Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Autonomous movement in mixed metal based soft-oxometalates induced by CO2 evolution and topological effects on their propulsion†
Apabrita Mallick and
Soumyajit Roy *
*
EFAML, Materials Science Centre, Department of Chemistry, Indian Institute of Science Education and Research, Kolkata-741246, India. E-mail: s.roy@iiserkol.ac.in
First published on 21st November 2016
Abstract
In current nanoscience, the synthesis of autonomously moving nanomotors proves to be an immediate challenge. In this work we have reported the synthesis of soft-oxometalate (SOM) based nanomotors comprising vanadium and molybdenum oxoanions which show autonomous movement in response to a chemical fuel like that of an aqueous solution of sodium bicarbonate. CO2 produced from bicarbonate in an acidic environment created by the SOMs is solely responsible for creating the chemical potential gradient which induces motion in these nanomotors. We have explained this motion qualitatively and also shown how chemical anisotropy and size of these nanoparticles influence such autonomous motion.
Introduction
Nanomachines1,2 play a significant role in today's nanotechnology as they can perform tailored tasks using energy. Nanomotors prove to be a step towards realisation of such nanomachines.3,4 Nanomotors may be defined as nano scale objects which harness energy from their environment and convert it to motion and force. In nature various biological motors are present.5 For instance, eukaryotic cells contain several powerful biomotors which convert chemical energy from hydrolysis of ATP to mechanical work.6 Other examples of biomotors include cytoskeletal molecular motors and enzymatic motors which are involved in the processing of RNA and DNA.7,8 Taking inspiration from these biological machines various groups across the world have started working with nano machines. The first attempt of preparing such small-scale machines was taken by Whitesides et al. in 2002.9 They used Pt catalyst to propel millimetre sized plastic disks. In 2004–2005 the group of Sen, Mallouk and Ozin contributed further to this field by preparing micrometre sized motors which constituted of Pt–Au and Ni–Au bimetallic rods of length 2–3 μm.10,11 These were chemically driven micromotors and the fuel used in these cases was primarily H2O2 which catalytically decomposed to form H2O and O2.The motion of these nanomotors is dominated by viscous force and thermal fluctuations which are converted to Brownian motion.12,13 In order to overcome the viscous force in low Reynold's number regime and also to induce motion in the nanomotors some form of energy input must be provided.14 This is because of the fact that the mechanism of motion of nanoscale objects differs from that of macro objects.15,16 A macro particle can maintain its motion for a certain period of time even without continuous supply of energy but a nanoscale object cannot continue its motion due to the dominance of viscous drag. To control the motion of the nano objects a continuous driving force and an external stimulus are necessary. The external stimulus may be in the form of light,17–19 chemical gradient,20–22 magnetic field,23–25 electric field,26,27 ultrasonic sound.28 Depending on the interaction between the particle and the input energy source the movement can be translation,29,30 rotation,31 rolling,32 contraction,33 delivery34 or collective motion.35
In the present work we have mainly focused on chemically propelled nanomotors. The motion of the chemical nanomotors can be explained by generation of surface tension gradients,36 bubble propulsion,37 osmosis38 or self-electrophoresis.39 Chemical propulsion depends on the chemical fuel used and till date the most extensively used chemical fuel is H2O2.40,41 The shape10 and symmetry42 of the nanomotor also plays a vital role in determining the mechanism of the motion.
Soft state of oxometalates comprising of superstructures of oxometalates are known as soft-oxometalates (SOMs)43–51 and since they are soft in nature they can be rendered motile deliberately using correct chemical fuel or by any other suitable external energy. We have already induced motion in SOMs using both physical as well as chemical external energy sources.17,52 In one of our previous works mesoscopic asymmetric peapod shaped SOM's50 controlled motion along complex paths using tailor-made sophisticated optical potentials was reported.17 In another work motion was induced in heptamolybdate based SOMs using dithionite as a fuel from which SO2 was generated to propel the SOMs.52 As a next step we try to exploit the intrinsic acidity of the SOMs and propel them by exposing them to a bicarbonate rich environment which can produce CO2 which can propel the SOMs. The SOM used here as the nanomotor is composed of oxoanions of two metals – molybdenum and vanadium. The morphology of these SOMs can be tuned by varying the loading of the two metal oxoanions which helps us achieving control in the movement of the SOMs. As mentioned earlier, the primary design principle of the SOM nanomotors is based on the fact that the SOMs are heteropolyacids which evolve CO2 (propelling the SOMs) from bicarbonate which is used as the fuel. Here we first describe its synthesis and characterization and later its motion.
