min. [( Green) phenol, ( Green) phenol, ( red) catechol, ( red) catechol, ( blue) pyrogallol]. blue) pyrogallol]. | |
3.5. Effect of temperature
Preliminary experiments suggested that a sufficiently high temperature is required to achieve mono-methylation without degrading the ionic liquid. The effect of temperature was studied from 140 °C to 200 °C (Fig. 6) because beyond 220 °C, phosphonium-based ionic liquids start to degrade. The ionic liquid could be reused when there is no degradation and to be on the safer side, a temperature of 180 °C was used. It was observed that the conversion increases substantially with increasing temperature, which suggested that the reaction was intrinsically kinetically controlled. Compared to all other processes, such as vapor phase and heterogeneously catalyzed processes, this process gives good results at lower temperatures and hence it becomes an energy saving and economic process.
 |
| | Fig. 6 Effect of temperature. Reaction conditions: mole ratio of reactants 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (phenol/catechol/pyrogallol 6 (phenol/catechol/pyrogallol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC), agitation speed 200 rpm, catalyst concentration (trihexyl (tetradecyl) phosphonium bromide) 0.88 × 10−5 mol cm−3, time duration 60 min. [( DMC), agitation speed 200 rpm, catalyst concentration (trihexyl (tetradecyl) phosphonium bromide) 0.88 × 10−5 mol cm−3, time duration 60 min. [( Green) phenol, ( Green) phenol, ( red) catechol, ( red) catechol, ( blue) pyrogallol]. blue) pyrogallol]. | |
3.6. Reusability of the catalyst
The reusability of the catalyst trihexyl (tetradecyl) phosphonium bromide (PB) was studied according to the procedure reported by McNulty and co-workers.27 PB was separated by adding dichloromethane (DCM) plus different methanol to water volume ratios. At first, the methanol to water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 V/V) mixture was added to the reaction mixture and then the same volume of DCM was added. Two separate layers formed. PB was extracted from the DCM layer and the remaining reaction mixture remained in the other methanol–water mixture. The layers were separated. Thereafter the DCM layer was subjected to vacuum distillation to separate PB as a bottom product. The IL recovered, along with a makeup quantity for handling losses, was reused in the next reaction and the conversion–time profile was generated. The final conversions were within ±3% (Fig. 7). There was no considerable change (drop) in the final conversions with used PB and thus catalyst was reusable. The phosphonium-based quaternary salts have ion-pairs in their structure and therefore complex with the reagent. Any decrease in concentration due to degradation will lead to reduced rates and conversion. Since practically the same conversions were obtained, it was concluded that there was no degradation of the catalyst and hence it was reusable.
2 V/V) mixture was added to the reaction mixture and then the same volume of DCM was added. Two separate layers formed. PB was extracted from the DCM layer and the remaining reaction mixture remained in the other methanol–water mixture. The layers were separated. Thereafter the DCM layer was subjected to vacuum distillation to separate PB as a bottom product. The IL recovered, along with a makeup quantity for handling losses, was reused in the next reaction and the conversion–time profile was generated. The final conversions were within ±3% (Fig. 7). There was no considerable change (drop) in the final conversions with used PB and thus catalyst was reusable. The phosphonium-based quaternary salts have ion-pairs in their structure and therefore complex with the reagent. Any decrease in concentration due to degradation will lead to reduced rates and conversion. Since practically the same conversions were obtained, it was concluded that there was no degradation of the catalyst and hence it was reusable.
