A simple, one-pot synthesis of molybdenum oxide-reduced graphene oxide composites in supercritical methanol and their electrochemical performance†
Jieun Hwanga,
Dohyeon Yoona,
Boyoung Kweona,
Wonyoung Changb and
Jaehoon Kim*ac
aSchool of Mechanical Engineering, Sungkyunkwan University, 2066 Seobu-ro, Jangan-gu, Suwon, Gyeonggi-do 16419, Republic of Korea. E-mail: jaehoonkim@skku.edu; Fax: +82-31-290-5889; Tel: +82-31-299-4843
bCenter for Energy Convergence, Korea Institute of Science and Technology, Hwarang-ro 14-gil 5, Seongbuk-gu, Seoul, 02792, Republic of Korea
cSungkyun Advanced Institute of Nano Technology (SAINT), Sungkyunkwan University, 2066 Seobu-ro, Jangan-gu, Suwon, Gyeonggi-do 16419, Republic of Korea
First published on 7th November 2016
Abstract
A simple and green supercritical methanol (scMeOH) route is developed to tightly anchor molybdenum oxide (MoO2) nanoparticles on reduced graphene oxide (RGO). In scMeOH, graphene oxide is reduced, and MoO2 nanoparticles with sizes of 10–20 nm are simultaneously deposited on the basal plane of RGO in a short time without using any reducing agents or additives. When tested as an anode in lithium ion batteries, the MoO2–RGO composites show enhanced electrochemical performance compared to bare MoO2. The composite with a MoO2 loading of 37.0 wt% delivers a high reversible discharge capacity of 793 mA h g−1 at 50 mA g−1 and an excellent rate performance of 205 mA h g−1 at 2.5 A g−1. After 100 cycles of high rate testing of up to 50 A g−1, the MoO2–RGO composite recovers most of its initial capacity. The improved electrochemical performance of MoO2–RGO can be attributed to the tight anchoring of nanosized MoO2 on RGO and the mesoporous structure of the composite. Consequently, the transport length of Li diffusion into the MoO2 phase is shortened, charge transfer kinetics at the electrode–electrolyte interface is facilitated, and the volume expansion associated with the conversion reaction can be accommodated.
1. Introduction
There are various types of electrochemical energy storage and conversion systems such as fuel cells, supercapacitors, and lithium-ion batteries (LiBs).1–4 Among these, LiBs are considered the most promising choice for energy storage and utilization in large-scale applications including electric bikes, electric vehicles, and energy storage systems because of their high energy density and long lifetime. However, the typical commercially available graphite-based anode material for portable electronic devices would not meet the fast-growing demand for large-scale battery applications because of its low theoretical capacity (372 mA h g−1), low density (∼2.0 g cm−3), and potential safety concerns.5,6 Hence, the development of alternative active materials with high specific energy density, improved safety, better stability, and inexpensive starting materials is required. Molybdenum dioxide (MoO2) has been considered a promising anode material because of its high chemical and thermal stability, high density (6.5 g cm−3), and high theoretical capacity (838 mA h g−1).7,8 In particular, the high theoretical capacity associated with MoO2 originates from its ability to accommodate four lithium ions for each MoO2 unit by Li ion insertion into the octahedral interstitial sites (xLi + xe− + MoO2 ↔ LixMoO2, 0 < x < 0.98) and the subsequent conversion of Li0.98MoO2 to Mo (Li0.98MoO2 + 3.02Li+ + 3.02e− → Mo + 2Li2O).7,9,10 However, the intrinsically sluggish electron transport kinetics often allow only one electron transfer to the bulk MoO2 phase under normal charge–discharge conditions,7,11,12 which hinders the conversion reaction and thus delivers much lower capacities than the theoretical capacity. In addition, the huge volume change and cell pulverization during the conversion reaction decrease the electrical conductivity, leading to rapid capacity fading upon cycling.The two most promising ways to alleviate the low cycle performance and the capacity loss associated with bulk MoO2 particles are the design of nanostructured MoO2 and the combination of MoO2 with carbonaceous materials. The improved electrochemical performance of nanostructured MoO2 (e.g., nanoparticles,13 nanorods,14 ordered mesoporous structures,7 core–shell microcapsules,15 and hierarchical mesoporous structures16) has been confirmed by many studies; by fabricating nanostructured MoO2 materials, the diffusion length of Li ions and electrons can be shortened and the electrolyte–electrode contact area can increase, which can enhance the kinetics of Li insertion/deinsertion. Furthermore, forming MoO2–carbon composites (e.g., amorphous carbon-coated MoO2 nanocrystals,17 MoO2/multiwall carbon nanotube hybrids,18 MoO2/ordered mesoporous carbon composites,19 and ultrathin MoO2 nanosheets encapsulated in a carbon matrix20) can overcome the huge volume change and the degradation of cell integrity. Recently, MoO2–graphene composites containing MoO2 nanoparticles deposited on a 2D graphene layer have drawn considerable interest because the excellent electrical conductivity, the chemical stability, and the unique mechanical flexibility of graphene can improve the electrochemical performance of MoO2.8,10,21–30 The unique properties of graphene make it an excellent matrix for MoO2 composite, or more generally, for other conversion-based electrode materials.31 Graphene can act as an excellent buffer layer, accommodating the huge volume changes during the conversion reaction. In addition, the high electrical conductivity of graphene and homogeneous distribution of the MoO2 phase on the graphene layer would ensure fast and uniform electron and Li ion transport to the MoO2 phase. In addition to acting as a support for MoO2 particles, graphene itself can accommodate Li ions with capacities of 500–1000 mA h g−1 in various sites on edges, defects, functional groups, nanopores, and gaps between layers.32,33 Various approaches have been investigated for the synthesis of MoO2–graphene composites including a hydrazine-based reduction,8 a hydrothermal22,26,27,29,30 or solvothermal28 approach, heat-treatment,23,24 and chemical vapor deposition.25 Although previous work has demonstrated that the electrochemical performance of MoO2–graphene is highly promising, most of the previously developed synthetic routes require long reaction times (6–24 h), toxic reducing agents and chemicals (e.g., hydrazine hydrates), complicated synthetic procedures, multi-step reactions, and the requirement of additional calcination, which would hinder the practical applications of the MoO2–graphene composites. To the best of our knowledge, there are few efforts to develop a simple, green and fast synthetic route for fabricating of the MoO2–graphene composite structure.
In this study, a simple, one-pot supercritical methanol (scMeOH) route for the synthesis of MoO2-reduced graphene oxide (RGO) composites is investigated. Graphene oxides (GO) are reduced, and MoO2 nanoparticles are simultaneously deposited on the RGO in the supercritical methanol medium without using any external reducing agents or additional heat-treatment. In a previous study, we demonstrated that supercritical alcohols are an effective reducing agent for deoxygenation of various types of oxygen functionalities present in GO.34,35 In addition, supercritical alcohols are excellent media for the synthesis of various types of nanostructured metal oxides with enhanced electrochemical performance (e.g., TiO2,36 Li4Ti5O12,37–39 ZnO,40 and LiFePO4 (ref. 41)) because of the unique synthetic conditions associated with supercritical alcohols such as extremely fast nucleation rates, the retardation of particle growth by surface modification with alcohol moieties, and the ability to form hierarchical mesoporous particles without using templates. During the one-pot reaction investigated here, tight anchoring of heterogeneously nucleated MoO2 particles near the basal plane of Supercritical alcohol-Reduced Graphene Oxide (SRGO) results in robust MoO2–SRGO composites. When tested as an anode in LiBs, the MoO2–SRGO composites exhibit excellent reversible capacity (793.0 mA h g−1 at 50 mA g−1 after 50 cycles) and high-rate performance (204.5 mA h g−1 at 2.5 A g−1).