Experimental
Materials and methods
All the reagents were purchased from commercial sources (Merck) and were used without further purification. The glassware were cleaned in an acid bath, base bath and then rinsed with isopropanol followed by acetone and kept in hot air oven for 48 hours prior use.Synthesis of V/Mo soft-oxometalate (SOM)
Ammonium heptamolybdate tetrahydrate (800 mg, 0.687 mmol) was dissolved in distilled water (4 mL) and heated until simmering hot. A clear dispersion of ammonium heptamolybdate was formed which was kept in refrigerator for 10 minutes. This dispersion was then brought back to room temperature. To this solution a specific amount of sodium metavanadate was added separately to prepare dispersions such that the molar ratio of sodium metavanadate to ammonium heptamolybdate (V/Mo loading) were 0.08, 0.10, 0.12, 0.14, 0.17, 0.24, 0.50, 0.70 and 1.00 respectively. After addition of metavanadate the mixture was sonicated for 15 minutes using an ultrasonicator to form a stable dispersion. The dispersion was brought back to room temperature and kept undisturbed for 24 hours for equilibration. Beyond the V/Mo loading ratio of 0.50 precipitate was obtained after 24 hours.Preparation of sodium bicarbonate solutions
Calculated amounts of sodium bicarbonate were dissolved in 10 mL of distilled water to prepare bicarbonate solutions of concentrations 0.0119 mol L−1, 0.0238 mol L−1, 0.0416 mol L−1, 0.0595 mol L−1, 0.0952 mol L−1, 0.1190 mol L−1, 0.1428 mol L−1, 0.1785 mol L−1, 0.2142 mol L−1, 0.2381 mol L−1, 0.2976 mol L−1, which were used as fuels.Instrumental analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio is maintained. The microscope was again focused at objective 40× and the videos were recorded using DSIC camera attached with the microscope. This procedure was repeated 3 times each for all concentrations of sodium bicarbonate as well as for all variations of V/Mo loading to ensure the reproducibility of the process and to get more number of data points for analysis.
1 volume ratio is maintained. The microscope was again focused at objective 40× and the videos were recorded using DSIC camera attached with the microscope. This procedure was repeated 3 times each for all concentrations of sodium bicarbonate as well as for all variations of V/Mo loading to ensure the reproducibility of the process and to get more number of data points for analysis.Analysis of motion of SOMs
Results and discussion
We first describe the formation of the vanadate–molybdate SOM and later its characterization with the help of Scanning Electron Microscopy (SEM), Energy Dispersive X-ray Analysis (EDAX), Dynamic Light Scattering (DLS), Powder X-ray Diffraction (PXRD), Horizontal Attenuated Total Reflectance-Infra Red (HATR-IR) Spectroscopy and Raman spectroscopy.Different morphologically distinctive aqueous dispersions of SOMs are made from the initial dispersion of heptamolybdate in water52 and sodium metavanadate, by suitably adjusting the molar ratio of vanadate and molybdate (V/Mo loading = 0.08, 0.10, 0.12, 0.14, 0.17, 0.24, 0.35 and 0.50) followed by sonication for 15 minutes and thermal equilibration at 25 °C for another 24 hours.
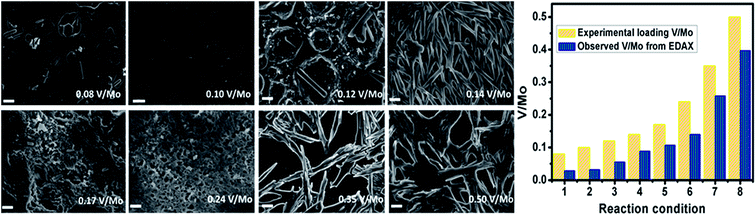
We first check the stability of the dispersions. To do so, we employed the relation D = D0(1 + kDC) where D = diffusion coefficient measured from hydrodynamic radii (from DLS), D0 = diffusion coefficient at zero concentration, C = sample concentration in mol L−1, kD is a complex function depending on hydrodynamic radii which predicts the repulsive electrostatic interactions among SOMs.54 The positive value of kD confirms the stability of the SOMs, with increasing negative value of kD the SOMs become unstable. From the kD vs. V/Mo loading graph (Fig. 1) dispersions with V/Mo loading of 0.14, 0.17 and 0.24 are found to be more stable than others. DLS size distribution experiments (Fig. S1†) show that for V/Mo loading of 0.08 to 0.12 the average hydrodynamic diameter (Dh) of SOMs vary from 500–740 nm (Fig. 1) and from SEM we observe that in such SOMs the rods of {Mo7} are nested within the cavity of vanadate. With further increase in vanadate concentration, i.e.; for V/Mo loading of 0.14–0.24 there is a morphological transformation back to nanorods, with hydrodynamic diameter decreasing to 330 nm as evident from Fig. 1. At and beyond V/Mo loading of 0.35 the system becomes unstable and microcrystalline sharp needles (Fig. 2) of vanadate–molybdate precipitate. The graph in Fig. 2 compares the experimental loading of V/Mo ratios in the SOMs with the ratios obtained from EDAX mapping (Fig. S2†). The V/Mo ratios obtained from EDAX are slightly less than the experimental loading ratios but it follows the same trend as the experimental ones.