 |
| | Fig. 7 Catalyst reusability. Reaction conditions: temperature 180 °C, mole ratio of reactants 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (phenol/catechol/pyrogallol 6 (phenol/catechol/pyrogallol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC), agitation speed 200 rpm, catalyst concentration (trihexyl (tetradecyl) phosphonium bromide) 0.88 × 10−5 mol cm−3, time duration 60 min. [( DMC), agitation speed 200 rpm, catalyst concentration (trihexyl (tetradecyl) phosphonium bromide) 0.88 × 10−5 mol cm−3, time duration 60 min. [( Green) phenol, ( Green) phenol, ( red) catechol, ( red) catechol, ( blue) pyrogallol]. blue) pyrogallol]. | |
4. Reaction kinetics and mechanism
4.1. Reaction mechanism for O-methylation of pyrogallol
The reaction mechanism for O-methylation of pyrogallol is proposed (Scheme 4). In this process, pyrogallol and the catalyst (IL) come in contact to form a catalyst IL–pyrogallol complex. The IL–pyrogallol complex attacks on the CH3 bond of DMC yielding 3-hydroxy-guaiacol (or 2,3-di-hydroxy anisole). Further, in the next step, 3-hydroxy guaiacol interacts with IL to form the next complex and there is a series of reaction leading to the formation of 3-hydroxy veratrole. Similarly, the subsequent reaction proceeds to give tri-methoxy benzene (Scheme 3).
 |
| | Scheme 4 Reaction mechanism for the methylation of pyrogallol. | |
4.2. Model development
In the case of the O-methylation of pyrogallol, there are three consecutive reactions (Scheme 3). The overall rate of the reaction of DMC (B) is given by the summation of the rates of the individual steps. Here, at first, pyrogallol (A) reacts with DMC (B) to produce 3-hydroxy guaiacol (C). Further, 3-hydroxy guaiacol reacts with DMC to produce 3-hydroxy veratrole, (D) which further reacts with DMC to produce tri-methoxy benzene (E).| | 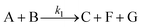 | (1) |
| |  | (2) |
| |  | (3) |
where A–G are pyrogallol, DMC, 3-hydroxy guaiacol, 3-hydroxy veratrole, tri-methoxy benzene, methanol and CO2, respectively.
Since the equilibrium is disturbed by evolution of CO2, there is a change in the number of moles. So volume change must be considered. Since methanol was used as a solvent, it is assumed that the change in volume is insignificant.
| |  | (4) |
| |  | (5) |
| |  | (6) |
| |  | (7) |
| |  | (8) |
| |  | (9) |
| | 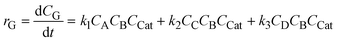 | (10) |
Overall stoichiometry gives the following:
| | |
−k1CACBCCat + k1CACBCCat − k2CCCBCCat + k2CCCBCCat − k3CDCBCCat + k3CDCBCCat = 0
| (11) |
In other words:
| |  | (12) |
At t = 0,
| | | CE = 0, CD = 0, CC = 0, CA = CA0, CF = CF0, CG = 0 | (13) |
Only methanol will be non-zero since it was taken as a solvent.
| |  | (14) |
Volume changes due to reaction, since 3 moles are generated by reacting 2 moles.
Thus, there will be shrinkage. In the case of the current system, the reaction is a liquid phase reaction and methanol was added as a solvent and hence it is assumed that there is no significant volume change.
| | | CE + CD + CC = CA0 − CA = CA0XA | (15) |
where
XA is the fractional conversion of A.
So if the equations are solved for C, D and E, the relationship can be used in conjunction with the fractional conversion of A to find out the concentrations of A–G.
As the concentration of B used was in excess compared to the limiting reactant A, the concentration of B in bulk can be considered as constant.
As CB = CB0 = constant,
Therefore,
| |  | (16) |
Where,
k1′ =
k1CB0CCatSimilarly,
| |  | (18) |
The concentration of C produced at any time can be calculated as
| |  | (19) |
Similarly,
| |  | (20) |
The concentration of D produced at any time can be calculated as
| |  | (21) |
And the concentration of E produced at any time can be calculated by rearranging
eqn (15)| | | CE = CA0 − CA − CC − CD | (22) |
Now from
eqn (11),
| |

| (23) |
| |

| (24) |
Integrating the above equation,
Concentration profiles as function of time were plotted by using eqn (17), (19), (21) and (22) (Fig. 8). Further using Polymath, eqn (4), (6) and (7) were solved and then the values of k1–k3 were calculated. The values of k1–k3 (k1 > k2 > k3) indicate that the formation of C can be maximized, which can also be confirmed by the concentration profiles. From the Arrhenius plots, the activation energies were calculated to be 13.1, 11.8 and 9.2 kcal mol−1 for the first, second and third steps, respectively (Fig. 9). The lower values of activation energy for the second and third steps indicate that the reaction will proceed to form the undesired product easily if the desired product is not separated.