2. Experimental
2.1. Materials
Natural graphite flakes (size < 45 μm, 99.99% purity), bis(acetylacetonato) dioxomolybdenum(VI) (MoO2(acac)2) and methanol (anhydrous, 99.8% purity) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Distilled and de-ionized (DDI) water was prepared by an AQUAMax™-Basic 360 water purification system (Younglin instrument Co., Ltd., Anyang, Korea). Mixed cellulose ester membrane filters (pore size of 0.45 μm) and FP Vericel® membrane filters (pore size of 0.45 μm) were supplied by Advantec (Toyo Roshi Kaisha, Ltd., Japan) and by the Pall Corporation (New York, NY, USA), respectively. Poly(vinylidene difluoride) (PVDF, Kureha Chemical Industry Co., Tokyo, Japan), acetylene black (DENKA Co. Ltd., Tokyo, Japan), and N-methyl-2-pyrrolidone (NMP, purity ≥ 98 wt%, Alfa-Caesar, MA, USA) were used without further purification.2.2. Synthesis of MoO2–SRGO composite
Fig. 1 shows a schematic of the synthesis the MoO2–SRGO composites. GO was synthesized using the modified Hummers method.42,43 A detailed description of the synthesis of GO given in our previous publications.35,44 The synthesis of bare MoO2 and MoO2–SRGO composites, and the reduction of GO to SRGO was conducted in scMeOH using a SUS 316 tube reactor manufactured by the Hanyang Precision Company (Seoul, Korea) with an inner volume of 11 mL. In a typical synthesis of the MoO2–SRGO composites, measured amounts of MoO2(acac)2 and GO were introduced in methanol at room temperature with vigorous stirring, and the whole solution was sonicated for 10 min. Then, 4 mL of the mixed suspension was transferred to the tube reactor. After being tightly sealed, the reactor was immersed into a molten salt bath whose temperature was maintained at 400 °C. After the desired reaction time of 30 min, the reactor was removed from the salt bath and quenched in a cold water bath. The produced composites in the reactors were collected by washing with ethanol, were purified by five cycles of decantation and centrifugation using ethanol, and filtered using a membrane filter. The wet composites were dried in a vacuum oven at 70 °C for 12 h. As listed in Table 1, three composite samples with different loadings of MoO2 in the range of 27.2–55.6 wt% were prepared by adjusting the ratio of MoO2(acac)2 and GO in the methanol solution.| Sample code | MoO2(acac)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GO weight ratio GO weight ratio |
MoO2 contenta (wt%) | MoO2 crystallite sizeb (nm) | BET surface area (m2 g−1) | Average pore size diameter (nm) | Total pore volume (cm3 g−1) | Porosityc (%) | Reversible capacity at 50 mA g−1 (mA h g−1) | Reversible capacity at 1 A g−1 (mA h g−1) |
|---|---|---|---|---|---|---|---|---|---|
| a Estimated using TGA (see Fig. 5).b Estimated using (211) peak in the XRD patterns and the Scherrer's equation.c Estimated under the assumption that the density of graphene is 2 g cm−3 and the density of MoO2 is 6.47 g cm−3. | |||||||||
| MoO2–SRGO-1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
27.2 | 19.07 | 15.2 | 13.1 | 0.051 | 16.4 | 491.7 | 256.8 |
| MoO2–SRGO-2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
37.0 | 19.12 | 14.3 | 11.4 | 0.041 | 15.0 | 899.1 | 401.1 |
| MoO2–SRGO-3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
55.6 | 19.22 | 19.2 | 10.8 | 0.052 | 23.3 | 822.3 | 264.6 |
2.3. Material characterization
The phase structure of the samples was analysed using an X-ray diffractometer (D8 ADVANCE, Bruker Corporation, MA, USA) with Cu Kα radiation in the 2θ range from 3° to 90° at 40 kV and 100 mA. The functional groups on the surface of the samples were characterized using a Fourier-transform infrared (FT-IR) spectrometer (IFS-66/S, Bruker, MA, USA). X-ray Photoelectron Spectroscopy (XPS) of the samples was performed using a PHI 5000 Versa Probe (ULVAC-PHI Inc., Kanagawa, Japan) spectrometer. The morphology of the samples was measured using a field emission scanning electron microscope equipped with energy dispersive spectrometer (FE-SEM/EDS, JSM7500F, JEOL, Tokyo, Japan) and a Tecnai-G2 high-resolution transmission electron microscope (HR-TEM, FEI Co. Ltd., OR, USA). For the HR-TEM measurements, the samples were dispersed in dimethylformamide (DMF) using ultrasonication and were deposited on to the copper grid with holey carbon film. The Brunauer–Emmett–Teller (BET) surface area was acquired using a Belsorp-mini II apparatus (BEL Inc., Osaka, Japan). The thermal properties of the samples were examined using a TGA Q50 thermogravimetric analyzer (TGA, TA Instruments, DE, USA) at temperatures ranging from 30–700 °C with a heating rate of 10 °C min−1 and an air flow rate of 60 mL min−1.2.4. Electrochemical characterization
The electrochemical properties of the MoO2–SRGO composite electrode materials were measured in a CR2032 type coin-cell configuration. The working electrode was composed of the active material (70 wt%), acetylene black as a conducting material (10 wt%), and PVDF as a binder (20 wt%). After blending in NMP, the slurry was cast uniformly on a Cu foil to prepare the electrode film. The film was then dried in an oven at 80 °C for 24 h to remove NMP. The electrode film was punched into 14 mm-diameter discs (with an area of 1.54 cm2) and weighed. The test cells were fabricated with the composite anode and lithium metal as a counter electrode. The electrolyte was 1 M LiPF6 dissolved in ethylene carbonate (EC)/dimethyl carbonate (DMC)/ethylmethyl carbonate (EMC) solvent (with a volume ratio for EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC
DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC = of 1
EMC = of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). A microporous polypropylene membrane (Celgard 2500, Celgard LLC., NC, USA) was used as the separator. The cell assembly was conducted in a glove box filled with ultrahigh purity argon gas. Cyclic voltammetry (CV) tests of the cells were performed using a model ZIVE MP1 potentiostat analyzer (WonATech Corp., Korea) at room temperature. The CV tests were performed at a scanning rate of 1.0 mV s−1 between 0.001–3.0 V (hereafter vs. Li/Li+) for the initial 10 charge–discharge cycles. The cells were galvanostatically charged and discharged in the voltage range from 0.005 to 3.0 V using a model WBCS 3000 battery test system (WonAtech Corp., Seoul, Korea) at room temperature. The cyclability was recorded at a current density of 50 mA g−1 for up to 50 cycles, and the current density was varied from 50 mA g−1 to 50 A g−1 for the rate performance measurements. Electrochemical impedance spectroscopy (EIS) tests were performed using a ZIVE MP1 impedance analyzer (WonATech Corp., Korea) over a range of from 100 kHz to 10 mHz.
1). A microporous polypropylene membrane (Celgard 2500, Celgard LLC., NC, USA) was used as the separator. The cell assembly was conducted in a glove box filled with ultrahigh purity argon gas. Cyclic voltammetry (CV) tests of the cells were performed using a model ZIVE MP1 potentiostat analyzer (WonATech Corp., Korea) at room temperature. The CV tests were performed at a scanning rate of 1.0 mV s−1 between 0.001–3.0 V (hereafter vs. Li/Li+) for the initial 10 charge–discharge cycles. The cells were galvanostatically charged and discharged in the voltage range from 0.005 to 3.0 V using a model WBCS 3000 battery test system (WonAtech Corp., Seoul, Korea) at room temperature. The cyclability was recorded at a current density of 50 mA g−1 for up to 50 cycles, and the current density was varied from 50 mA g−1 to 50 A g−1 for the rate performance measurements. Electrochemical impedance spectroscopy (EIS) tests were performed using a ZIVE MP1 impedance analyzer (WonATech Corp., Korea) over a range of from 100 kHz to 10 mHz.
