Structure engineering of a core/shell Si@Ta3N5 heterojunction nanowires array for photoelectrochemical water oxidation†
Abstract
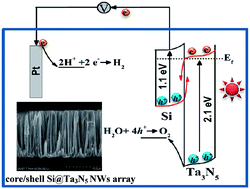
Structure-tuned core/shell n-Si@n-Ta3N5 nanowires array heterojunction photoanodes were prepared on a wafer scale by electroless metal-assisted etching of an Si wafer, spin coating of a Ta(OC2H5)5 sol precursor solution, and ammonia treatment to convert it to Ta3N5. The length of the Si NWs and the thickness of the Ta3N5 shell were critical variables to control the high photoelectrochemical water splitting performance of the photoanode. The photocurrent density of the optimum core/shell Si@Ta3N5 nanowires array was 2.5 times higher than that of the planar Si@Ta3N5 composite at 1.23 VRHE under 1 sun illumination. The Ta3N5 nanoshell served as a protection layer to significantly improve the chemical stability of the Si photoelectrode, and acted as a component to form a heterojunction with Si to promote the transport and separation of photoexcited charge carriers.

 Please wait while we load your content...
Please wait while we load your content...