One-pot microwave-assisted in situ reduction of Ag+ and Au3+ ions by Citrus limon extract and their carbon-dots based nanohybrids: a potential nano-bioprobe for cancer cellular imaging
P. A. Sajida,
S. Shashank Chettya,
S. Praneethaa,
A. Vadivel Murugan*a,
Yogesh Kumarb and
Latha Periyasamyb
aAdvanced Functional Nanostructured Materials Laboratory, Centre for Nanoscience & Technology, Madanjeet School of Green Energy Technologies, Pondicherry University (A Central University), Dr R. V. Nagar, Kalapet, Puducherry 605014, India. E-mail: avmrajeshwar@gmail.com; avmurugan.nst@pondiuni.edu.in
bDepartment of Biochemistry and Molecular Biology, Pondicherry University (A Central University), Dr R. V. Nagar, Kalapet, Puducherry 605014, India
First published on 25th October 2016
Abstract
In the present study, we demonstrate a rapid, in situ reduction of metal (Ag+ and Au3+) ions and subsequent carbonization of Citrus limon (lemon) extract to synthesize highly luminescent carbon-dots (C-dots) and their metal nanohybrids (MCNs) using one-pot microwave (MW) assisted technique within 6 min. In principle, L-ascorbic acid, citric acid and flavonoids reduce metal ions into corresponding metal nanoparticles followed by carbonization of carbohydrates in the lemon extract along with externally added ethylenediamine (EDA) to form EDA-functionalized C-dots based nanohybrids. The synthesized pristine C-dots, Ag/C-dot and Au/C-dot nanohybrids (<5 nm) colloidal solution exhibited bright photoluminescence (PL) at ∼515 nm with significant quantum yield (QY) of 48.3%, 46.2%, 62.2% and PL emission lifetime of 3.6 ns, 9.4 ns and 9.0 ns respectively. The reduced PL intensity and QY for Ag/C-dot nanohybrids could be due to fluorescence resonance energy transfer (FRET) while enhanced PL intensity and QY for Au/C-dot nanohybrids could be attributed to surface plasmonic resonance (SPR), when compared to pristine C-dots. These C-dots-based nanohybrids exhibited no signs of cytotoxicity in colon cancer cell lines (SW-480) and were easily internalized for fluorescence bioimaging. Thus, the MW-assisted sustainable synthesis of pristine C-dots and metal/C-dot nanohybrids derived from natural lemon extract can be exhibited as eco-friendly intense potential nano-bioprobes for cancer cellular imaging applications.
Introduction
Carbon dots (C-dots) with size <10 nm have been proposed as a fascinating class of zero-dimensional, quasi-spherical, carbonaceous photonic-nanomaterials.1–6 They have engendered significant research interest owing to their intrinsic properties such as size and wavelength-dependent photoluminescence (PL), low photo-bleaching, excellent water-solubility, biocompatibility and environmental amiability which collectively contribute to their vital applications in bioimaging.7–11 Due to the abundance of primary oxygen functionalities on their surface which account for its hydrophilicity, C-dots have been utilized in preparing optical sensors for detecting biomolecules, sub-cellular organelles and environmentally hazardous free radicals, thus extending their biological applications.1,2,12–14On the other hand, metal nanoparticles – Ag and Au in particular, have attracted massive interest due to their physicochemical and optoelectronic properties, resulting in their widespread use in photovoltaic, catalytic and biological applications, particularly in medicinal and anti-microbial activities.15–19 The size, shape, crystal structure and nanoparticle composition simultaneously contribute to determine their properties and subsequent interaction with incident light.15 When the size of nanoparticle is considerably smaller than the wavelength of incident light, the particle gets subjected to uniform electric field. This leads to collective oscillation of electrons in the conduction band, resulting in energy-dependent resonance known as Surface Plasmon Resonance (SPR).15 SPR accounts for effective light scattering that enhances surface plasmon absorption bands and localized electromagnetic fields. This causes overlapping of localized electromagnetic fields of carbon core excitons and surface plasmons, leading to coupling effect and stimulating enhanced radiative emission.20 However, in nanocomposites like C-dots/silver nanoparticles, a non-radiative phenomenon known as fluorescence resonance energy transfer (FRET) also occurs, resulting in reduced PL. Due to long-range dipole–dipole interactions between excited-state donor and ground-state acceptor lying in proximity, energy transfer occurs. As a result, a substantial quantity of radiant energy is dissipated as heat. In FRET, energy transfer rate is influenced by the extent of spectral overlapping between emission spectrum of excited donor and absorption spectrum of acceptor, resulting in effective fluorescence quenching and reduced PL.21
Recently, to enhance the optimal PL and biological properties of C-dots, surface-functionalizing strategies were developed.5,22,23 Similarly, incorporating noble metal nanoparticles onto carbon surface for Surface Enhanced Raman Scattering (SERS) studies, bactericidal and ratiometric fluorescence bioimaging applications have been recently reported.10,24,25 Metal/Carbon-Nanohybrids (MCNs) are advantageous over long-established metal nanoparticles (MNPs) due to C-dots being excellent electron donors and capable of reducing metal salts to corresponding metal nanoparticles on the surface and simultaneously serving as ideal adsorbent layer and stabilizing agent for MNPs.
