Raw product of rare-earth ore works as a high-k gate insulator for low-voltage operable organic field-effect transistors†
Abstract
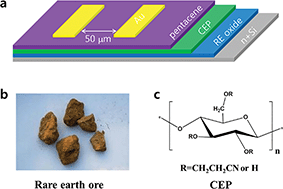
Rare earth metal oxides were found to be good candidates for high-k gate insulators in field-effect transistors. However, refinement of individual elements of rare earth metals either requires complicated processes, or discards a large fraction of the components, which drastically increases the fabrication cost and wastes natural resources. We demonstrate here the successful use of rare-earth raw product to fabricate high-quality gate insulators, which contain a number of different elements without strict refinement. The oxide dielectric thin film showed a very high dielectric constant (k) value of 35 and high dielectric strength >1 MV cm−1, which even rivals those of pure rare-earth oxides. High-k gate insulators are essential for low-voltage operated organic field-effect transistors (OFETs). Capping with a high-k cyanoethylated pullulan (CEP) polymer layer further increased the film quality and created a favorable semiconductor/dielectric interface, and benefits the stacking of overgrown semiconductor molecules. The OFETs were successfully operated at low voltage of 2–4 V, exhibiting nice mobility ∼0.5 cm2 V−1 s−1, on/off current ratio >104, and a steep subthreshold slope of 0.096 V dec−1. The utilization of rare-earth raw product as a source material would drastically reduce the production cost of gate insulators in OFETs, and reduce environmental pollution, which is meaningful in view of green chemistry.

 Please wait while we load your content...
Please wait while we load your content...