Preparation of magnetic activated carbon from waste rice husk for the determination of tetracycline antibiotics in water samples
Abstract
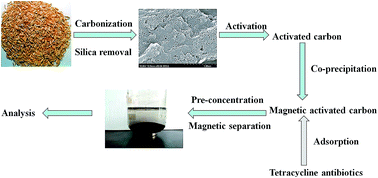
In this work, the silica in rice husk was removed to create a natural template to prepare porous activated carbon. The preparation process of the activated carbon (AC) from waste rice husk consisted of carbonization, silica removal and activation. Factors affecting the preparation of AC, including the mass ratio of ZnCl2 and rice husk carbon (RHC), activation temperature and time were investigated. Based on the Brunauer–Emmett–Teller (BET) results, the specific surface area of AC prepared under optimized conditions was as high as 1673.25 m2 g−1. Then, magnetic activated carbon (mag-AC) was synthesized by in situ co-precipitation of rice husk-based activated carbon and Fe3O4. The specific surface area and average pore diameter of mag-AC were respectively 986.97 m2 g−1 and 3.62 nm, respectively. It was shown that mag-AC was a viable adsorbent for magnetic extraction of tetracycline antibiotics in water samples followed by quantification by HPLC-MS/MS. The results indicated satisfactory correlation coefficients (R2) ranging from 0.9955 to 0.9998. Limits of detection (LOD) and quantization (LOQ) were achieved in the range of 0.10 to 0.95 and 0.35 to 2.85 μg L−1 in water samples, respectively.


 Please wait while we load your content...
Please wait while we load your content...