3. Results and discussion
3.1. Characteristics of MoO2–SRGO composites
The MoO2–SRGO composites with different MoO2 loadings were synthesized by controlling the ratio of the MoO2 precursor and GO in the methanol suspension solution. The weight ratios of MoO2(acac)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GO tested in this study are listed in Table 1. The differences in the morphology of the synthesized composites with varying the MoO2 loading were examined using SEM and HR-TEM, and representative images are shown in Fig. 2. The SEM images of the bare MoO2 particles, synthesized in scMeOH in the absence of GO, are shown in Fig. S1.† The bare MoO2 exhibits hierarchically porous micron-sized spheres comprised of nanosized particles with diameters of 30–80 nm. The formation of hierarchically porous nanometer- and micron-sized particles in scMeOH is because of the unique surface modifications of the growing MoO2 particles by the organic moieties generated from scMeOH and the interactions between the particles. A similar morphology of hierarchically porous microspheres in supercritical alcohols was observed previously in case of TiO2,36,45 ZnO,46,47 CeO2,48–51 and Li4Ti5O12.38,52 As shown in the low magnification SEM image of MoO2–SRGO-1 (with a MoO2 loading of 27.2 wt%) in Fig. S2a,† MoO2 particles with much less interparticle aggregation were deposited on the surface of SRGO when compared to the deposited particles of the bare MoO2 sample. The high-magnification SEM and TEM images of MoO2–SRGO-1 (Fig. 2a) show that the MoO2 particles with sizes of 10–20 nm were uniformly deposited on the basal plane of SRGO. Clearly, the presence of GO increases the heterogeneous nucleation rate of MoO2 particles near the surface of GO over the homogenous nucleation in the bulk supercritical fluid phase. In the MoO2–SRGO-2 composite (with a MoO2 loading of 37.0 wt%), more complete coverage of the SRGO sheets with the MoO2 particles and a slight increase in particle size to 20–50 nm were observed (Fig. 2b). The EDS mapping of the composite (Fig. S3†) shows the uniform distribution of MoO2 particles on the surface of SRGO sheets. In some areas (as indicated by the arrow in Fig. 2b), the aggregation of growing particles on the SRGO sheets results in larger particles of size ∼30–40 nm. In the MoO2–SRGO-3 composite (with a MoO2 loading of 55.6 wt%), the MoO2 particles are in close contact with neighboring particles (Fig. 2c). As shown in Fig. S2b,† the MoO2–SRGO-3 sample retains the micron-sized, spherically shaped MoO2 particles that are loosely attached to the graphene layer, which probably formed in the bulk supercritical phase by the homogeneous nucleation mechanism. The close contact between MoO2 particles formed on the surface of SRGO and the micron-sized MoO2 particles formed in the supercritical fluid phase would deteriorate cycling stability because of the huge volume change during the insertion/deinsertion of the Li ions, which will be discussed in a later section. In the selected area electron diffraction (SAED) pattern of the MoO2–SRGO samples, shown in the inset of HR-TEM images in Fig. 2; the concentric diffraction rings could be indexed to the (011), (
GO tested in this study are listed in Table 1. The differences in the morphology of the synthesized composites with varying the MoO2 loading were examined using SEM and HR-TEM, and representative images are shown in Fig. 2. The SEM images of the bare MoO2 particles, synthesized in scMeOH in the absence of GO, are shown in Fig. S1.† The bare MoO2 exhibits hierarchically porous micron-sized spheres comprised of nanosized particles with diameters of 30–80 nm. The formation of hierarchically porous nanometer- and micron-sized particles in scMeOH is because of the unique surface modifications of the growing MoO2 particles by the organic moieties generated from scMeOH and the interactions between the particles. A similar morphology of hierarchically porous microspheres in supercritical alcohols was observed previously in case of TiO2,36,45 ZnO,46,47 CeO2,48–51 and Li4Ti5O12.38,52 As shown in the low magnification SEM image of MoO2–SRGO-1 (with a MoO2 loading of 27.2 wt%) in Fig. S2a,† MoO2 particles with much less interparticle aggregation were deposited on the surface of SRGO when compared to the deposited particles of the bare MoO2 sample. The high-magnification SEM and TEM images of MoO2–SRGO-1 (Fig. 2a) show that the MoO2 particles with sizes of 10–20 nm were uniformly deposited on the basal plane of SRGO. Clearly, the presence of GO increases the heterogeneous nucleation rate of MoO2 particles near the surface of GO over the homogenous nucleation in the bulk supercritical fluid phase. In the MoO2–SRGO-2 composite (with a MoO2 loading of 37.0 wt%), more complete coverage of the SRGO sheets with the MoO2 particles and a slight increase in particle size to 20–50 nm were observed (Fig. 2b). The EDS mapping of the composite (Fig. S3†) shows the uniform distribution of MoO2 particles on the surface of SRGO sheets. In some areas (as indicated by the arrow in Fig. 2b), the aggregation of growing particles on the SRGO sheets results in larger particles of size ∼30–40 nm. In the MoO2–SRGO-3 composite (with a MoO2 loading of 55.6 wt%), the MoO2 particles are in close contact with neighboring particles (Fig. 2c). As shown in Fig. S2b,† the MoO2–SRGO-3 sample retains the micron-sized, spherically shaped MoO2 particles that are loosely attached to the graphene layer, which probably formed in the bulk supercritical phase by the homogeneous nucleation mechanism. The close contact between MoO2 particles formed on the surface of SRGO and the micron-sized MoO2 particles formed in the supercritical fluid phase would deteriorate cycling stability because of the huge volume change during the insertion/deinsertion of the Li ions, which will be discussed in a later section. In the selected area electron diffraction (SAED) pattern of the MoO2–SRGO samples, shown in the inset of HR-TEM images in Fig. 2; the concentric diffraction rings could be indexed to the (011), (![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 11), (
11), (![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 12), (
12), (![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 02), (
02), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 33), and (033) lattice planes of the monoclinic structure of MoO2.
33), and (033) lattice planes of the monoclinic structure of MoO2.
The crystalline structures of the MoO2–SRGO composites are shown in Fig. 3a. The XRD patterns of the bare MoO2 synthesized in scMeOH, the calcined MoO2 at 600 °C under a flow of 5% H2/Ar (designated as C–MoO2), and SRGO shown in the figures for comparison purpose. The MoO2 phase in the composites and in C–MoO2 exhibit a monoclinic MoO2 phase (PDF#73-1807); the characteristic lattice parameters are listed in Table S1.† The broad peak centered at 24° in the bare MoO2 could originate from MoO3 impurities, which may be caused by the incomplete reduction of the Mo6+ precursor to a Mo4+ phase in scMeOH.16 When the as-synthesized MoO2 was calcined at 600 °C under a flow of 5% H2/Ar, the formation of phase-pure MoO2 is observed (C–MoO2). In the MoO2–SRGO composites, the (011) plane of MoO2 centered at 26° is much broader than that of C–MoO2, which could be because of the overlap with the (002) plane of the SRGO layers. As listed in Table 1, the crystallite sizes in the composite sample, estimated using the Scherrer's equation and the (211) plane, do not change much with the MoO2 loading: 19.07 nm for MoO2–SRGO-1, 19.12 nm for MoO2–SRGO-2, and 19.22 nm for MoO2–SRGO-3. These values are also larger than the crystallite size of bare MoO2 (10.05 nm) synthesized in the absence of GO. The rich number of heterogeneous nucleation sites associated with the oxygen functionalities of GO could enhance the nucleation rates at the basal plane of GO, resulting in the larger crystallite sizes for the composite samples.