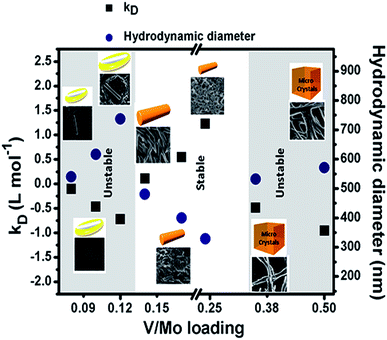 | ||
| Fig. 1 Stability study of V/Mo SOMs at different loading of V/Mo from kD value and hydrodynamic diameter obtained from DLS study. | ||
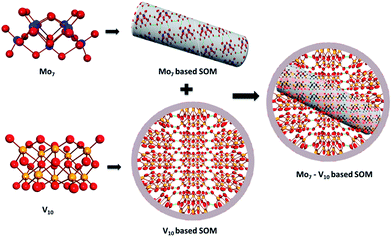
We now speculate on the reasons for obtaining various morphologies in the dispersions. Dispersing ammonium heptamolybdate (0.687 mmol) in 4 mL of water gives rise to a pH of 5.5. Vanadate in between pH range of 4–9 exists as decavanadate.55 Hence, all the above morphologies are created by self-assembly of heptamolybdate and decavanadate which we have confirmed from HATR-IR later. Though the exact structure of the (Mo7–V10) based SOMs cannot be obtained we provide a model of the formation of the SOMs from the constituent polyoxometalates–heptamolybdate and decavanadate via hydrogen bonding (Fig. 3). The above mentioned SOMs have been synthesized via sonication which is a versatile technique for creating nanostructures. Ultrasonic waves generate hollow structures via subsequent generation, growth and collapse of bubbles in aqueous solution.56 These bubbles create a liquid–air interface which acts as a template for the growth of hollow spherical vanadate SOMs.57 Heptamolybdate rods have higher surface energy than vanadate spheres as spheres are the lowest energy structures. We believe that to minimise the magnitude of electrostatic repulsion between the heptamolybdate rods they take shelter within the vanadate cavity which is responsible for the embedded morphologies of rods in shells for the V/Mo loadings of 0.08, 0.10 and 0.12.58 Till now this system is under thermodynamic control, on addition of further electrolyte in the form of sodium metavanadate the spheres get transformed to {Mo7–V10} rods. For V/Mo loadings higher than 0.12 we believe the added electrolyte minimizes the electrostatic repulsion thus stabilizing the kinetically controlled rods and kD values are positive implying stability of SOMs.59 Finally for V/Mo ratios beyond 0.24 the system becomes unstable again and it flocculates in the form of microcrystals, which we have further investigated with powder X-ray diffraction (PXRD) (Fig. 4).
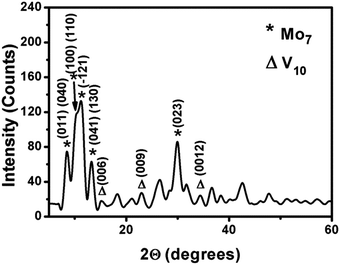 | ||
| Fig. 4 Powder XRD of the V/Mo composite at molar ratio of 0.35. The peaks with * represent the planes of {Mo7} and those with Δ represent the planes of {V10}. | ||
Fig. 4 shows the powder X-ray diffraction (PXRD) pattern of vanadate–molybdate composite with intense peaks centered at 8.5290, 10.2060, 11.1330, 13.2360, 15.1780, 23.0000, 29.9160, 34.4610 with d-spacings of 1.036, 0.866, 0.794, 0.668, 0.583, 0.386, 0.298, 0.260 nm respectively. The peaks at 15.1780, 23.0000, 34.4610 may be assigned to the Bragg's reflections from (006), (009), (0012) planes of decavanadate respectively.60 The peaks at 8.5290, 10.2060, 11.1330, 13.2360, 29.9160 may be from [(011), (040)], [(100), (110)], (−121), [(041), (130)], (023) planes of heptamolybdate respectively.61 The peaks are broadened due to the formation of micron sized crystals. In this process of composite formation, lattices of both the oxoanions do not remain intact which could be clearly understood from the change in the d values as compared to literature.60,61 A new lattice might have been formed in the process with probable interpenetration of the vanadate and molybdate lattices, exact structure of which is beyond the scope of this paper.