Since the serendipitous discovery of C-dots, systematic and extensive studies regarding synthetic strategies and applications were carried out due to their inherent properties. However, these methods had many drawbacks such as requirement of unsustainable and costly raw materials, toxic reagents, complex experimental set-up, multi-step synthesis processes, acid/alkali/oxidant treatment, higher temperature and greater time consumption which were rendered incompatible to the concept of green chemistry.2,9,26 Thus, it has become a necessity to synthesize highly fluorescent C-dots from earth-abundant and low-cost natural resources. In this regard, bottom-up approaches such as pyrolysis,23,27 silica-supported synthesis,28 solvo/hydrothermal carbonization of carbohydrates obtained from synthetically derived precursors2,29 or natural sources such as pomelo-peel, egg-shell membrane ashes, coffee grounds, date molasses, cow milk and orange juice were demonstrated to synthesize C-dots.2,3,30–33 Among them, microwave (MW) assisted synthesis method is one of the most energy-efficient, sustainable synthesis process and relatively requires lesser temperature and time with better product yield.23,30,32 Of recent, Ag and Au nanoparticles have been synthesized using Citrus limon extracts.34,35 Regarding the synthesis of MCNs, Luo et al. electrochemically synthesized C-dots to prepare Au/C-dot nanocomposites for 80 min.10 Similarly, Shen et al. synthesized C-dots from chitosan to reduce Ag-nanoparticles using multistep processes for nearly 39 h.12 Essner and co-workers successfully synthesized Ag–Au@C-dot bimetallic nanoparticles using thermal degradation of solid citric acid for 2 h with both Ag and Au nanoparticles prepared at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.15 Very recently, gold-carbon dots were prepared from glucose, chloroauric acid and glutathione by Zhang et al. using microwave method within 20 min for ratiometric cellular imaging applications.25 In our laboratory, microwave-assisted solvo/hydrothermal methods have been successfully developed for the synthesis of several nanostructured hybrid materials.36–39
1.15 Very recently, gold-carbon dots were prepared from glucose, chloroauric acid and glutathione by Zhang et al. using microwave method within 20 min for ratiometric cellular imaging applications.25 In our laboratory, microwave-assisted solvo/hydrothermal methods have been successfully developed for the synthesis of several nanostructured hybrid materials.36–39
Here we demonstrate a rapid, in situ reduction of Ag+ and Au3+ ions using natural and earth abundant Citrus limon (lemon) extract and its subsequent carbonization along with ethylenediamine (EDA) functionalization to synthesize highly luminescent pristine carbon-dots (C-dots) and metal/C-dot nanohybrids (MCNs) using one-pot, sustainable microwave-assisted technique within 6 min as illustrated in Fig. 1. Unlike most of the natural resources, lemon extract is easily procurable and requires zero-processing for the synthesis of C-dots-based nanohybrids. The lemon extract is a rich and natural source of L-ascorbic acid (vitamin-C) which constitutes about 64%, 6% citric acid besides 2.5% sugars and flavonoid derivatives.40 Both L-ascorbic acid and citric acid serve as carbon precursors and predominant reducing agents for developing MCNs with enhanced physico-chemical and optical properties. It is hypothesized that the tautomeric transformations of flavonoids from enol-form to keto-form release reactive hydrogen atom that may also aid in reduction of metal ions to metal nanoparticle.41 The EDA-functionalization on MCNs enables the formation of emissive trap sites on C-dot surface, thus controlling the particle size and subsequently enhancing the fluorescence.23 The nanohybrids derived from natural lemon extract here exhibit intense potential as eco-friendly nano-bioprobes for cancer cellular imaging applications.