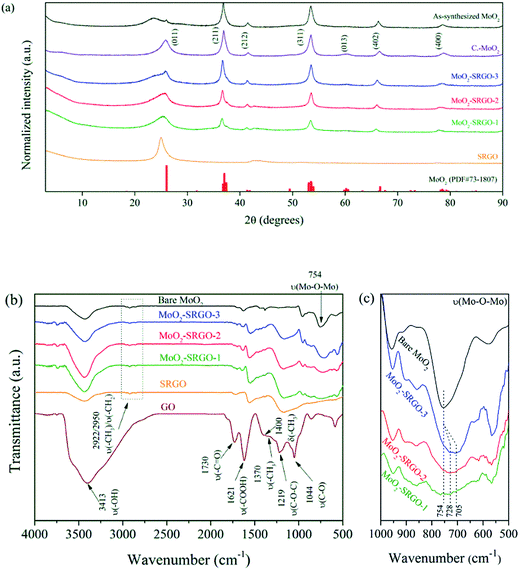 | ||
| Fig. 3 (a) XRD patterns, (b) the overall FT-IR spectra, and (c) the magnified FT-IR spectra for ν(Mo–O–Mo) of the bare MoO2, C–MoO2, GO, SRGO, and the MoO2–SRGO composites. | ||
The FT-IR spectra of GO, SRGO, and the MoO2–SRGO composites are shown in Fig. 3b. In the GO sample, various types of functional groups such as hydroxyl (ν-C–OH, 3413 cm−1), carbonyl (ν-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 1730 cm−1), carboxyl (ν-COOH, 1621 cm−1), methyl (ν-CH3, 1400/1370 cm−1), epoxy (ν-C–O–C, 1219 cm−1), and C–O groups (ν-C–O, 1044 cm−1) are observed, indicating that the graphene sheets were oxidized. After the reduction of GO in scMeOH, most of the oxygen functional groups disappear because of the unique deoxygenating ability of scMeOH.34,35 As in the case of the bare SRGO, the peak intensity of the oxygen functional groups of GO decreases significantly during the MoO2–SRGO composite formation, indicating that scMeOH reduces GO to SRGO and that simultaneously MoO2 particles are deposited on the surface of the SRGO sheets. As shown in Fig. 3c, the Mo–O–Mo stretching of the bare MoO2 sample is observed at 754 cm−1. Some of the Mo–O–Mo stretching band shifts to the lower wavenumber regions of 728 and 705 cm−1 in the MoO2–SRGO composite samples, suggesting a weakening of the Mo–O–Mo bond. A closer inspection of the FT-IR spectra indicates more complete coverage of MoO2 particles on the SRGO surface red-shifts the Mo–O–Mo bond in higher degree. This weakening of the Mo–O–Mo bond can be beneficial for facile Li ion uptake into the MoO2 region through the conversion reaction.53
O, 1730 cm−1), carboxyl (ν-COOH, 1621 cm−1), methyl (ν-CH3, 1400/1370 cm−1), epoxy (ν-C–O–C, 1219 cm−1), and C–O groups (ν-C–O, 1044 cm−1) are observed, indicating that the graphene sheets were oxidized. After the reduction of GO in scMeOH, most of the oxygen functional groups disappear because of the unique deoxygenating ability of scMeOH.34,35 As in the case of the bare SRGO, the peak intensity of the oxygen functional groups of GO decreases significantly during the MoO2–SRGO composite formation, indicating that scMeOH reduces GO to SRGO and that simultaneously MoO2 particles are deposited on the surface of the SRGO sheets. As shown in Fig. 3c, the Mo–O–Mo stretching of the bare MoO2 sample is observed at 754 cm−1. Some of the Mo–O–Mo stretching band shifts to the lower wavenumber regions of 728 and 705 cm−1 in the MoO2–SRGO composite samples, suggesting a weakening of the Mo–O–Mo bond. A closer inspection of the FT-IR spectra indicates more complete coverage of MoO2 particles on the SRGO surface red-shifts the Mo–O–Mo bond in higher degree. This weakening of the Mo–O–Mo bond can be beneficial for facile Li ion uptake into the MoO2 region through the conversion reaction.53
The removal of oxygen functionalities of GO during the one-pot synthesis of the MoO2–SRGO composites was further investigated using XPS. The high resolution C 1s XPS spectra of the GO and MoO2–SRGO-2 are shown in Fig. 4a and b, respectively. The C 1s peaks were deconvoluted into seven types of peaks: sp2 graphitic carbon (284.5 eV), sp3 carbon (285.5 eV), C–OH (hydroxyl group, 286.0 eV), C–O/epoxy (ether and epoxy group, 286.6 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carbonyl group, 287.6 eV), O
O (carbonyl group, 287.6 eV), O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O (carboxyl group, 288.7 eV), and π–π* shake-up (satellite from sp2 graphitic carbon, 289.9 eV).54 The quantification of these peaks is listed in Table S2.† The oxidation of graphite using the modified Hummers method generates peaks associated with the oxygen functionalities (Fig. 4a). The peak area% of the oxygen functionalities in MoO2–SRGO-2 decreases significantly, while that of sp2 graphitic carbon increases, suggesting the restoration of the aromatic structure. A similar trend is observed in the MoO2–SRGO-1 and MoO2–SRGO-3 samples (Fig. S4a and c†). When compared to the bare SRGO, the MoO2–SRGO composite samples have a slightly lower area% of sp2 carbon and higher area% of sp3 carbon, indicating that the simultaneous formation of MoO2 with the GO reduction inhibits the restoration of aromatic structure to some degree. In addition, the area% of π–π* shake-up peak at around 289.9 eV in the MoO2–SRGO-2 is 1.3%, a value that is much lower than that for the bare SRGO (∼10 area%).35 This may imply that during the composite formation in scMeOH, the growing MoO2 phase near the surface of the SRGO effectively retards restacking of the RGOs. The Mo 3d peak of the MoO2–SRGO samples can be deconvoluted into four peaks at the binding energies of 230.0 eV, 232.6 eV, 233.1 eV, and 235.8 eV, which can be attributed to Mo4+ 3d5/2, the Mo4+ 3d3/2, Mo6+ 3d5/2, and Mo6+ 3d3/2, respectively (Fig. 4c). The presence of Mo6+ 3d5/2 and Mo6+ 3d3/2 in the spectrum, which is associated with hexavalent MoO3 phase, may result from the slight surface oxidation of metastable MoO2 when exposed to air.18,21,27
C–O (carboxyl group, 288.7 eV), and π–π* shake-up (satellite from sp2 graphitic carbon, 289.9 eV).54 The quantification of these peaks is listed in Table S2.† The oxidation of graphite using the modified Hummers method generates peaks associated with the oxygen functionalities (Fig. 4a). The peak area% of the oxygen functionalities in MoO2–SRGO-2 decreases significantly, while that of sp2 graphitic carbon increases, suggesting the restoration of the aromatic structure. A similar trend is observed in the MoO2–SRGO-1 and MoO2–SRGO-3 samples (Fig. S4a and c†). When compared to the bare SRGO, the MoO2–SRGO composite samples have a slightly lower area% of sp2 carbon and higher area% of sp3 carbon, indicating that the simultaneous formation of MoO2 with the GO reduction inhibits the restoration of aromatic structure to some degree. In addition, the area% of π–π* shake-up peak at around 289.9 eV in the MoO2–SRGO-2 is 1.3%, a value that is much lower than that for the bare SRGO (∼10 area%).35 This may imply that during the composite formation in scMeOH, the growing MoO2 phase near the surface of the SRGO effectively retards restacking of the RGOs. The Mo 3d peak of the MoO2–SRGO samples can be deconvoluted into four peaks at the binding energies of 230.0 eV, 232.6 eV, 233.1 eV, and 235.8 eV, which can be attributed to Mo4+ 3d5/2, the Mo4+ 3d3/2, Mo6+ 3d5/2, and Mo6+ 3d3/2, respectively (Fig. 4c). The presence of Mo6+ 3d5/2 and Mo6+ 3d3/2 in the spectrum, which is associated with hexavalent MoO3 phase, may result from the slight surface oxidation of metastable MoO2 when exposed to air.18,21,27
 | ||
| Fig. 4 C 1s high-resolution XPS spectra and peak deconvolution results of (a) GO, (b) SRGO, and (c) the Mo 3d high-resolution XPS spectra of MoO2–SRGO-2. | ||
The MoO2 loading and the oxidation stability of the MoO2–SRGO composites are examined using TGA under a flow of air, and the results are shown in Fig. 5. All the samples show marginal mass loss at temperatures below 200 °C in the TGA profiles, indicating that mass loss from vaporization of adsorbed water or other volatile species is negligible. The bare MoO2 has a weight gain of 5.6 wt%, which is caused by the oxidation of MoO2 to a MoO3 phase. The theoretical weight gain of phase-pure MoO2 without any organic moieties in the particle is approximately 12 wt%.9 The much smaller weight gain observed in the bare MoO2 particles prepared in scMeOH could be caused by weight loss from the oxidation of organic moieties attached on the MoO2 surface and the presence of a MoO3 phase in the sample, as discussed in previous section. The weight of the SRGO approached zero at temperatures above 600 °C, indicating almost complete combustion of graphene sheets to CO or CO2 under a flow of air. All the composite samples have different weight loss values depending on the Mo(acac)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GO ratio. By considering the weight gain of MoO2 during the oxidative TGA measurements, the MoO2 loading in the composites is estimated to be 27.2 wt% (MoO2–SRGO-1), 37.0 wt% (MoO2–SRGO-2), and 55.6 wt% (MoO2–SRGO-3) under the assumption that each sample has same amount of MoO3 and organic moieties on the surface of the MoO2 particles. The MoO2 loading in the composites is also estimated using the atomic concentration of molybdenum and carbon in the survey scans of XPS (see Fig. S5†); the MoO2 loadings are calculated to be 24.4 wt% (MoO2–SRGO-1), 32.8 wt% (MoO2–SRGO-2), and 53.1 wt% (MoO2–SRGO-3), which are quite comparable to the MoO2 loading values estimated using TGA. The differential thermogravimetric (DTG) profiles of the composite samples shows that the main oxidative decomposition temperatures of SRGO in the composites are lower (485–500 °C) than that of bare SRGO (560 °C), indicating a decrease in oxidation stability in the composite samples (Fig. 5b). Previous work has shown that the oxidative decomposition of carbon increases when metal oxide nanoparticles are tightly bound to the carbon support because of the catalytic effect of the metal oxide nanoparticles.55–58 Thus, the decrease in the oxidation stability of the MoO2–SRGO composites compared to the bare SRGO implies that the MoO2 particles are tightly anchored to the basal plane of SRGO, enhancing the oxidative decomposition of SRGO. The fine contact between the MoO2 particles and graphene sheets can effectively facilitate the electron transfer to the MoO2 phase during the charge and discharge process.
