Largely-increased length of silver nanowires by controlled oxidative etching processes in solvothermal reaction and the application in highly transparent and conductive networks†
Abstract
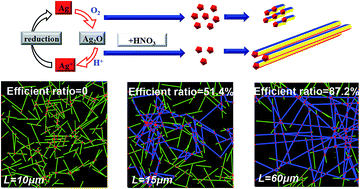
To increase the aspect ratio of metal nanowires and meanwhile understand its effect on the conducting behavior of transparent and conductive films is important for the optoelectronic application of these nanowires. Here a strategy is disclosed to control nanowire length and hence the aspect ratio by importing HNO3 into reactions. With a small amount of HNO3 added, the length of silver nanowires was increased by 50 fold, from several micrometers to hundreds of micrometers, and the average aspect ratio reached 821. Carefully designed experiments show that the improved nanowire length is due to oxidative behavior between HNO3 and O2, which screens nuclei in the solution and thus only a small part of them could grow into longer nanowires. Transparent & conductive films were fabricated using these long nanowires. Longer nanowires not only showed better transparency (+10%), reaching 92.1% (transmittance@550 nm) and 12.6 ohm sq−1 (sheet resistance), but also presented better stability against heating, even in open air. An inert gas environment like N2 is preferred for the heat treatment of silver nanowires. Atom re-arrangement was observed due to Rayleigh instability. Moreover, Monte Carlo based simulation is performed on networks that consisted of randomly arranged silver nanowires. Efficient ratio of nanowire length that contributes to conduction is simulated in cases of varied nanowire length, and the effect of the efficient ratio on electrical conductivity of the network is studied. At similar transparency, longer nanowires could help to form more conductive routes in the networks, which is beneficial to the conductivity of the network.

 Please wait while we load your content...
Please wait while we load your content...