Materials and methods
Materials
All the materials were used without further purification. All the experiments were carried out using double distilled water. Fresh grade lemons were procured from vegetable market. Ethylenediamine (EDA) was purchased from Merck. Silver nitrate (AgNO3) and chloroauric acid (HAuCl4) were purchased from Fischer Scientific Co. and Sigma-Aldrich respectively. Dulbecco modified Eagle medium (DMEM), fetal bovine serum (FBS), penicillin and streptomycin for preparing 1% antibiotic solution, phosphate buffer saline (PBS) and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) were all purchased from Himedia. Dimethyl sulfoxide (DMSO) was purchased from Merck Biosciences.Synthesis of pristine C-dots from lemon extract
Briefly, 50 mL of fresh lemon extract was filter-prepared with sterile hand-squeezing and 9 mM (603 μL) EDA was added. The lemon juice–EDA mixture was instantly transferred into domestic microwave oven (800 W) and irradiated for 6 min. During irradiation, the colour of the solution changed from white turbid to dark brown indicating the formation of pristine amine-functionalized C-dots. The C-dots powder was stored at room temperature for further characterization.Synthesis of silver/carbon-dot (Ag/C-dot) nanohybrids
20 mL of lemon extract was mixed thoroughly with 15 mL of 1 mM AgNO3 solution, 15 mL of deionized water and 9 mM EDA. The solution mixture was transferred into microwave oven (800 W) and irradiated for 6 min. During irradiation, the solution colour changed from white turbid solution to glittering yellow, indicating the reduction of Ag+ ions to Ag nanoparticles and finally to dark brown colour, indicating the formation of amine-functionalized Ag/C-dot nanohybrids. The Ag/C-dot nanohybrids powder was stored at room temperature for further characterization. The mechanism involving reduction of Ag+ ions to Ag nanoparticles has been explained as follows:42,43Synthesis of gold/carbon-dot (Au/C-dot) nanohybrids
Au/C-dot nanohybrids were synthesized using same procedure as used for the preparation of Ag/C-dot nanohybrids. Here, 20 mL of filter-prepared lemon extract was mixed thoroughly with 15 mL of 1 mM chloroauric acid (HAuCl4) solution, 15 mL of deionized water and 9 mM EDA. The solution mixture was transferred into microwave oven (800 W) and irradiated for 6 min. During irradiation, the solution colour changed from white turbid to purple, indicating the reduction of Au3+ ions to Au nanoparticles and finally to dark brown colour, indicating the formation of amine-functionalized Au/C-dot nanohybrids. The Au/C-dots nanohybrid powder was stored at room temperature for further characterization. The reaction mechanism involving reduction of Au3+ ions to Au nanoparticles has been explained as follows:44Material characterization and instruments
Structural characterization of synthesized pristine C-dots and MCNs was carried out using PANalytical X'pert powder X-ray diffraction system (Phillips) with Cu Kα radiation (λ = 1.54 Å), operating at 200 mA and an X-ray acceleration voltage of 40 kV for scanning the diffraction angles from 10–80°. The chemical composition as well as functional groups was confirmed using Bruker-Alpha Fourier Transform Infrared (FTIR) spectrophotometer. The absorbance spectra were recorded using UV-3600 plus spectrophotometer (Shimadzu). The steady-state emission spectra and time-resolved PL (TRPL) spectra were recorded using Fluorolog spectrophotometer (Jobin Yvon) with excitation wavelength ranging from 300 to 480 nm and spectral resolution of 3 nm. High Resolution Transmission Electron Microscopy (HRTEM) images were obtained using Jeol/JEM 2100 microscope with lanthanum hexaborate (LaB6) as electron source and an accelerating voltage of 200 kV and magnification from 2000–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×. The Energy Dispersive X-ray (EDX) analysis was carried out using Bruker-Alpha analyzer.
000×. The Energy Dispersive X-ray (EDX) analysis was carried out using Bruker-Alpha analyzer.