GO ratio. By considering the weight gain of MoO2 during the oxidative TGA measurements, the MoO2 loading in the composites is estimated to be 27.2 wt% (MoO2–SRGO-1), 37.0 wt% (MoO2–SRGO-2), and 55.6 wt% (MoO2–SRGO-3) under the assumption that each sample has same amount of MoO3 and organic moieties on the surface of the MoO2 particles. The MoO2 loading in the composites is also estimated using the atomic concentration of molybdenum and carbon in the survey scans of XPS (see Fig. S5†); the MoO2 loadings are calculated to be 24.4 wt% (MoO2–SRGO-1), 32.8 wt% (MoO2–SRGO-2), and 53.1 wt% (MoO2–SRGO-3), which are quite comparable to the MoO2 loading values estimated using TGA. The differential thermogravimetric (DTG) profiles of the composite samples shows that the main oxidative decomposition temperatures of SRGO in the composites are lower (485–500 °C) than that of bare SRGO (560 °C), indicating a decrease in oxidation stability in the composite samples (Fig. 5b). Previous work has shown that the oxidative decomposition of carbon increases when metal oxide nanoparticles are tightly bound to the carbon support because of the catalytic effect of the metal oxide nanoparticles.55–58 Thus, the decrease in the oxidation stability of the MoO2–SRGO composites compared to the bare SRGO implies that the MoO2 particles are tightly anchored to the basal plane of SRGO, enhancing the oxidative decomposition of SRGO. The fine contact between the MoO2 particles and graphene sheets can effectively facilitate the electron transfer to the MoO2 phase during the charge and discharge process.
To investigate the textural properties of the MoO2–SRGO composites, nitrogen adsorption–desorption isotherms of each sample are obtained, as shown in Fig. S6.† All the isotherms show distinct hysteresis between adsorption and desorption at a P/P0 of 0.5–1.0, indicating the presence of a mesoporous structure. The pore size distributions were characterized using the Barrette–Joynere–Halenda (BJH) method, and the results are shown in the inset of the figures. The composites samples have a wide distribution of pore sizes in the range of 2–100 nm with average pore size diameters of 11–13 nm. The unique porous structure can be beneficial for the facile penetration of the electrolyte, for good accessibility of lithium ions to the active sites, and for buffering of the volume expansion during the conversion process.
3.2. Electrochemical properties
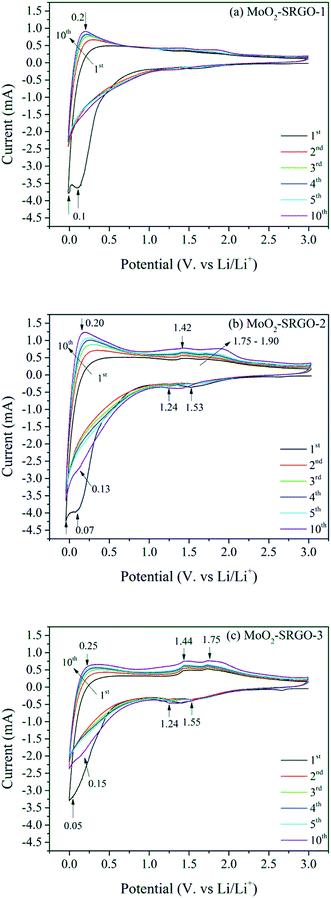
The electrochemical properties of the MoO2–SRGO composites were examined using the cyclic voltammetry (CV) and galvanostatic charge and discharge cycling performance. Fig. 6 shows CV curves of the MoO2–SRGO composites measured at a scan rate 1.0 mV s−1 in the voltage range of 0.001–3.0 V. At the high MoO2 loading of 55.6 wt% (MoO2–SRGO-3, Fig. 6c), two redox couples at 1.24/1.44 V and 1.55/1.75 V became more prominent when compared to the MoO2–SRGO composites with the lower MoO2 loadings. The highly reversible reduction peaks at 1.55 V and 1.24 V during the cathodic (or Li insertion) scan is related to the phase transition of partially lithiated LixMoO2 from monoclinic to orthorhombic and then from orthorhombic back to monoclinic, respectively.9,11 After the 10th cycle, a broad peak at around 0.15 V becomes more prominent during the cathodic scan, suggesting the transformation of MoO2 to Mo and Li2O by the conversion reaction mechanism described above.14 An additional irreversible peak at below 0.05 V during the first cathodic scan is associated with the formation of a solid electrolyte interface (SEI) caused by the decomposition of electrolytes15,59 and irreversible Li-ion attachment to the residual oxygen functionalities.60,61 When the MoO2 loading decreases to 37.0 wt% (MoO2–SRGO-2, Fig. 6b), the two redox couple peaks associated with the phase transition of partially lithiated LixMoO2 are less distinct, and the peak associated with SEI formation becomes more prominent when compared to MoO2–SRGO-3. Additionally, MoO2–SRGO-2 exhibits a clear cathodic peak near 0 V and an anodic peak at 0.2 V, which can be ascribed to the Li ion insertion/deinsertion in the graphene layer.62 At the low MoO2 loading of 27.2 wt% (MoO2–SRGO-1, Fig. 6a), the two redox couple peaks of partially lithiated LixMoO2 and the deep conversion peak are almost invisible, suggesting the MoO2 contribution to the electrochemical reaction is much less significant.Fig. 7a–d shows galvanostatic charge–discharge profiles and cycling retention for up to 50 cycles of the MoO2–SRGO composites at a current density 50 mA g−1 in the potential window of 0.005–3.0 V. The capacity values in the composite are calculated based on the total mass of MoO2 and SRGO. The charge–discharge profiles of C–MoO2, shown in Fig. S7,† indicate the presence of two voltage plateaus at 1.6 and 1.3 V during the initial discharge process, and at 1.4 and 1.7 V during the initial charge process. These plateaus correspond to the two redox couples observed in the CV profiles. The long voltage tail at below 0.5 V in the discharge process can be ascribed to the deep conversion reaction of partially lithiated LixMoO2.14 The MoO2–SRGO composites do not have clear voltage plateaus associated with the MoO2 phase possibly because of the contribution of the carbon phase. A similar trend is observed in MoO2-ordered mesoporous carbon with a carbon content of 37 wt%.19 The cycling performance of the C–MoO2 sample and the MoO2–SRGO composites for up to 50 cycles is shown in Fig. 7d. The initial discharge and charge capacities of C–MoO2 are 904 and 635 mA h g−1, respectively, with a coulombic efficiency of 70%. The initial capacity loss could be caused by the formation of an SEI layer, the decomposition of electrolytes, and/or the irreversible trapping of some Li ion in the MoO2 matrix.19,63 During the initial 15 cycles, the discharge capacity increases to 1094 mA h g−1 potentially because of the change in the MoO2 lithiation mechanism from intercalation of Li ion to the MoO2 phase (xLi + xe− + MoO2 ↔ LixMoO2, 0 < x < 0.98) to conversion of Li0.98MoO2 to Mo and Li2O (Li0.98MoO2 + 3.02Li+ + 3.02e− → Mo + 2Li2O).9,19,64 In the following 35 cycles, the discharge capacity decreases significantly to 547 mA h g−1 probably because of cell disintegration caused by the volume change. In contrast, the MoO2–SRGO composites show much better cycling stability. The MoO2–SRGO-2 sample has an initial discharge capacity of 1601 mA h g−1 and a charge capacity of 956 mA h g−1 with a coulombic efficiency of 60%. This value is lower than that of C–MoO2 possibly because of additional irreversible Li ion trapping in the SRGO matrix.44 The discharge capacity decreases to ∼900 mA h g−1 after the first five cycles and then stabilizes at ∼800 mA h g−1 for the remaining fifty cycles. The coulombic efficiency reached 94% after the third cycle and then over 97% for the next fifty cycles, indicating that the MoO2–SRGO-2 has a high reversible capacity with a good electrochemical stability. Similar cycling behavior is observed in the MoO2–SRGO-3 sample with high MoO2 loading under low current density. The MoO2–SRGO-1 sample shows much lower capacity (512 mA h g−1) than that of the MoO2–SRGO composites with higher MoO2 loadings because of the increased contribution of SRGO (with a low reversible capacity of ∼340 mA h g−1 as previously reported44) to the total capacity of the composite.