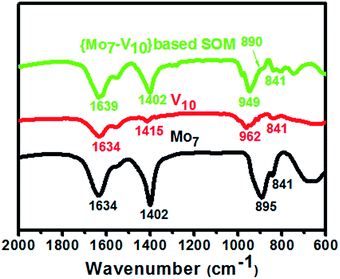
In order to confirm that the SOM is composed of vanadate–molybdate we have characterized the heptamolybdate dispersion, decavanadate dispersion and the SOM dispersion by HATR-IR spectroscopy. From the HATR-IR spectra (Fig. 5) we have observed characteristic peaks of ammonium heptamolybdate tetrahydrate at 1634 cm−1 due to stretching of O–H bond. The peak at 1402 cm−1 is due to bending of N–H bond of the ammonium ion and the peaks at 895 cm−1 and 841 cm−1 are due to symmetric and asymmetric stretching modes of the vibration in MoO42−. For decavanadate the peaks were observed at 1634 cm−1 for symmetric stretching of O–H bond, at 962 cm−1 and 841 cm−1 for symmetric and asymmetric stretching vibrations of V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. For the SOM dispersion, peaks were observed at 1639 cm−1 for O–H bond stretching, 1402 cm−1 for N–H bending, 890 cm−1 and 841 cm−1 for symmetric and asymmetric Mo
O bond. For the SOM dispersion, peaks were observed at 1639 cm−1 for O–H bond stretching, 1402 cm−1 for N–H bending, 890 cm−1 and 841 cm−1 for symmetric and asymmetric Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, 949 cm−1 and 841 cm−1 for symmetric and asymmetric stretching vibrations of V
O stretching, 949 cm−1 and 841 cm−1 for symmetric and asymmetric stretching vibrations of V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. We observe a shift in O–H bond stretching for the SOM dispersion which is due to hydrogen bonding type interaction between the interacting vanadate and molybdate clusters. Shifts in Mo
O bond. We observe a shift in O–H bond stretching for the SOM dispersion which is due to hydrogen bonding type interaction between the interacting vanadate and molybdate clusters. Shifts in Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and V
O and V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations indicate weak interactions between molybdate and vanadate. From these peaks we infer that the SOM is indeed composed of vanadate and molybdate.
O stretching vibrations indicate weak interactions between molybdate and vanadate. From these peaks we infer that the SOM is indeed composed of vanadate and molybdate.
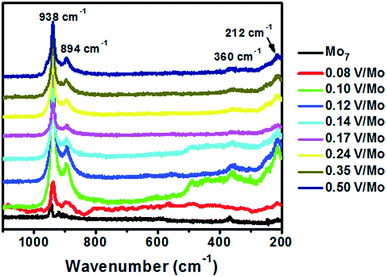
From Raman spectroscopy (Fig. 6) we observe that decavanadate is Raman inactive. {Mo7} alone possesses Raman bands at 944, 918, 590, 365 and 214 cm−1 which may be attributed to Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric and asymmetric stretching vibrations, Mo–O–Mo symmetric stretching (weak band), Mo
O symmetric and asymmetric stretching vibrations, Mo–O–Mo symmetric stretching (weak band), Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bending and Mo–O–Mo deformations respectively. In case of SOMs with different V/Mo loading the Raman bands are shifted to 938, 894, 360 and 212 cm−1. This shift to lower wavenumbers may be due to higher polarizability of the bonds as they are attached to oxoanions of vanadium.
O bending and Mo–O–Mo deformations respectively. In case of SOMs with different V/Mo loading the Raman bands are shifted to 938, 894, 360 and 212 cm−1. This shift to lower wavenumbers may be due to higher polarizability of the bonds as they are attached to oxoanions of vanadium.
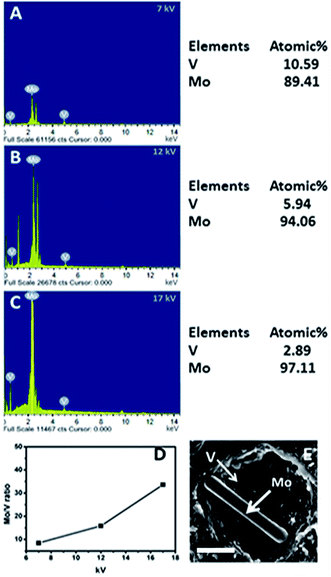
We now analyze the morphologies obtained for V/Mo loadings of 0.08 to 0.12. We observe for V/Mo loading of 0.08 to 0.12 a structure of a rod nested within a hollow cavity. To prove the nanorods are of heptamolybdate and the cavity consists of vanadate we varied the energy density of the probing electron beam in the scanning electron microscope. This is done by increasing the accelerating voltage from 7–17 kV while performing energy dispersive X-ray analysis (Fig. 7). Mo/V ratio is measured at a particular location and it is observed that on accelerating energy the Mo/V ratio also increases from 8.44 to 33.60. This is because at lower energy density the electron beam can penetrate only up to a few layers while higher energy beam penetrates deep inside the cavity. This shows that the surface of the structure is rich in vanadates while deeper structure where rod is present is rich in molybdates. This clearly shows that {Mo7} rich rods are cradled inside the cavities rich in vanadates.