 |
| | Fig. 8 Concentration profile for the O-methylation of pyrogallol. Reaction conditions: temperature 200 °C, mole ratio of reactants 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (pyrogallol 6 (pyrogallol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC), agitation speed 200 rpm, catalyst concentration (trihexyl (tetradecyl) phosphonium bromide) 0.88 × 10−5 mol cm−3. DMC), agitation speed 200 rpm, catalyst concentration (trihexyl (tetradecyl) phosphonium bromide) 0.88 × 10−5 mol cm−3. | |
 |
| | Fig. 9 Arrhenius plot for the three steps of methylation of pyrogallol. | |
4.3. For O-methylation of catechol
The reaction mechanism for the O-methylation of catechol can be explained by the first two steps shown for the methylation of pyrogallol (Scheme 4). In the case of the O-methylation of catechol, there are two consecutive reactions occurring. At first, catechol (A) reacts with DMC (B) to produce guaiacol (C). Further, guaiacol reacts with DMC to produce veratrole (D).
Similarly, the overall rate of the reaction can be predicted by adding the rates of two individual steps and as discussed earlier, the concentrations of A, C and D at any point of time ‘t’ can be calculated by using eqn (17), (19) and (21), respectively, and the concentration profiles can be plotted as shown in Fig. 10. Similarly, the net rate of the reaction can be calculated by addition of the individual step rate equations. As discussed above, the values of k1 and k2 can be calculated and the values of k1 and k2 (i.e. k1 > k2) indicate that the formation of C can be maximized, which can also be confirmed by the concentration profiles. The kinetic rate constants for each step were plotted and the activation energy was calculated to be 7.8 and 6.7 kcal mol−1 for the first and second steps, respectively (Fig. 11). The values of the activation energy indicate that the reaction will proceed to form the undesired product very easily as there is not a big difference in the activation energies of two steps.
 |
| | Fig. 10 Concentration profile for the O-methylation of catechol. Reaction conditions: temperature 200 °C, mole ratio of reactants 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (catechol 6 (catechol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC), agitation speed 200 rpm, catalyst concentration (trihexyl (tetradecyl) phosphonium bromide) 0.88 × 10−5 mol cm−3. DMC), agitation speed 200 rpm, catalyst concentration (trihexyl (tetradecyl) phosphonium bromide) 0.88 × 10−5 mol cm−3. | |
 |
| | Fig. 11 Arrhenius plot for the two steps of methylation of catechol. | |
4.4.
O-Methylation of phenol
The reaction mechanism for the O-methylation of phenol is similar to the first step discussed in the case of the O-methylation of pyrogallol.
The plot of ln(CA0/CA) versus time at different temperatures gives straight lines passing through the origin which confirms that the reaction is of pseudo first order with respect to the limiting reactant (Fig. 12). The Arrhenius plot was plotted to determine the activation energy as 5.9 kcal mol−1 (Fig. 13).
