Synthesis of quinolines through copper-catalyzed intermolecular cyclization reaction from anilines and terminal acetylene esters†
Zhilei Zheng,
Guobo Deng* and
Yun Liang*
Key Laboratory of the Assembly and Application of Organic Functional Molecules, Hunan Normal University, Changsha, Hunan 410081, China. E-mail: gbdeng@hunnu.edu.cn; yliang@hunnu.edu.cn; Fax: +86 731 88872533
First published on 24th October 2016
Abstract
A simple and convenient copper-catalyzed intermolecular cyclization reaction for the synthesis of quinolines from anilines and terminal acetylene esters has been developed. This methodology constructs the C–N and C–C bonds successively via a cascade process, and provides the desired products in moderate to good yields.
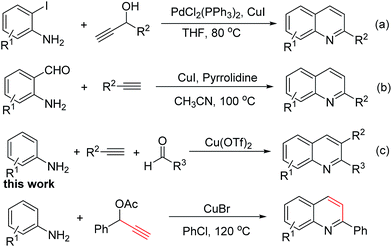
The quinoline skeleton is a very important nitrogen-containing heterocyclic unit featuring prominently in compounds showing antimalarial, antiinflammatory, antineoplastic, antifungal, antiseptic, and analgesic properties.1 Accordingly, considerable attention has been paid to developing efficient methods for its synthesis. The classical methods for constructing quinolines include the Skraup reaction,2 Combes synthesis,3 Gould–Jacobs reaction,4 Friedländer synthesis,5 and Doebner–von Miller reaction.6 In recent years, significant progress has been made in the development of transition metal-catalyzed methods because of their functional group tolerance, stereo- and regioselectivity and significant yields under mild reaction conditions.7 Especially, alkyne and anilines have been ubiquitously employed in quinolines syntheses through transition metal-catalyzed C–N/C–C bonds formation. For example, Cho and co-workers reported a palladium-catalyzed method for the synthesis of quinolines from terminal acetylenic carbinols with 2-iodoanilines in high yields (Scheme 1a).8 Patil group developed a copper-catalyzed tandem addition/cycloisomerization reaction giving access to quinolines using 2-aminobenzaldehydes and terminal alkynes as substrate (Scheme 1b).9 Subsequently, a copper-catalyzed three-component coupling reaction for the synthesis of quinoline derivatives in a one-pot procedure was reported (Scheme 1c).10 Using other metal such as Ti,11 Ni12 and Au13 as catalyst for synthesis of quinoline methods were reported too. In addition, transition metal catalysts have been widely applied in the reaction of some prefunctionalized substrates, such as 2-aminophenyl-boronates,14 trialkylamines,15 styrene oxides,16 and phenylacetaldehyde17 for the synthesis of quinolines. These methods have good progress in the synthesis of quinoline derivatives, but development of novel and expeditious methods are still desired. Herein, we describe a copper-catalyzed C–N/C–C bond formation reaction synthesis of substituted quinolines using terminal acetylene esters and anilines as substrate under air atmosphere.
Initially, the reaction of aniline 1a and 1-phenylprop-2-ynyl acetate 2a was chosen as a model reaction to optimize the reaction by diverse catalysts, and solvents. The results were summarized in Table 1. A preliminary study was implemented with the aim of searching an appropriate catalyst to promote the reaction between aniline 1a and 1-phenylprop-2-ynyl acetate 2a. Copper catalyst was chosen as the initial experimental catalyst due to reports revealing copper's friendly applications in such intramolecular and intermolecular cyclic reactions. Firstly, when a solution of 1a and 2a in toluene was stirred in the presence of a catalytic amount of various copper catalysts (such as CuI, CuCl, CuBr, Cu(OAc)2, Cu(OTf)2, CuCl2, entries 1–6, respectively), it was observed that CuBr provided the desired 2-phenylquinoline 3a in 47% isolated yield (entry 3), which was found to be the best among all the considered copper catalysts. Then, the reaction conditions were further investigated, the influence of different solvents on the outcome of the model reaction was screened. It was established that it can be run in a number of solvents (Table 1, entries 7–13). We found that the chlorobenzene was the best solvent (Table 1, entry 11). Next, some ligands and bases were tested (see ESI†). In the end, we increased the amount of catalyst to 100% could not benefit the yield (Table 1, entry 14). Thus, the optimized reaction conditions were as follows: 1a (0.2 mmol), 2a (0.3 mmol), CuBr (20 mol%), in PhCl (2 mL) under air atmosphere at 120 °C.