Now we investigate the motion of SOMs with varying V/Mo loading and for different concentrations of fuel. As mentioned earlier, in this paper these SOMs are used as the nanomotors and an aqueous solution of sodium bicarbonate is used as the fuel. The dispersion of heptamolybdate SOMs (i.e., V/Mo loading of 0) is found to be acidic with a pH of 5.5. These heteroacid SOMs evolve CO2 from bicarbonate solution according to the following reactions:
| HCO3− + H+ (SOM) → H2CO3 |
| H2CO3 → H2O + CO2 (g)↑ |
Even though evolution of CO2 is observed from the heptamolybdate SOM dispersion, these SOMs alone do not exhibit any autonomous motion. We believe that due to absence of any anisotropy in the structure of these SOMs, the evolution of CO2 is non-directional and symmetric and hence it cannot induce motion. So we ask – can some asymmetry be induced in these particles to make them active as anisotropy plays a crucial role in the movement of active nanomotors? On introduction of vanadate in the molybdate SOMs i.e.; with varying V/Mo loading the desired anisotropy is obtained which in turn renders motility in these nanoparticles. However, for only vanadate containing SOM dispersion no effervescence is observed on addition of bicarbonate solution. Hence it may be inferred that the carbon dioxide is generated from the molybdate part of the SOMs and the motility is induced due to asymmetry introduced in these SOMs by the vanadates.
To elucidate the motion of the SOMs qualitatively we propose that a mobile diffused boundary is generated between the starting SOM and the species generated after reaction. This interface in turn generates an osmotic boundary where CO2 gas is produced which again gives rise to a slip velocity between the SOM surface and the continuous medium.
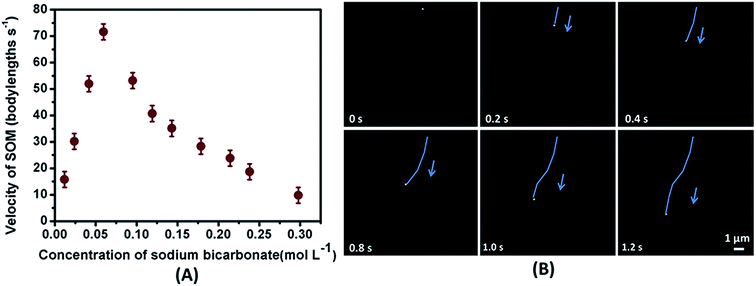
To interpret the effect of the fuel on the mechanism of motion we varied the loading of concentration of the fuel on a given SOM dispersion with a fixed V/Mo loading of 0.17. It is observed that the propulsion velocity of these SOMs increases with increasing concentration of the fuel which is obvious as more and more CO2 is generated that amplifies the chemical potential gradient near the SOM surface leading to greater propulsion. The propulsion velocity reaches a maximum of 72 body lengths s−1 at 0.0595 mol L−1 of sodium bicarbonate concentration. After the tipping point and past this fuel concentration, the propulsion diminishes (Fig. 8A). This is due to the fact that some CO2 gets adsorbed on the free SOM surface thereby saturating the reaction sites which exerts an additional viscous drag on the SOMs.
Consequently, the velocity of the SOMs dwindles even though we increase the concentration of the fuel. To analyse the individual movement of each SOM particle we have calculated the velocity of the SOM at different concentrations of bicarbonate (Fig. 8A) and shown the time lapse images of the moving SOM in 0.0595 mol L−1 bicarbonate solution.
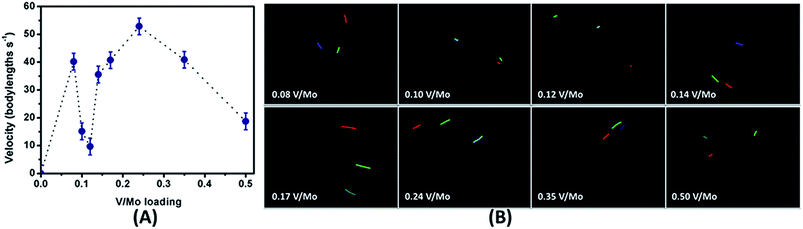
Now to perceive the impact of asymmetry on actuation of these nanomotors eight SOM dispersions were prepared by altering the loading of V/Mo (Fig. 9A). For the first dispersion having an interpenetrating rod encapsulated in a shell of vanadate where V/Mo loading was 0.08 the mean propulsion velocity was found to be 40 body lengths s−1 (Fig. 9A). We have also obtained the trajectories of the motile SOMs for time duration of 1 second with ImageJ and TrackPy (Fig. 9B).
The coloured lines indicate the distance travelled by each SOM for 1 second in bicarbonate solutions.
With increase in vanadate loading in the SOM (for V/Mo loading of 0.08 to 0.12) the extent of encapsulation of molybdate by vanadate increases thereby decreasing the interaction between the heptamolybdate rods and the bicarbonate solution. Hence less CO2 is produced and the propulsion velocities of the SOMs reduce till V/Mo loading reaches 0.12. Now for V/Mo loading of 0.14 to 0.24 the encapsulation of molybdate by vanadate is not seen anymore.