 |
| | Fig. 12 Pseudo first order rate equation for the methylation of phenol ( 200 °C, 200 °C,  180 °C, 180 °C,  160 °C, and 160 °C, and  140 °C). 140 °C). | |
 |
| | Fig. 13 Arrhenius plot for the O-methylation of phenol. | |
5. Conclusions
The phosphonium-based ionic liquids were found to be promising catalysts over other reported homogeneous and heterogeneous catalysts for the O-methylation of phenolic substances with DMC. Among all the studied phosphonium-based ionic liquids, trihexyl (tetradecyl) phosphonium bromide (PB) was found to be the best suited catalyst for the O-methylation of phenol, catechol and pyrogallol. It was found that the speed of agitation has no effect and hence no mass transfer resistance was present. With the increase in the catalyst concentration there was an increase in the rate of reaction. The reaction is intrinsically kinetically controlled and the temperature has a significant effect on the rate of reaction. With an increase in temperature there was increase in the rate of reaction, but the selectivity to the mono-methylated product is affected. The maximum conversion and selectivity were found at 200 °C, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 mole ratio of reactant to DMC and at a catalyst concentration of 0.88 × 10−5 mol cm−3. The overall order of the reactions was found to be pseudo first order. The ionic liquid catalyst IL-102 can be easily separated and reused. Kinetic models were developed based on the proposed reaction mechanism. The activation energy for each step of the reaction was also calculated.
6 mole ratio of reactant to DMC and at a catalyst concentration of 0.88 × 10−5 mol cm−3. The overall order of the reactions was found to be pseudo first order. The ionic liquid catalyst IL-102 can be easily separated and reused. Kinetic models were developed based on the proposed reaction mechanism. The activation energy for each step of the reaction was also calculated.
Nomenclature
| A | Mono-, di- or tri-hydroxy benzene |
| B | Dimethyl carbonate |
| C | Mono methylated product |
| D | Di methylated product |
| E | Tri methylated product |
| F | Methanol |
| G | Carbon dioxide |
| M | Mole ratio |
|
C
A
| Concentration of A, mol cm−3 |
|
C
B
| Concentration of B, mol cm−3 |
|
C
A0
| Initial concentration of A, mol cm−3 |
|
C
C
| Concentration of C, mol cm−3 |
|
C
D
| Concentration of D, mol cm−3 |
|
C
E
| Concentration of E, mol cm−3 |
|
C
cat
| Concentration of the catalyst, mol cm−3 |
|
k
1, k2, k3 | Reaction rate constants, s−1 |
|
E
| Activation energy, kcal mol−1 |
|
t
| Reaction time, s |
|
X
A
| Fractional conversion of A |
Acknowledgements
This work was done under the collaborative project “Sustainable Catalytic Syntheses of Chemicals using Carbon Dioxide as Feedstock (GreenCatCO2)” supported by the Department of Science and Technology, Government of India (DST-GOI) and the Academy of Finland. GDY received support from the R. T. Mody Distinguished Professor Endowment and J. C. Bose National Fellowship from DST-GoI.
References
-
Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, 2002 Search PubMed.
- S. Keskin, D. Kayrak-Talay, U. Akman and Ö. Hortaçsu, J. Supercrit. Fluids, 2007, 43(1), 150–180 CrossRef CAS.
- T. Welton, Ionic liquids in catalysis, Coord. Chem. Rev., 2004, 248(21), 2459–2477 CrossRef CAS.
- G. D. Yadav and P. M. Bisht, J. Mol. Catal. A: Chem., 2004, 223(1), 93–100 CrossRef CAS.
- G. D. Yadav and B. G. Motirale, Ind. Eng. Chem. Res., 2008, 47, 9081–9089 CrossRef CAS.
- G. D. Yadav and S. P. Tekale, Org. Process Res. Dev., 2010, 14, 722–727 CrossRef CAS.
- G. D. Yadav and S. V. Lande, J. Mol. Catal. A: Chem., 2006, 244, 271–277 CrossRef CAS.
- M. Badri, J.-J. Brunet and R. Perron, Tetrahedron Lett., 1992, 33, 4435–4438 CrossRef CAS.
- S. Keskin, D. Kayrak-Talay, U. Akman and Ö. Hortaçsu, J. Supercrit. Fluids, 2007, 43, 150–180 CrossRef CAS.
- K. B. Wiberg and K. A. Saegebarth, J. Org. Chem., 1960, 25, 832–833 CrossRef CAS.
- R. Bal and S. Sivasanker, Appl. Catal., A, 2003, 246, 373–382 CrossRef CAS.
- A. Loupy, J. Sansoulet and F. Vaziri-Zand, Bull. Soc. Chim. Fr., 1987, 6, 1027–1035 Search PubMed.