| Entry | Catalyst | Solvent | Yield/3ab |
|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), catalyst (20 mol%), PhCl (2 mL) in sealed Schlenk tube, at 120 °C for 12 h.b Isolated yields.c CuBr (1 equiv.). | |||
| 1 | CuI | Toluene | Trace |
| 2 | CuCl | Toluene | 35 |
| 3 | CuBr | Toluene | 47 |
| 4 | Cu(OAc)2 | Toluene | Trace |
| 5 | Cu(OTf)2 | Toluene | 18 |
| 6 | CuCl2 | Toluene | 20 |
| 7 | CuBr | DCE | 53 |
| 8 | CuBr | DMF | 26 |
| 9 | CuBr | o-Xylene | 57 |
| 10 | CuBr | 1,4-Dioxane | 48 |
| 11 | CuBr | PhCl | 60 |
| 12 | CuBr | DMSO | 17 |
| 13 | CuBr | NMP | 29 |
| 14c | CuBr | PhCl | 49 |
With the optimized reaction conditions in hand, we next investigated the scope of this transformation by different aromatic anilines and 1-phenylprop-2-ynyl acetate 2a (Table 2). A variety of different aniline derivatives were smoothly converted to the corresponding 2-arylated quinolines products in moderate yield. First of all, when an electron-donating group at the different position, such as the o-methyl, m-methyl and p-methyl substituted aniline were obtained in 61%, 76%, and 53% yield respectively. However, two regioisomers generated from substrates 1c, in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Meanwhile, substrates 1e also afforded the desired product in acceptable yields. Then, anilines bearing double substituted groups on the aromatic ring, could moderately react with 1a, and provided the expected products. Although aniline 1a obtained 2-arylated quinoline 3a with a moderate 60% yield, the 4-fluorobenzenamine and 4-iodobenzenamine were less reactive, affording their target products in poor yields. Next, substrates containing carboxyl and amide groups also could lead to the formation of desired products in acceptable yields. Unfortunately, the substrate 4-nitro aniline 1m did not detect the corresponding products 6-nitro-2-phenylquinoline, only attained the intermediate propargyl amine 3m. It revealed that the reaction might undergo an electrophilic cyclic process. These results indicated that an electronic effect on the substituted group played a significant role in the reaction.
1 ratio. Meanwhile, substrates 1e also afforded the desired product in acceptable yields. Then, anilines bearing double substituted groups on the aromatic ring, could moderately react with 1a, and provided the expected products. Although aniline 1a obtained 2-arylated quinoline 3a with a moderate 60% yield, the 4-fluorobenzenamine and 4-iodobenzenamine were less reactive, affording their target products in poor yields. Next, substrates containing carboxyl and amide groups also could lead to the formation of desired products in acceptable yields. Unfortunately, the substrate 4-nitro aniline 1m did not detect the corresponding products 6-nitro-2-phenylquinoline, only attained the intermediate propargyl amine 3m. It revealed that the reaction might undergo an electrophilic cyclic process. These results indicated that an electronic effect on the substituted group played a significant role in the reaction.
To expand the scope of this cyclization methodology, the different substituted naphthylamines and ynyl acetate substrates were screened (Table 3). Initially, both α-naphthylamine and β-naphthylamine were explored, which were also tolerated in this transformation generating corresponding 3n and 3o in 70% and 40% yield. Then, a various of phenylprop-2-ynyl acetates reacted with the α-naphthylamine, both donating groups (such as Me, OMe) and electron-withdrawing (such as F, Cl and Br) were tolerated, and they could smoothly transform into the desired products in moderate to good yields. These results showed that electronic nature of the additional substituent had little influence on regulating the reaction. Substrates 2g with two substituents at the 2 and 5 positions were also viable for constructing 3u in 64% yields. Gratifyingly, the reaction of 1-(thiophen-3-yl)prop-2-yn-1-yl acetate 2h gave 3v in 64% yield, thus making this method useful for the preparation of heterocycle-containing pharmaceuticals and natural products. Finally, 9-phenanthryl substituted ynyl acetate 2i also underwent cyclization reaction, to provide 2-(anthracen-9-yl)benzo[h]quinoline 3w in 49% yield.