Instead we see only nanorods that are chemically anisotropic and are composed of both vanadate and molybdate. Hence for SOMs with V/Mo loading of 0.14 and above the bicarbonate solution can directly interact with the molybdate present in the nanorods with vanadate imparting asymmetry thereby enhancing the propulsion velocity. For 0.17 and 0.24 loading of V/Mo, the size of the nanorods diminishes ensuing acceleration of the SOMs. For SOMs with V/Mo loading of 0.35 and 0.50 microcrystalline rods of increasing size are produced and consequently the velocity drops again. Beyond the V/Mo loading of 0.5 the dispersion of V/Mo SOM becomes unstable. Now we discuss the effect of size on the propulsion velocity of mixed vanadate–molybdate SOMs. For the vanadate encapsulated molybdate rods at V/Mo loading of 0.12 the hydrodynamic diameter is 740 nm and the propulsion velocity is the lowest ∼9 body lengths s−1. For the vanadate–molybdate rods at V/Mo loading of 0.24 the hydrodynamic diameter reduces to 330 nm and the velocity is the highest i.e.; 53 body lengths s−1. It is also to be noted that in low Reynold's number regime, the axial propulsion force of the nanomotors calculated by Stokes' equation is compensated by the drag force of the fluid on the nanomotors.62,63 For the nanorod encapsulating vesicular SOMs the drag force is (0.285 ± 0.012) fN while for the rod-like SOMs it is (0.736 ± 0.009) fN which explains the higher propulsion velocity of the rod-like SOMs.
So the following points emerge from our study:
(1) Although molybdate is responsible for effervescence of CO2 from bicarbonate, alone molybdate based SOMs cannot show motility when treated with fuel bicarbonate. Instead induction of chemical anisotropy in the form of mixed metalate SOM with V and Mo is required for observing motility.
(2) However for a SOM with chemical anisotropy, i.e., SOMs with vanadate and molybdate, the hydrodynamic radius of the SOM inversely influences the velocity of propagation. It is observed that smaller the hydrodynamic radius, higher is the velocity of the SOM.
From all the above results we thus infer that the autonomous motion of the nanomotors is dependent on the anisotropy of SOMs, and the size of the individual nanomotors, for a given concentration of the fuel.
Conclusions
To summarize, in this work we have reported a decavanadate and heptamolybdate based soft-oxometalate (SOM) system and employed sodium bicarbonate solution as fuel to render these nano particles motile. The decomposition of bicarbonate to form CO2 in the presence of acidic dispersion of heptamolybdate is the driving force behind the motion of the SOMs. Vanadate induces asymmetry in these SOMs which provides directionality to the translation. In this work we have clearly demonstrated that size and chemical anisotropy of SOMs play a crucial role in their movement which is reflected in the magnitude of their propulsion velocity. Thus we can tune the movement of the SOMs either by controlling their size or by varying the molar ratio of the chemical constituents – vanadate and molybdate and hence possibilities exist for controlling their motion and using them as devices for transport and delivery.64–66Acknowledgements
SR gratefully acknowledges the grants from IISER-Kolkata, India, DST-fast track, BRNS-DAE grant.Notes and references
- J. Wang, Nanomachines: fundamentals and applications, John Wiley & Sons, 2013 Search PubMed.
- J. Wang, Lab Chip, 2012, 12, 1944–1950 RSC.
- J. Li, W. Gao, R. Dong, A. Pei, S. Sattayasamitsathit and J. Wang, Nat. Commun., 2014, 5, 5026 CrossRef CAS PubMed.
- K. Kim, X. Xu, J. Guo and D. Fan, Nat. Commun., 2014, 5, 3632 Search PubMed.
- R. D. Vale and R. A. Milligan, Science, 2000, 288, 88–95 CrossRef CAS PubMed.
- A. Upadhyaya and A. van Oudenaarden, Curr. Biol., 2003, 13, R734–R744 CrossRef CAS PubMed.
- G. Woehlke and M. Schliwa, Nat. Rev. Mol. Cell Biol., 2000, 1, 50–58 CrossRef CAS PubMed.
- H. Yin, M. D. Wang, K. Svoboda and R. Landick, Science, 1995, 270, 1653 CAS.
- R. F. Ismagilov, A. Schwartz, N. Bowden and G. M. Whitesides, Angew. Chem., Int. Ed., 2002, 41, 652–654 CrossRef CAS.
- W. F. Paxton, K. C. Kistler, C. C. Olmeda, A. Sen, S. K. S. Angelo, Y. Cao, T. E. Mallouk, P. E. Lammert and V. H. Crespi, J. Am. Chem. Soc, 2004, 126, 13424–13431 CrossRef CAS PubMed.
- S. Fournier-Bidoz, A. C. Arsenault, I. Manners and G. A. Ozin, Chem. Commun., 2005, 4, 441–443 RSC.