- F. Toda and K. Okuda, J. Chem. Soc., Chem. Commun., 1991, 1212–1214 RSC.
- F. Toda, H. Takumi and M. Akehi, J. Chem. Soc., Chem. Commun., 1990, 1270–1271 RSC.
- G. Sarala Devi, D. Giridhar and B. Reddy, J. Mol. Catal. A: Chem., 2002, 181, 173–178 CrossRef CAS.
- Z. H. Fu and Y. Ono, Catal. Lett., 1993, 21, 43–47 CrossRef CAS.
- A. Bomben, M. Selva and P. Tundo, Ind. Eng. Chem. Res., 1999, 38, 2075–2079 CrossRef CAS.
- U. Tilstam, Org. Process Res. Dev., 2012, 16, 1974–1978 CrossRef CAS.
- U. Tilstam, Org. Process Res. Dev., 2012, 16, 1150–1153 CrossRef CAS.
- S. Ouk, S. Thiebaud, E. Borredon, P. Legars and L. C. Lecomte, Tetrahedron Lett., 2002, 43, 2661–2663 CrossRef CAS.
- P. Tundo, F. Trotta, G. Moraglio and F. Ligorati, Ind. Eng. Chem. Res., 1988, 27, 1565–1571 CrossRef CAS.
- F. Bautista, J. Campelo, A. Garcia, D. Luna, J. Marinas and A. Romero, React. Kinet. Catal. Lett., 1998, 63, 261–269 CrossRef CAS.
- T. Jyothi, T. Raja, M. Talawar and B. Rao, Appl. Catal., A, 2001, 211, 41–46 CrossRef CAS.
- Z. L. Shen, X. Z. Jiang, W. M. Mo, B. X. Hu and N. Sun, Green Chem., 2005, 7, 97–99 RSC.
- P. J. Das, J. Das, J. Mol. Liq., 2015, 209, 94–98 CrossRef CAS.
- J. Q. Nie, H. W. Chen, Q. H. Song, B. Liao and Q. X. Guo, Energy Fuels, 2010, 24(10), 5722–5726 CrossRef CAS.
- J. McNulty, A. Capretta, S. Cheekoori, J. A. Clyburne and A. Robertson, Chim. Oggi, 2004, 22, 13–16 CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  *a
*a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 mole ratio of reactant to DMC, and trihexyl (tetradecyl) phosphonium bromide (PB) as catalyst. A reaction mechanism is proposed and discussed to deduce the kinetics.
6 mole ratio of reactant to DMC, and trihexyl (tetradecyl) phosphonium bromide (PB) as catalyst. A reaction mechanism is proposed and discussed to deduce the kinetics.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC mole ratio: 1
DMC mole ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) at 180 °C with the trihexyl (tetradecyl) phosphonium bromide catalyst with a concentration of 1.18 × 10−5 mol cm−3. It was observed that the speed of agitation had no effect on the conversion of any of the phenolics. It would mean that there was no external mass transfer resistance. There was natural circulation produced because of the high temperature and CO2 generated in situ. All experiments thereafter were conducted at 200 rpm so as to have well mixed reactants and uniform temperature throughout the reactor.