The rate performance of the MoO2–SRGO composites is evaluated under various current densities of 0.05–50 A g−1, and the results are shown in Fig. 7e. At the low current density regime of 0.1–1.0 A g−1, the capacities of the composites decrease in the order MoO2–SRGO-2 > MoO2–SRGO-3 > MoO2–SRGO-1. When the current density increases to 2.5 A g−1, the capacities of the composite with the highest MoO2 loading decreases more drastically, and thus the composites with the low-to-medium MoO2 loading have higher capacities than MoO2–SRGO-3; for example, at 2.5 A g−1, the order of capacities changes to MoO2–SRGO-2 (205 mA h g−1) > MoO2–SRGO-1 (164 mA h g−1) > MoO2–SRGO-3 (131 mA h g−1). When compared to the C–MoO2 results (Fig. S8,† for example 93 mA h g−1 at 1.67 A g−1), the MoO2–SRGO composites show exceptionally improved electrochemical performance. Under the ultrafast current density of 10–50 A g−1 (charge–discharge cycles of a few seconds), the capacities of the composites with the low-to-medium MoO2 loading are less than 30 mA h g−1, but when the current density is lowered to 0.05 A g−1 after 100 cycles, the composite samples recover most of their initial capacities with marginal capacity loss (3% loss in case of MoO2–SRGO-1; 7% loss in case of MoO2–SRGO-2). From these results, the excellent cell integrity of the composite electrode is maintained even after enduring ultrafast charge–discharge cycles. Although MoO2–SRGO-3 has a larger capacity than MoO2–SRGO-1 at the initial low current densities of 0.05–0.5 A g−1, its capacities quickly degrade at higher current densities of 1–2.5 A g−1, and the initial high capacity are not completely recovered after 100 cycles (24% loss). In addition, the capacities MoO2–SRGO-3 at 0.05 A g−1 continuously decrease from 663 to 561 mA h g−1 when the number of cycles increases from 100 to 130. This poor cycling performance is due to the severe volume change, which may be caused by the aggregation of the MoO2 particles in close contact in the high MoO2 loading sample.
To understand the enhanced electrochemical performance of the MoO2–SRGO composites, the EIS tests of the C–MoO2 and MoO2–SRGO-2 were carried out under different cycling conditions; the data was collected prior to cycling, after the first charge–discharge cycle at 3.0 V, and after 100 cycles at a current density of 50 mA g−1 and 3.0 V. The results in Fig. 8a show that prior to cycling, the Nyquist plots of the samples before cycling can be divided into two parts: a semicircle in the high-to-middle frequency region (which corresponds to a charge transfer process) and an oblique straight line in the low frequency region (which is related to the Li ion diffusion within the electrode). The Nyquist plot data were fitted using an equivalent circuit model with electrolyte resistance (Re), charge transfer resistance (Rct), and Li ion diffusion into the active material phase (Zw) as the parameters,65 with the fitting results shown in Table 2. Prior to cycling, Re values of C–MoO2 and MoO2–SRGO-2 are very similar. The semicircle for the MoO2–SRGO-2 composite electrode is larger than that of the C–MoO2 electrode, suggesting that the Rct of MoO2–SRGO-2 (279.94 Ω) is higher than that of MoO2 (106.54 Ω). On the other hand, the slope of the line for the MoO2–SRGO-2 electrode is steeper than that of C–MoO2, indicating that the MoO2–SRGO-2 has a higher rate of Li ion diffusion into the active materials, confirming that SRGO provides a more conductive pathway for fast Li ion transport than C–MoO2. After the first charge–discharge cycle, the Nyquist plots are divided in three parts, two semicircles and one straight line (Fig. 8b). SEI layer formation on the electrode surface after the first charge–discharge cycle contributes to an additional semicircle located in the higher-frequency region. The data were fitted using four parameters, Re, Rct, SEI resistance (Rf), and Zw with the results shown in Table 2. As can be seen in Fig. 7 and S7,† MoO2–SRGO-2 exhibits a larger degree of initial capacity loss when compared to C–MoO2, indicating that presence of SRGO enhances SEI formation possibly because of residual oxygen functionalities and defects in the SRGO.44 Consequently, the Rf of MoO2–SRGO-2 electrode (118.98 Ω) is larger than that of C–MoO2 (59.02 Ω). After a hundred cycles, the resistance values are significantly different from the initial values and the values after the 1st cycle; as listed in Table 2, Rct and Rf of the MoO2–SRGO-2 electrode are an order of magnitude lower than those of C–MoO2, indicating that SRGO can improve charge transfer kinetics to the surface of the composite electrode and retard the formation of the SEI layer upon cycling. In addition, the slope of the line for the MoO2–SRGO-2 electrode is much steeper than that of C–MoO2, suggesting a larger Li ion diffusion coefficient in the MoO2–SRGO-2 composite.