- P. Fischer and A. Ghosh, Nanoscale, 2011, 3, 557–563 RSC.
- T. R. Kline, W. F. Paxton, T. E. Mallouk and A. Sen, Angew. Chem., Int. Ed., 2005, 44, 744–746 CrossRef CAS PubMed.
- M. Guix, C. C. Mayorga-Martinez and A. Merkoçi, Chem. Rev., 2014, 114, 6285–6322 CrossRef CAS PubMed.
- S. Nakata, Y. Doi and H. Kitahata, J. Colloid Interface Sci., 2004, 279, 503–508 CrossRef CAS PubMed.
- G. Zhao, T. H. Seah and M. Pumera, Chem.–Eur. J., 2011, 17, 12020–12026 CrossRef CAS PubMed.
- B. Roy, N. Ghosh, P. K. Panigrahi, A. Banerjee, A. Sahasrabudhe, B. Parasar and S. Roy, J. Mol. Eng. Mater., 2014, 2, 1440006 CrossRef.
- W. Duan, M. Ibele, R. Liu and A. Sen, Eur. Phys. J. E: Soft Matter Biol. Phys., 2012, 35, 1–8 CrossRef PubMed.
- Y. Li, F. Mou, C. Chen, M. You, Y. Yin, L. Xu and J. Guan, RSC Adv., 2016, 6, 10697–10703 RSC.
- W. Gao, S. Sattayasamitsathit, J. Orozco and J. Wang, J. Am. Chem. Soc, 2011, 133, 11862–11864 CrossRef CAS PubMed.
- N. S. Zacharia, Z. S. Sadeq and G. A. Ozin, Chem. Commun., 2009, 39, 5856–5858 RSC.
- Q. Xiao, J. Li, J. Han, K.-X. Xu, Z.-X. Huang, J. Hu and J.-J. Sun, RSC Adv., 2015, 5, 71139–71143 RSC.
- L. Zhang, T. Petit, Y. Lu, B. E. Kratochvil, K. E. Peyer, R. Pei, J. Lou and B. J. Nelson, ACS Nano, 2010, 4, 6228–6234 CrossRef CAS PubMed.
- S. Tottori, L. Zhang, F. Qiu, K. K. Krawczyk, A. Franco-Obregón and B. J. Nelson, Adv. Mater., 2012, 24, 811–816 CrossRef CAS PubMed.
- I. Kavre, G. Kostevc, S. Kralj, A. Vilfan and D. Babič, RSC Adv., 2014, 4, 38316–38322 RSC.
- S. T. Chang, V. N. Paunov, D. N. Petsev and O. D. Velev, Nat. Mater., 2007, 6, 235–240 CrossRef CAS PubMed.
- G. Loget and A. Kuhn, J. Am. Chem. Soc, 2010, 132, 15918–15919 CrossRef CAS PubMed.
- V. Garcia-Gradilla, J. Orozco, S. Sattayasamitsathit, F. Soto, F. Kuralay, A. Pourazary, A. Katzenberg, W. Gao, Y. Shen and J. Wang, ACS Nano, 2013, 7, 9232–9240 CrossRef CAS PubMed.
- C. Stock, N. Heureux, W. R. Browne and B. L. Feringa, Chem.–Eur. J., 2008, 14, 3146–3153 CrossRef CAS PubMed.
- M. Manjare, B. Yang and Y.-P. Zhao, Phys. Rev. Lett., 2012, 109, 128305 CrossRef PubMed.
- J. Gibbs and Y. P. Zhao, Small, 2009, 5, 2304–2308 CrossRef CAS PubMed.
- Y. He, J. Wu and Y. Zhao, Nano Lett., 2007, 7, 1369–1375 CrossRef CAS PubMed.
- J. C. Nawroth, H. Lee, A. W. Feinberg, C. M. Ripplinger, M. L. McCain, A. Grosberg, J. O. Dabiri and K. K. Parker, Nat. Biotechnol., 2012, 30, 792–797 CrossRef CAS PubMed.
- D. Kagan, R. Laocharoensuk, M. Zimmerman, C. Clawson, S. Balasubramanian, D. Kang, D. Bishop, S. Sattayasamitsathit, L. Zhang and J. Wang, Small, 2010, 6, 2741–2747 CrossRef CAS PubMed.
- J. Berna, D. A. Leigh, M. Lubomska, S. M. Mendoza, E. M. Pérez, P. Rudolf, G. Teobaldi and F. Zerbetto, Nat. Mater., 2005, 4, 704–710 CrossRef CAS PubMed.
- H. Zhang, W. Duan, L. Liu and A. Sen, J. Am. Chem. Soc, 2013, 135, 15734–15737 CrossRef CAS PubMed.
- A. A. Solovev, S. Sanchez, M. Pumera, Y. F. Mei and O. G. Schmidt, Adv. Funct. Mater., 2010, 20, 2430–2435 CrossRef CAS.