8) at 180 °C with the trihexyl (tetradecyl) phosphonium bromide catalyst with a concentration of 1.18 × 10−5 mol cm−3. It was observed that the speed of agitation had no effect on the conversion of any of the phenolics. It would mean that there was no external mass transfer resistance. There was natural circulation produced because of the high temperature and CO2 generated in situ. All experiments thereafter were conducted at 200 rpm so as to have well mixed reactants and uniform temperature throughout the reactor.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (Fig. 5). It was found that with an increase in the number of moles of DMC, the conversion of phenol/catechol/pyrogallol also increased. The current studies included three phenolics having 1, 2 and 3 hydroxyl groups in the benzene ring. The stoichiometry requires 2 moles of DMC per mole of substrate for 100% conversion. Thus, a 1
8 (Fig. 5). It was found that with an increase in the number of moles of DMC, the conversion of phenol/catechol/pyrogallol also increased. The current studies included three phenolics having 1, 2 and 3 hydroxyl groups in the benzene ring. The stoichiometry requires 2 moles of DMC per mole of substrate for 100% conversion. Thus, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 mol ratio was required with reference to pyrogallol and all substrates were reacted for 1 h for production of mono-ether as the main product. Methanol was also used as a solvent in this reaction. Both methanol and DMC could be distilled and reused. Considering a large scale operation at an industrial level with higher concentrations of DMC, a large amount of DMC will need to be separated and recycled. In order to avoid this problem, the lowest possible mole ratio of reactants was considered for further study. It was observed that at the mole ratio of 1
6 mol ratio was required with reference to pyrogallol and all substrates were reacted for 1 h for production of mono-ether as the main product. Methanol was also used as a solvent in this reaction. Both methanol and DMC could be distilled and reused. Considering a large scale operation at an industrial level with higher concentrations of DMC, a large amount of DMC will need to be separated and recycled. In order to avoid this problem, the lowest possible mole ratio of reactants was considered for further study. It was observed that at the mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, the reaction gives good conversion and maximum selectivity towards the mono O-methylated product.
6, the reaction gives good conversion and maximum selectivity towards the mono O-methylated product.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 V/V) mixture was added to the reaction mixture and then the same volume of DCM was added. Two separate layers formed. PB was extracted from the DCM layer and the remaining reaction mixture remained in the other methanol–water mixture. The layers were separated. Thereafter the DCM layer was subjected to vacuum distillation to separate PB as a bottom product. The IL recovered, along with a makeup quantity for handling losses, was reused in the next reaction and the conversion–time profile was generated. The final conversions were within ±3% (Fig. 7). There was no considerable change (drop) in the final conversions with used PB and thus catalyst was reusable. The phosphonium-based quaternary salts have ion-pairs in their structure and therefore complex with the reagent. Any decrease in concentration due to degradation will lead to reduced rates and conversion. Since practically the same conversions were obtained, it was concluded that there was no degradation of the catalyst and hence it was reusable.
2 V/V) mixture was added to the reaction mixture and then the same volume of DCM was added. Two separate layers formed. PB was extracted from the DCM layer and the remaining reaction mixture remained in the other methanol–water mixture. The layers were separated. Thereafter the DCM layer was subjected to vacuum distillation to separate PB as a bottom product. The IL recovered, along with a makeup quantity for handling losses, was reused in the next reaction and the conversion–time profile was generated. The final conversions were within ±3% (Fig. 7). There was no considerable change (drop) in the final conversions with used PB and thus catalyst was reusable. The phosphonium-based quaternary salts have ion-pairs in their structure and therefore complex with the reagent. Any decrease in concentration due to degradation will lead to reduced rates and conversion. Since practically the same conversions were obtained, it was concluded that there was no degradation of the catalyst and hence it was reusable.
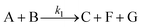








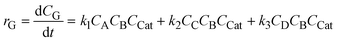










 200 °C,
200 °C,  180 °C,
180 °C,  160 °C, and
160 °C, and  140 °C).
140 °C).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 mole ratio of reactant to DMC and at a catalyst concentration of 0.88 × 10−5 mol cm−3. The overall order of the reactions was found to be pseudo first order. The ionic liquid catalyst IL-102 can be easily separated and reused. Kinetic models were developed based on the proposed reaction mechanism. The activation energy for each step of the reaction was also calculated.
6 mole ratio of reactant to DMC and at a catalyst concentration of 0.88 × 10−5 mol cm−3. The overall order of the reactions was found to be pseudo first order. The ionic liquid catalyst IL-102 can be easily separated and reused. Kinetic models were developed based on the proposed reaction mechanism. The activation energy for each step of the reaction was also calculated.


