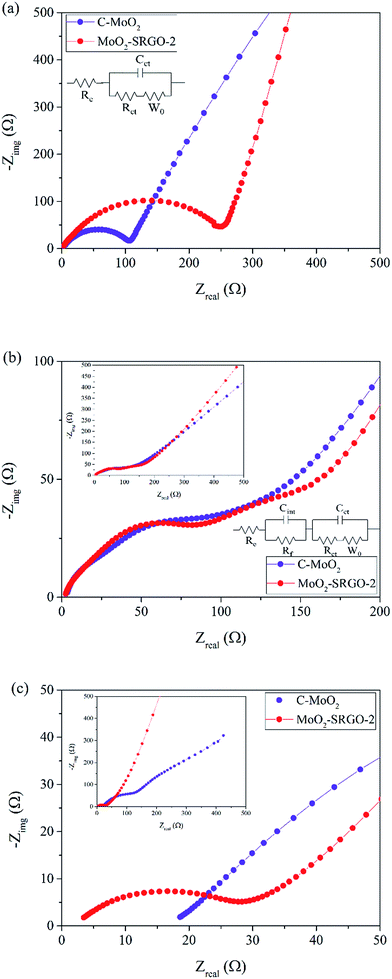 | ||
| Fig. 8 Nyquist plots of C–MoO2 and MoO2–SRGO-2 (a) prior to cycling, (b) after the 1st cycle, and (c) after 100 cycles at 50 mA g−1. | ||
| Sample | Rea (Ω) | Rfb (Ω) | Rctc (Ω) | |
|---|---|---|---|---|
| a Re: electrolyte resistance.b Rf: SEI resistance.c Rct: charge-transfer resistance. | ||||
| Prior to cycling | C–MoO2 | 2.87 | — | 106.54 |
| MoO2–SRGO-2 | 1.58 | — | 279.94 | |
| After the 1st cycles | C–MoO2 | 3.54 | 59.02 | 119.49 |
| MoO2–SRGO-2 | 2.51 | 118.98 | 109.62 | |
| After 100 cycles | C–MoO2 | 18.57 | 169.94 | 172.78 |
| MoO2–SRGO-2 | 3.47 | 26.26 | 66.97 | |
The morphology of the MoO2–SRGO-2 and the C–MoO2 electrodes before cycling and after 100 cycles was investigated to gain further insight into the enhanced electrochemical performance of the composite sample, as shown in Fig. S9.† Before cycling, well-dispersed MoO2–SRGO powders and micron-sized hierarchical spheres are observed in the MoO2–SRGO-2 and the C–MoO2 samples, respectively (Fig. S9a and d†). After 100 cycles, even the surface of the MoO2–SRGO-2 electrode is covered with the SEI layer, small distinct particles still remain with expanded size because of the tight anchoring of MoO2 particles on the RGO sheet (Fig. S9b and c†). However, heavily agglomerated particles are observed in the cycled C–MoO2 sample due to the large volume expansion during the conversion reaction (Fig. S9e†). The change in XRD patterns of MoO2–SRGO-2 before and after cycling is shown in Fig. S10.† The peaks associated with MoO2 are maintained after the 100 cycles even though the peaks become broad. The lattice constants of the MoO2 particles in the composite electrode before cycling are estimated to be a = 5.5133 Å, b = 4.5449 Å, c = 5.5809 Å and the angle between a and c lattices in the monoclinic structure is β = 120.31°. After 100 cycles, the lattice constants and the angle in MoO2–SRGO-2 become slightly larger (a = 5.6579 Å, b = 4.8261 Å, c = 5.6478 Å, β = 122.17°). Only 8.12% of MoO2 unit cell volume is expanded (from 0.12073 to 0.13054 nm3) after the 100 cycles. The SEM and XRD data suggests that the MoO2 particles on the surface of SRGO can maintain the structural integrity during cycling, which results in the excellent long-term stability.
According to the results discussed thus far, the improved discharge capacity, high-rate performance and cycling stability of the MoO2–SRGO composites compared with those of bare MoO2 particles can be attributed to the unique nano-to-mesoscale architecture of the composites. During the composite formation in scMeOH, the enhanced heterogeneous nucleation on the basal plane of SRGO over homogeneous counterpart can tightly anchor the nano-sized MoO2 particles on the carbon matrix. The smaller sized MoO2 particles deposited on the SRGO can shorten the transport length of Li ion diffusion into the MoO2 particles, and the highly conductive SRGO network can facilitate electron transport from the current collector to the active material phase, leading to faster Li ion uptake. Furthermore, the mesoporous structure of the MoO2–SRGO composites can allow sufficient penetration of electrolytes and enlarges the electrolyte–electrode interface, which can increase the rate of charge transfer. In addition, SRGO can provide a robust network to accommodate the volume expansion during Li insertion/deinsertion, which can maintain the high cycling stability.62,66,67 Therefore, the one-pot, supercritical route is a very promising approach for the synthesis of MoO2-reduced graphene oxide composites with enhanced electrochemical performance.
4. Conclusion
In summary, we developed a simple and fast, one-pot supercritical methanol route for the deposition of MoO2 nanoparticles on the RGO surface. The unique deoxygenation and particle formation properties of scMeOH reduces of GO to RGO and simultaneously tightly anchors MoO2 particles with sizes of 10–20 nm to the RGO surface. The electrochemical performance of the MoO2–SRGO composites is highly dependent on the MoO2 loading. With a MoO2 loading of 37.0 wt%, the MoO2–SRGO composites deliver a high reversible discharge capacity (793 mA h g−1 after the 50th cycle at 50 mA g−1), good cyclability/cell integrity, and excellent rate performance (205 mA g−1 at a high current density of 2.5 A g−1) when compared to the calcined MoO2 without the graphene layer. This study demonstrates that the simple scMeOH route can open new possibilities for synthesizing MoO2–graphene composites with enhanced electrochemical performance for use in lithium ion batteries.Acknowledgements
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (No. 2016R1A2B3008800). Additional support from the Human Resources Development program (No. 20124010203270) in the form of a grant from the Korea Institute of Energy Technology Evaluation and Planning (KETEP) funded by the Korean government Ministry of Trade, Industry, and Energy and by the Korea Institute of Science and Technology (KIST) Institutional Program (Project No. 2E26330/2E26292) is also appreciated.References
- Y. Yan, H. Xu, W. Guo, Q. Huang, M. Zheng, H. Pang and H. Xue, Inorg. Chem. Front., 2016, 3, 791–797 RSC.
- Y. Yan, B. Li, W. Guo, H. Pang and H. Xue, J. Power Sources, 2016, 329, 148–169 CrossRef CAS.
- Y. Yan, T. Wang, X. Li, H. Pang and H. Xue, Inorg. Chem. Front., 2016 10.1039/c6qi00199h.
- B. Li, M. Zheng, H. Xue and H. Pang, Inorg. Chem. Front., 2016, 3, 175–202 RSC.
- L. Lu, X. Han, J. Li, J. Hua and M. Ouyang, J. Power Sources, 2013, 226, 272–288 CrossRef CAS.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- Y. Shi, B. Guo, S. A. Corr, Q. Shi, Y.-S. Hu, K. R. Heier, L. Chen, R. Seshadri and G. D. Stucky, Nano Lett., 2009, 9, 4215–4220 CrossRef CAS PubMed.
- Y. Sun, X. Hu, W. Luo and Y. Huang, ACS Nano, 2011, 5, 7100–7107 CrossRef CAS PubMed.
- H. J. Zhang, J. Shu, K. X. Wang, X. T. Chen, Y. M. Jiang, X. Wei and J. S. Chen, J. Mater. Chem. A, 2014, 2, 80–86 CAS.
- W. Tang, C. X. Peng, C. T. Nai, J. Su, Y. P. Liu, M. Reddy, M. Lin and K. P. Loh, Small, 2015, 11, 2446–2453 CrossRef CAS PubMed.
- J. Dahn and W. McKinnon, Solid State Ionics, 1987, 23, 1–7 CrossRef CAS.
- J. Auborn and Y. Barberio, J. Electrochem. Soc., 1987, 134, 638–640 CrossRef CAS.
- Y. Liu, H. Zhang, P. Ouyang and Z. Li, Electrochim. Acta, 2013, 102, 429–435 CrossRef CAS.
- B. Guo, X. Fang, B. Li, Y. Shi, C. Ouyang, Y.-S. Hu, Z. Wang, G. D. Stucky and L. Chen, Chem. Mater., 2012, 24, 457–463 CrossRef CAS.
- X. Y. Zhao, M. H. Cao, B. Liu, Y. Tian and C. W. Hu, J. Mater. Chem., 2012, 22, 13334–13340 RSC.
- J. Hwang, D. Min, D. Yoon, W. Chang and J. Kim, Chem.–Eng. J., 2016, 290, 335–345 CrossRef CAS.
- L. Zhou, H. B. Wu, Z. Wang and X. W. Lou, ACS Appl. Mater. Interfaces, 2011, 3, 4853–4857 CAS.
- A. Bhaskar, M. Deepa and T. N. Rao, ACS Appl. Mater. Interfaces, 2013, 5, 2555–2566 CAS.
- L. Zeng, C. Zheng, C. Deng, X. Ding and M. Wei, ACS Appl. Mater. Interfaces, 2013, 5, 2182–2187 CAS.
- J. Ni, Y. Zhao, L. Li and L. Mai, Nano Energy, 2015, 11, 129–135 CrossRef CAS.
- F. F. Xia, X. L. Hu, Y. M. Sun, W. Luo and Y. H. Huang, Nanoscale, 2012, 4, 4707–4711 RSC.