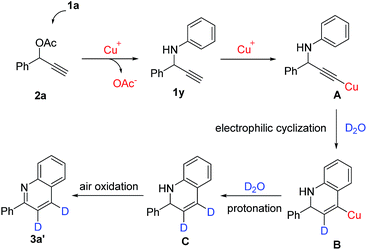
To shed light on the possible mechanism of the reaction, several control experiments were carried out, as shown in Scheme 2. Inspired by unpredictable product 3m which was obtained from substrate 1m, N-(1-phenylprop-2-yn-1-yl)aniline (1y) was treated under standard reaction conditions. To our delight, 3a was isolated in 80% yield (Scheme 2a). This result indicated that 1y should be the intermediate among the cyclization process. Subsequently, using substrate 2a′ to react with aniline under standard conditions. To our surprise, the reaction product was 3a, which did not have deuterium at any position of the quinoline ring (Scheme 2b). Next, we conducted the third control experiment in the presence of D2O under standard conditions. Notably, the product 3a′ had the deuterium at the 3 and 4 position of the quinoline ring (Scheme 2c). Meanwhile, N-(1,3-diphenylprop-2-yn-1-yl)aniline (1z) could not be detected the corresponding products under standard conditions (Scheme 2d). According to these control experiments, this result indicated that copper(I) acetylide should be a key intermediate in this intermolecular cyclization reaction.
Based on the above observations and previous mechanistic studies,18 a plausible mechanism is proposed in Scheme 3. First, the terminal acetylene ester 2a undergoes intermolecular nucleophilic attack by the aromatic amine to produce intermediate 1y. CuBr-mediated enhancement of the alkyne acidity by coordination to the carbon–carbon triple bond, enabling the formation of the corresponding copper(I) acetylide A. Then, A generates intermediate B by electrophilic cyclization. Subsequently, B undergoes protonation to produce unstable 2-phenyl-1,2-dihydroquinoline C. Finally, an air oxidation of C affords the desired products 3a′.19
In summary, we have developed a simple method for the synthesis of substituted quinoline derivatives by copper-catalyzed annulation of anilines with terminal acetylene esters. In the reaction, the C–N and C–C bonds are formed via an annulation process. In addition, no ligands, no bases are required and it could be performed under air conditions. Efforts to extend the applications of the transformation in organic synthesis as well as screen for biological activity of these types of compounds are currently underway in our laboratory.
Acknowledgements
This work was supported by the Natural Science Foundation of China (21572051, 21602057), the Research Project of Chinese Ministry of Education (213027A), Scientific Research Fund of Hunan Provincial Education Department (15A109).Notes and references
- (a) J. P. Michael, Nat. Prod. Rep., 2007, 24, 223 RSC; (b) J. P. Michael, Nat. Prod. Rep., 2008, 25, 166 RSC; (c) G. Gakhar, T. Ohira, A. Shi, D. H. Hua and T. A. Nguyen, Drug Dev. Res., 2008, 69, 526 CrossRef CAS; (d) A. Lilienkampf, J. Mao, B. Wan, Y. Wang, S. G. Franzblau and A. P. Kozikowski, J. Med. Chem., 2009, 52, 2109 CrossRef CAS PubMed; (e) M. V. N. de Souza, K. C. Pais, C. R. Kaiser, M. A. Peralta, M. de L. Ferreira and M. C. S. Lourenco, Bioorg. Med. Chem., 2009, 17, 1474 CrossRef CAS PubMed; (f) S. Chen, R. Chen, M. He, R. Pang, Z. Tan and M. Yang, Bioorg. Med. Chem., 2009, 17, 1948 CrossRef CAS PubMed; (g) J. Datta, K. Ghoshal, W. A. Denny, S. A. Gamage, D. G. Brooke, P. Phiasivongsa, S. Redkar and S. T. Jacob, Cancer Res., 2009, 69, 4277 CrossRef CAS PubMed; (h) K. Kaur, M. Jain, R. P. Reddy and R. Jain, Eur. J. Med. Chem., 2010, 45, 3245 CrossRef CAS PubMed; (i) V. J. Venditto and E. E. Simanek, Mol. Pharmaceutics, 2010, 7, 307 CrossRef CAS PubMed.
- Z. H. Skraup, Ber. Dtsch. Chem. Ges., 1880, 13, 2086 Search PubMed.
- A. Combes, Bull. Soc. Chim. Fr., 1883, 49, 89 Search PubMed.
