Synthesis of TiO2@C nanospheres with excellent electrochemical properties
Abstract
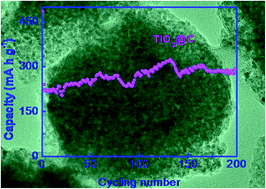
TiO2@C nanospheres are synthesized through annealing a mixture of tetrabutyl titanate and oleic acid, as the titanium and carbon source, respectively. The as-synthesized TiO2@C with higher carbon content presents a superior specific capacity (286.5 mA h g−1 at 100 mA g−1 after 200 cycles) and higher rate performance (148 mA h g−1 at 500 mA g−1), when tested as the anode material for lithium ion batteries. The as-synthesized TiO2@C nanospheres are promising anode materials for next-generation LIBs.

 Please wait while we load your content...
Please wait while we load your content...