Quantum yield (QY) and PL lifetime measurement
The PL QY of pristine C-dots and MCNs was measured using Rhodamine-6G (dissolved in ethanol, QY = 0.95) as reference by the equation:36,45| Φs = (Fs/Fr)(Ar/As)(ns2/nr2)Φr |
The amplitude-weighted average lifetime (τav) was calculated using the equation:46–48
| τav = B1τ12 + B2τ22 + B3τ32/B1τ1 + B2τ2 + B3τ3 |
In vitro cytotoxicity studies
MTT assay, as described by Mossman49 was carried out to determine the cellular viability of the prepared MCNs. Cell line SW-480 (human colon primary adenocarcinoma) was purchased from National Centre for Cell Science (NCCS), Pune, India. The cells were cultured in DMEM supplemented with 10% FBS in 96-well flat-bottomed culture plates and incubated for 24 h in a humidified 5% CO2 incubator at 37 °C. The cells were seeded at a density of 5 × 103 cells per well per 200 μL of the culture media. After 24 h of incubation of SW-480 cells, they were treated with aqueous solutions of the MCNs at different concentrations (10, 50, 100, 250, 500 and 1000 μg mL−1 respectively) and incubated again for 24 h. After removing the culture medium, 20 μL of 5 mg mL−1 MTT solution was added and the plate was again incubated at 37 °C for 4 h in the dark. The purple-coloured formazan crystals formed were dissolved in 100 μL of dimethyl sulfoxide (DMSO). The quantity of formazan metabolite formed was measured as a function of its absorbance value attained at 570 nm. The absorbance values were determined using ELISA plate spectrophotometer (Molecular Devices Inc., Sunnyvale, CA, USA) and the cell-viability was calculated accordingly using the formula:46,49| Cell viability (%) = (As/Ac) × 100. |
In vitro cellular imaging
The SW-480 cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin antibiotic solution in tissue culture flasks. To carry out in vitro cellular imaging studies, the cells were seeded in a 6-well plate at a density of 104 cells per well and incubated for 24 h. The cells were incubated with fresh culture media containing 100 μL of 1 mg mL−1 concentration of pristine C-dots and MCNs for 4 h. Finally, the cells were washed thrice with PBS and observed under inverted fluorescence microscope.Results and discussions
The mechanism of pristine C-dots and MCNs synthesis from lemon extract involves in situ reduction of metal ions (Ag+, Au3+) and subsequent carbonization of its components. During synthesis, the microwave radiation directly interacts with the reactant molecules resulting in rapid and localized heat transfer. Lemon extract undergoes dehydration and sucrose decomposes to glucose and fructose and to intermediate products like furfural derivatives, organic acids, aldehydes and phenolic derivatives like flavonoids (FOH). The organic acids contain hydronium ion which catalyses the following decomposition steps. Polymerization and condensation of furfural derivatives and organic acids result in soluble polymers, which undergo aromatization to form aromatic clusters via aldol condensation, cycloaddition and hydroxymethyl-mediated furan resin condensation. These aromatic clusters get concentrated and undergo nucleation burst on attaining critical supersaturation point, resulting in the formation of carbon dots.2,33 EDA facilitates amine passivation at C-dot surfaces. However in MCNs preparation, the weak acids (citric acid, L-ascorbic acid) and flavonoid derivatives present in the lemon extract actively chelate and reduce metal ions into metal nanoparticles in addition to the microwave radiation. Also, the conversion of ketones to carboxylic functional groups is likely to be involved in Ag+/Au3+ ion reduction resulting in the stages of initiation of metal nanoparticle formation via nucleation assisted growth.41 These metal nanoparticles further result in the formation of metal/C-dot nanohybrids via carbonization of carbohydrates with other constituents present in the natural lemon extract. The mechanism involved in the reduction of metal ions to metal nanoparticles by flavonoids has been explained as follows:50Analytical characterization