- W. Gao, A. Pei, R. Dong and J. Wang, J. Am. Chem. Soc, 2014, 136, 2276–2279 CrossRef CAS PubMed.
- Y. Wang, R. M. Hernandez, D. J. Bartlett, J. M. Bingham, T. R. Kline, A. Sen and T. E. Mallouk, Langmuir, 2006, 22, 10451–10456 CrossRef CAS PubMed.
- A. Agrawal, K. K. Dey, A. Paul, S. Basu and A. Chattopadhyay, J. Phys. Chem. C, 2008, 112, 2797–2801 CAS.
- J. Gibbs and Y.-P. Zhao, Appl. Phys. Lett., 2009, 94, 163104 CrossRef.
- J. R. Howse, R. A. Jones, A. J. Ryan, T. Gough, R. Vafabakhsh and R. Golestanian, Phys. Rev. Lett., 2007, 99, 048102 CrossRef PubMed.
- S. Roy, Comments Inorg. Chem., 2011, 32, 113–126 CrossRef CAS.
- S. Roy, CrystEngComm, 2014, 16, 4667–4676 RSC.
- B. Roy, M. Arya, P. Thomas, J. K. Jurgschat, K. Venkata Rao, A. Banerjee, C. Malla Reddy and S. Roy, Langmuir, 2013, 29, 14733–14742 CrossRef CAS PubMed.
- S. Das, P. Thomas and S. Roy, Eur. J. Inorg. Chem., 2014, 2014, 4551–4557 CrossRef CAS.
- P. Thomas, C. Pei, B. Roy, S. Ghosh, S. Das, A. Banerjee, T. Ben, S. Qiu and S. Roy, J. Mater. Chem. A, 2015, 3, 1431–1441 CAS.
- K. Das and S. Roy, Chem.–Asian J., 2015, 10, 1884–1891 CrossRef CAS PubMed.
- S. Das, S. Biswas, T. Balaraju, S. Barman, R. Pochamoni and S. Roy, J. Mater. Chem. A, 2016, 4, 8875 CAS.
- S. Roy, M. T. Rijneveld-Ockers, J. Groenewold, B. W. Kuipers, H. Meeldijk and W. K. Kegel, Langmuir, 2007, 23, 5292–5295 CrossRef CAS PubMed.
- S. Roy, L. C. Bossers, H. J. Meeldijk, B. W. Kuipers and W. K. Kegel, Langmuir, 2008, 24, 666–669 CrossRef CAS PubMed.
- A. Mallick, D. Lai and S. Roy, New J. Chem., 2016, 40, 1057–1062 RSC.
- D. Allan, T. Caswell, N. Keim and C. van der Wel, TrackPy v0.3.2, 2016 Search PubMed.
- M. Anyfantakis, A. Bourlinos, D. Vlassopoulos, G. Fytas, E. Giannelis and S. K. Kumar, Soft Matter, 2009, 5, 4256–4265 RSC.
- N. Greenwood and A. Earnshaw, Chemistry of the Elements, Butterworth-Heinemann, 2nd edn, 1997 Search PubMed.
- R. K. Rana, Y. Mastai and A. Gedanken, Adv. Mater., 2002, 14, 1414–1418 CrossRef CAS.
- C. Deng, H. Hu, X. Ge, C. Han, D. Zhao and G. Shao, Ultrason. Sonochem., 2011, 18, 932–937 CrossRef CAS PubMed.
- L. Zhang, K. Yu and A. Eisenberg, Science, 1996, 272, 1777 CAS.
- L. Zhang and A. Eisenberg, Macromolecules, 1996, 29, 8805–8815 CrossRef CAS.
- R. G. Buchheit, H. Guan, S. Mahajanam and F. Wong, Prog. Org. Coat., 2003, 47, 174–182 CrossRef CAS.
- J. Wienold, R. E. Jentoft and T. Ressler, Eur. J. Inorg. Chem., 2003, 2003, 1058–1071 CrossRef.
- T. Ui, R. Hussey and R. Roger, Phys. Fluids, 1984, 27, 787–795 CrossRef CAS.
- S. Gilder and J. Glen, Science, 1998, 279, 72–74 CrossRef CAS PubMed.
- S. Ebbens, R. A. Jones, A. J. Ryan, R. Golestanian and J. R. Howse, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2010, 82, 015304 CrossRef PubMed.
- L. Baraban, D. Makarov, R. Streubel, I. Mönch, D. Grimm, S. Sanchez and O. G. Schmidt, ACS Nano, 2012, 6, 3383–3389 CrossRef CAS PubMed.
- L. L. del Mercato, M. Carraro, A. Zizzari, M. Bianco, R. Miglietta, V. Arima, I. Viola, C. Nobile, A. Sorarù and D. Vilona, Chem.–Eur. J., 2014, 20, 10910–10914 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24132h |
| This journal is © The Royal Society of Chemistry 2016 |