- R. G. Gould and W. A. Jacobs, J. Am. Chem. Soc., 1939, 61, 2890 CrossRef CAS.
- F. Friedländer, Ber. Dtsch. Chem. Ges., 1882, 15, 2572 CrossRef.
- O. Doebner and W. von Miller, Ber. Dtsch. Chem. Ges., 1881, 14, 2812 CrossRef.
- (a) R. Perez-Ruiz, L. R. Domingo, M. C. Jimenez and M. A. Miranda, Org. Lett., 2011, 13, 5116 CrossRef CAS PubMed; (b) H. Richter and O. G. Mancheno, Org. Lett., 2011, 13, 6066 CrossRef CAS PubMed; (c) R. N. Monrad and R. Madsen, Org. Biomol. Chem., 2011, 9, 610 RSC; (d) X. Jia, F. Peng, C. Qing, C. Huo and X. Wang, Org. Lett., 2012, 14, 4030 CrossRef CAS PubMed; (e) Y. Chen, J. Huang, T.-L. Hwang, T. J. Li, S. Cui, J. Chan and M. Bio, Tetrahedron Lett., 2012, 53, 3237 CrossRef CAS; (f) Z. Wang, S. Li, B. Yu, H. Wu, Y. Wang and X. Sun, J. Org. Chem., 2012, 77, 8615 CrossRef CAS PubMed; (g) R. Suresh, S. Muthusubramanian, R. Senthilkumaran and G. Manickam, J. Org. Chem., 2012, 77, 1468 CrossRef CAS PubMed; (h) A. V. Gulevich, A. S. Dudnik, N. Chernyak and V. Gevorgyan, Chem. Rev., 2013, 113, 3084 CrossRef CAS PubMed; (i) R. Rohlmann, T. Stopka, H. Richter and O. G. Mancheno, J. Org. Chem., 2013, 78, 6050 CrossRef CAS PubMed; (j) J. B. Bharate, S. B. Bharate and R. A. Vishwakarma, ACS Comb. Sci., 2014, 16, 624 CrossRef CAS PubMed; (k) L. Kong, Y. Zhou, H. Huang, Y. Yang, Y. Liu and Y. Li, J. Org. Chem., 2015, 80, 1275 CrossRef CAS PubMed; (l) J. Tummatorn, P. Poonsilp, P. Nimnual, J. Janprasit, C. Thongsornkleeb and S. Ruchirawat, J. Org. Chem., 2015, 80, 4516 CrossRef CAS PubMed.
- C. S. Cho, J. Organomet. Chem., 2005, 690, 4094 CrossRef CAS.
- N. T. Patil and V. S. Raut, J. Org. Chem., 2010, 75, 6961 CrossRef CAS PubMed.
- C. E. Meyet and C. H. Larsen, J. Org. Chem., 2014, 79, 9835 CrossRef CAS PubMed.
- S. Majumder, K. R. Gipson and A. L. Odom, Org. Lett., 2009, 11, 4720 CrossRef CAS PubMed.
- R. P. Korivi and C.-H. Cheng, J. Org. Chem., 2006, 71, 7079 CrossRef CAS PubMed.
- S. Cai, J. Zeng, Y. Bai and X.-W. Liu, J. Org. Chem., 2012, 77, 801 CrossRef CAS PubMed.
- J. Horn, S. P. Marsden, A. Nelson, D. House and G. G. Weingarten, Org. Lett., 2008, 10, 4117 CrossRef CAS PubMed.
- C. S. Cho, B. H. Oh, J. S. Kim, T.-J. Kim and S. C. Shim, Chem. Commun., 2000, 19, 1885 RSC.
- Y. Zhang, M. Wang, P. Li and L. Wang, Org. Lett., 2012, 14, 2206 CrossRef CAS PubMed.
- R. Yan, X. Liu, C. Pan, X. Zhou, X. Li, X. Kang and G. Huang, Org. Lett., 2013, 15, 4876 CrossRef CAS PubMed.
- P. Siemsen, R. C. Livingston and F. Diederich, Angew. Chem., Int. Ed., 2000, 39, 2632 CrossRef CAS.
- T. S. Symeonidis, I. N. Lykakis and K. E. Litinas, Tetrahedron, 2013, 69, 4612 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra23858k |
| This journal is © The Royal Society of Chemistry 2016 |