Fig. 2 represents X-ray diffraction (XRD) patterns of the synthesized pristine C-dots and MCNs. The XRD patterns displayed a broad (220) peak obtained at 16.59° for pristine C-dots and slightly shifted to 17.69° for both MCNs, indicating a highly amorphous carbon structure (ICDD 01-082-0505). Interestingly, the occurrence of (220) plane obtained for pristine C-dots and MCNs synthesized from lemon extract, denoting a cubic crystal structure was hardly reported unlike previously reported (002), (100) and (101) planes.7,15,33 However, in MCNs the presence of metal nanoparticles on C-dot surfaces indicated a transformation from amorphous to nanocrystalline phase, which was confirmed by the formation of metal-incorporated (111), (002), (022) and (113) peaks at 38°, 44°, 64° and 77° respectively and were also matching with standard reference codes for silver and gold (ICDD 98-008-3968 and ICDD 98-008-5071 respectively). The corresponding d-value for highly intense (111) metal peak was found to be 0.23 nm for both Ag/C-dot and Au/C-dot nanohybrids. | ||
| Fig. 2 XRD patterns of synthesized (a) Ag/C-dot nanohybrids and (b) Au/C-dot nanohybrids (right) with respect to pristine C-dots (green) from lemon extract. | ||
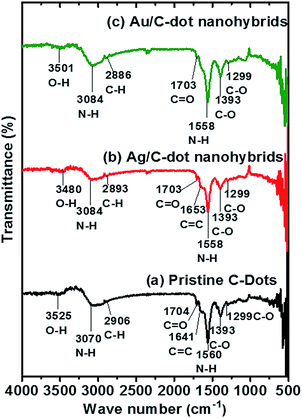
To clearly understand about the chemical composition and surface functional group present in the synthesized C-dots and MCNs, FTIR spectroscopy was carried out as shown in Fig. 3.
A feeble hydroxyl (–OH) peak was observed for pristine, Ag/C-dot and Au/C-dot nanohybrids at 3501 cm−1, 3480 cm−1 and 3525 cm−1 respectively caused by disintegration of most –OH moieties of sucrose during microwave pyrolysis.32 A prominent peak was found at 3024 cm−1, 3084 cm−1 and 3072 cm−1 respectively due to –NH2 stretching vibrations and at 1558 cm−1 for pristine C-dots and Ag/C-dot nanohybrids as well as at 1560 cm−1 for Au/C-nanohybrids due to deformed –NH2 vibrations caused by amine functionalization on C-dot surface. The C–H (methyl) stretching vibration was found at 2906 cm−1, 2893 cm−1 and 2886 cm−1 respectively. A faint C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration found at 1704 cm−1, 1703 cm−1 and 1697 cm−1 indicated the occurrence of weaker aromatic carbonyl linkages formed on the surface. The occurrence of vibrational C
O stretching vibration found at 1704 cm−1, 1703 cm−1 and 1697 cm−1 indicated the occurrence of weaker aromatic carbonyl linkages formed on the surface. The occurrence of vibrational C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bands caused by sp2 graphitic carbon atoms at 1653 cm−1 and 1641 cm−1 for Ag/C-dot and Au/C-dot nanohybrids respectively confirmed the formation of C-dots due to carbonization of precursors during microwave treatment. In addition, all the synthesized C-dots and MCNs exhibited C–H bend at 1393 cm−1 which was caused by sp3 carbon atoms present in C-dots. Aliphatic C–O stretching vibrations were found at 1299 cm−1 and 1293.7 cm−1.
C bands caused by sp2 graphitic carbon atoms at 1653 cm−1 and 1641 cm−1 for Ag/C-dot and Au/C-dot nanohybrids respectively confirmed the formation of C-dots due to carbonization of precursors during microwave treatment. In addition, all the synthesized C-dots and MCNs exhibited C–H bend at 1393 cm−1 which was caused by sp3 carbon atoms present in C-dots. Aliphatic C–O stretching vibrations were found at 1299 cm−1 and 1293.7 cm−1.
UV-Visible spectra of the synthesized pristine C-dots and MCNs were obtained as shown in Fig. 4(a). A broad peak was observed at around 350 nm due to n–π* transition. Similarly, a dip was observed near to 300 nm due to π–π* electronic transition caused by characteristic surface-bending of graphitic sp2 carbon sheets onto the surface of C-dot atoms.32 The band-gap energy was measured and was found to be 3.54 eV which is much greater than that of bulk graphitic carbon (−0.04 eV).