- Q. Yang, Q. Liang, J. Liu, S. Liang, S. Tang, P. Lu and Y. Lu, Mater. Lett., 2014, 127, 32–35 CrossRef CAS.
- L. Guo and Y. Wang, J. Mater. Chem. A, 2015, 3, 4706–4715 CAS.
- K. H. Seng, G. D. Du, L. Li, Z. X. Chen, H. K. Liu and Z. P. Guo, J. Mater. Chem., 2012, 22, 16072–16077 RSC.
- Z. X. Huang, Y. Wang, Y. G. Zhu, Y. Shi, J. I. Wong and H. Y. Yang, Nanoscale, 2014, 6, 9839–9845 RSC.
- Q. W. Tang, Z. Q. Shan, L. Wang and X. Qin, Electrochim. Acta, 2012, 79, 148–153 CrossRef CAS.
- A. Bhaskar, M. Deepa, T. N. Rao and U. V. Varadaraju, J. Power Sources, 2012, 216, 169–178 CrossRef CAS.
- Y. Xu, R. Yi, B. Yuan, X. F. Wu, M. Dunwell, Q. L. Lin, L. Fei, S. G. Deng, P. Andersen, D. H. Wang and H. M. Luo, J. Phys. Chem. Lett., 2012, 3, 309–314 CrossRef CAS PubMed.
- P. X. Han, W. Ma, S. P. Pang, Q. S. Kong, J. H. Yao, C. F. Bi and G. L. Cui, J. Mater. Chem. A, 2013, 1, 5949–5954 CAS.
- K. Palanisamy, Y. Kim, H. Kim, J. M. Kim and W.-S. Yoon, J. Power Sources, 2015, 275, 351–361 CrossRef CAS.
- Z.-S. Wu, G. Zhou, L.-C. Yin, W. Ren, F. Li and H.-M. Cheng, Nano Energy, 2012, 1, 107–131 CrossRef CAS.
- Á. Caballero and J. Morales, Nanoscale, 2012, 4, 2083–2092 RSC.
- C. Xu, B. Xu, Y. Gu, Z. Xiong, J. Sun and X. S. Zhao, Energy Environ. Sci., 2013, 6, 1388–1414 CAS.
- E. B. Nursanto, A. Nugroho, S. A. Hong, S. J. Kim, K. Y. Chung and J. Kim, Green Chem., 2011, 13, 2714–2718 RSC.
- M. Seo, D. Yoon, K. S. Hwang, J. W. Kang and J. Kim, Carbon, 2013, 64, 207–218 CrossRef CAS.
- A. D. C. Permana, A. Nugroho, K. Y. Chung, W. Chang and J. Kim, Chem. Eng. J., 2014, 241, 216–227 CrossRef CAS.
- A. Nugroho, D. Yoon, O.-S. Joo, K. Y. Chung and J. Kim, Chem. Eng. J., 2014, 258, 357–366 CrossRef CAS.
- A. Nugroho, S. J. Kim, W. Chang, K. Y. Chung and J. Kim, J. Power Sources, 2013, 244, 164–169 CrossRef CAS.
- A. Nugroho, W. Chang, S. Jin Kim, K. Yoon Chung and J. Kim, RSC Adv., 2012, 2, 10805–10808 RSC.
- J. Kim, S.-A. Hong and J. Yoo, Chem. Eng. J., 2015, 266, 179–188 CrossRef CAS.
- W. Li, J. Hwang, W. Chang, H. Setiadi, K. Y. Chung and J. Kim, J. Supercrit. Fluids, 2016, 116, 164–171 CrossRef CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- N. I. Kovtyukhova, P. J. Ollivier, B. R. Martin, T. E. Mallouk, S. A. Chizhik, E. V. Buzaneva and A. D. Gorchinskiy, Chem. Mater., 1999, 11, 771–778 CrossRef CAS.
- D. Yoon, K. Y. Chung, W. Chang, S. M. Kim, M. J. Lee, Z. Lee and J. Kim, Chem. Mater., 2015, 27, 266–275 CrossRef CAS.
- P. Wang, K. Ueno, H. Takigawa and K. Kobiro, J. Supercrit. Fluids, 2013, 78, 124–131 CrossRef CAS.
- N. S. Han, H. S. Shim, J. H. Seo, S. M. Park, B. K. Min, J. Kim and J. K. Song, Chem. Phys. Lett., 2011, 505, 51–56 CrossRef CAS.
- B. Veriansyah, J.-D. Kim, B. K. Min, Y. H. Shin, Y.-W. Lee and J. Kim, J. Supercrit. Fluids, 2010, 52, 76–83 CrossRef CAS.
- J. Kim, Y.-S. Park, B. Veriansyah, J.-D. Kim and Y.-W. Lee, Chem. Mater., 2008, 20, 6301–6303 CrossRef CAS.
- B. Veriansyah, H. Park, J.-D. Kim, B. K. Min, Y. H. Shin, Y.-W. Lee and J. Kim, J. Supercrit. Fluids, 2009, 50, 283–291 CrossRef CAS.
- C. D. Slostowski, S. Marre, O. Babot, T. Toupance and C. Aymonier, Langmuir, 2012, 28, 16656–16663 CrossRef CAS PubMed.
- C. Slostowski, S. Marre, O. Babot, T. Toupance and C. Aymonier, Langmuir, 2014, 30, 5965–5972 CrossRef CAS PubMed.
- A. Nugroho, K. Y. Chung and J. Kim, J. Phys. Chem. C, 2013, 118, 183–193 Search PubMed.
- G. Zhou, D.-W. Wang, L.-C. Yin, N. Li, F. Li and H.-M. Cheng, ACS Nano, 2012, 6, 3214–3223 CrossRef CAS PubMed.
- J. I. Paredes, S. Villar-Rodil, P. Solís-Fernández, A. Martínez-Alonso and J. M. D. Tascón, Langmuir, 2009, 25, 5957–5968 CrossRef CAS PubMed.
- B. Dernaika and D. Uner, Appl. Catal., B, 2003, 40, 219–229 CrossRef CAS.
- G. Neri, L. Bonaccorsi, A. Donato, C. Milone, M. G. Musolino and A. M. Visco, Appl. Catal., B, 1997, 11, 217–231 CrossRef CAS.
- J. van Doorn, J. Varloud, P. Mériaudeau, V. Perrichon, M. Chevrier and C. Gauthier, Appl. Catal., B, 1992, 1, 117–127 CrossRef CAS.
- H. Liu, K.-L. Choy and M. Roe, Nanoscale, 2013, 5, 5725–5731 RSC.
- X. Zhang, X. Song, S. Gao, Y. Xu, X. Cheng, H. Zhao and L. Huo, J. Mater. Chem. A, 2013, 1, 6858–6864 CAS.
- M. Pumera, A. Ambrosi and E. L. K. Chng, Chem. Sci., 2012, 3, 3347–3355 RSC.
- S.-L. Kuo, W.-R. Liu, C.-P. Kuo, N.-L. Wu and H.-C. Wu, J. Power Sources, 2013, 244, 552–556 CrossRef CAS.
- W. Li, D. Yoon, J. Hwang, W. Chang and J. Kim, J. Power Sources, 2015, 293, 1024–1031 CrossRef CAS.
- X. Zhao, M. Cao, B. Liu, Y. Tian and C. Hu, J. Mater. Chem., 2012, 22, 13334–13340 RSC.
- H. Gao, C.-L. Liu, Y. Liu, Z.-H. Liu and W.-S. Dong, Mater. Chem. Phys., 2014, 147, 218–224 CrossRef CAS.
- M. E. Orazem and B. Tribollet, Electrochemical impedance spectroscopy, John Wiley & Sons, 2011 Search PubMed.
- S.-M. Paek, E. Yoo and I. Honma, Nano Lett., 2009, 9, 72–75 CrossRef CAS PubMed.
- X. Zhou, Y.-X. Yin, L.-J. Wan and Y.-G. Guo, Adv. Energy Mater., 2012, 2, 1086–1090 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24632j |
| This journal is © The Royal Society of Chemistry 2016 |