Adsorption and decarbonylation of furfural over H-ZSM-5 zeolite: a DFT study†
Abstract
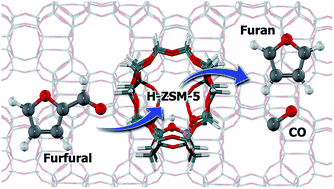
The conversion of low cost biomass and its derivatives is receiving considerable and growing attention as an alternative feedstock for fuel and chemicals production. Herein, the furfural adsorption and decarbonylation to furan on H-ZSM-5 zeolite are studied by density functional theory calculations. The furfural interacts with the zeolite active site through its three sites of methine carbon atoms, ring's oxygen and carbonyl group with the adsorption Gibbs free energies of −5.5, −8.3 and −15.7 kcal mol−1, respectively. Three pathways are considered for the decarbonylation of furfural. In the first pathway, the reaction starts with the protonation of a alpha-carbon leading to the arenium-ion like intermediate. This intermediate then eliminated the CO group to form the furan product. The earlier step is considered to be the rate-determining step of this pathway with an activation energy of 22.1 kcal mol−1. In the second pathway, it commences by protonation of furfural at the beta-carbon to produce the arenium-ion like intermediate. This intermediate then undergoes a 1,2-hydride shift and is subsequently decarbonated to eliminate the CO molecule and form the furan product. The first step of the furfural protonation has the highest activation energy, 27.7 kcal mol−1, and is therefore rate-determining. For the last path, the protonation at the furfural carbonyl group to the surface hydroxyalkyl species formation is the first step of this pathway. The reaction then proceeds through the migration of H to form the tertiary carbocation intermediates followed by the 1,2-hydride shift to form the secondary carbocation species. Finally the CO molecule is eliminated to generate the furan product. The rate-determining step of this pathway is the second step of the H migration with the activation energy of 45.8 kcal mol−1. Since the first pathway requires a lower rate-determining step activation barrier compared with the second and third pathways, the first pathway is therefore preferred for the furfural decarbonylation on H-ZSM-5 zeolite. The effect of the zeolite framework is also highlighted to greater stability of the intermediates and also transition state complexes.

 Please wait while we load your content...
Please wait while we load your content...