The steady state photoluminescent (PL) spectra of pristine C-dots, Ag/C-dot and Au/C-dot nanohybrids are shown in Fig. 4(b)–(d) respectively. The synthesized C-dots and MCNs commonly exhibited optimal excitation wavelength (λex) centered at 460 nm and the corresponding photoluminescence emission wavelength at 517 nm, 515 nm and 513 nm respectively. The synthesized C-dots exhibited excitation-wavelength dependent PL behaviour characteristic of fluorescent C-dots which was in turn attributed to the presence of emissive surface traps and aromatic conjugated structures present within the dots. The PL quantum yield was measured to be 48.3%, 46.2% and 62.2% for pristine C-dots, Ag/C-dot and Au/C-dot nanohybrids respectively.
The PL mechanism in synthesized C-dots and MCNs occurs predominantly due to two types of surface-states. First, oxygen-functional groups account for primary surface states on the carbon core formed due to partial oxidation of precursor molecules during C-dots synthesis. Secondly, amine functionalization on the C-dot surface results in surface-emissive trap sites with definite energy which get activated with optimal excitation energy.23 Thus, the carbon core and their functional groups (oxo and amine groups) undergo hybridization for synergetic emission. These functional groups on the C-dot surface develop several “surface states” with different energy-levels that result in excitation wavelength-dependent PL behaviour.23
In MCNs, surface plasmonic resonance (SPR) comes into act in addition to the carbon core and emissive trap sites present in C-dot surface. The PL mechanism of synthesized Ag/C-dot nanohybrids exhibited reduced fluorescence intensity and PL QY compared to pristine C-dots. This could be attributed to minimal level of FRET in addition to SPR, resulting in spectral overlapping between emission spectrum of C-dots and absorption spectrum of Ag nanoparticles.25
The energy transfer in the Ag/C-dot nanohybrids roots non-radiative recombination resulting in enhanced fluorescence quenching. Due to minimal concentration of Ag NPs, the effect of fluorescence quenching is much reduced. However, Au/C-dot nanohybrids exhibit significantly higher fluorescence intensity and PL QY with respect to both pristine and Ag/C-dot nanohybrids. Compared to silver (Ag+) ions (1.29 Å), gold (Au3+) ions have reduced ionic size of 0.85 Å. As SPR varies inversely with ionic size of the metal, the resulting Au/C-dot nanohybrids exhibit greater fluorescence intensity and significant QY.
The PL lifetime measurement of C-dots based nanohybrids using time-correlated single photon counting (TCSPC) method is shown in Fig. 5. The decay curves were tri-exponentially fitted and the average PL decay (exciton lifetime) of pristine C-dots, Ag/C-dot and Au/C-dot nanohybrids was calculated to be 3.6, 9.4 and 9.0 ns respectively. The pristine C-dots exhibited a shorter life-time due to radiative recombination of carbon core excitons.45,47 Moreover, MCNs showed significantly enhanced exciton lifetime due to SPR coupled photoluminescence.20
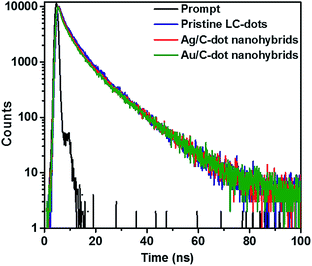 | ||
| Fig. 5 Time-resolved photoluminescence (TR-PL) decay spectra of pristine C-dots (blue), Ag/C-dot (red) and Au/C-dot (green) nanohybrids. | ||
To understand the size, morphology and the presence of metal nanoparticles embedded on the surface of synthesized MCNs, HRTEM images were taken as shown in Fig. 6. The metal nanoparticles were dispersed on the surface of carbon matrix and denoted a spheroidal shape with size < 5 nm. The interlayer spacing of metal nanoparticles (Au and Ag) were measured to be 0.24 nm, which was closely attributed to d-spacing value of (111) plane obtained from XRD (Fig. 2a and b).
 | ||
| Fig. 6 HRTEM images of (a) Ag/C-dot and (b) Au/C-dot nanohybrids with metal nanoparticle size of <5 nm with interlayer spacing of 0.24 nm. | ||
The presence of noble metals in the MCNs was further confirmed using EDX analysis as shown in Fig. 7. Carbon element exhibited predominant weight percentage of 59.59% and 56.4% in synthesized Ag/C-dot and Au/C-dot nanohybrids respectively, due to carbonization of precursors. Nitrogen element was 10.73% and 11.82%, respectively due to the functionalization of amine groups on the C-dots surface. Oxygen content was 28.5% and 31.64%, due to the occurrence of oxo-functionalized group in consistent to FTIR spectra. Significantly, the hybridization of metal in C-dots was confirmed with 1.19% of silver in Ag/C-dot and 0.14% of gold in Au/C-dot nanohybrids.
 | ||
| Fig. 7 Energy dispersive X-ray (EDX) analysis spectra of Ag/C-dot (top) and Au/C-dot nanohybrids (bottom). Ag/C-dots showed 1.19% wt percentage of silver whereas Au-LC dots denoted 0.14% of gold. | ||
Biological characterization
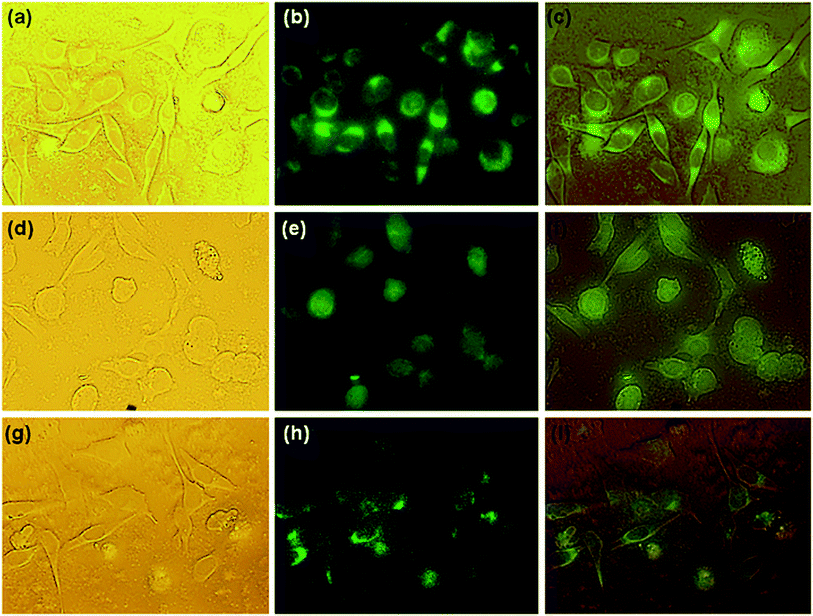
The cancer cellular imaging potential of synthesized C-dots-based nanohybrids has been shown in Fig. 9. The bright field microscopic images exhibited well defined cellular morphology, fluorescence microscopic images displayed green fluorescence and superimposed image indicated the facile internalization of pristine C-dots and MCNs through cellular membrane and distribution within the cytoplasmic region via endocytosis.52
Conclusions
In summary, we have successfully demonstrated “green” synthesis of C-dots-based nanohybrids via in situ reduction of noble metal ions (Ag+ and Au3+) by citric acid, L-ascorbic acid and flavonoids present in lemon extracts using one-pot microwave irradiation technique without requiring any acid/alkali treatment. The XRD spectra showed amorphous and disordered graphitic structure with phase transformation to nanocrystalline due to the presence of metal nanoparticles in C-dots-based nanohybrids. FTIR analysis revealed the stretching vibrations of carbon–carbon (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), amine (–NH2), acyl (C–O) and hydroxyl (–OH) groups. The synthesized samples showed characteristic absorbance peaks due to π–π* electronic transition and excitation wavelength-dependent PL behaviour.
C), amine (–NH2), acyl (C–O) and hydroxyl (–OH) groups. The synthesized samples showed characteristic absorbance peaks due to π–π* electronic transition and excitation wavelength-dependent PL behaviour.
The C-dots-based nanohybrids exhibit significant PL QY and dynamic lifetime. The reduced PL for Ag/C-dot nanohybrids could be attributed to FRET whereas the enhanced PL for Au/C-dot nanohybrids was due to SPR, primary oxo-functional groups and surface-emissive trap sites present in the carbon core. SPR constitutes enhanced PL lifetime for Ag/C-dot (9.4 ns) and Au/C-dot nanohybrids (9.0 ns), compared to pristine C-dots (3.6 ns). The synthesized MCNs exhibited a spheroidal morphology with size < 5 nm. The EDX analysis confirmed the presence of metals in C-dot nanohybrids. MTT assay exhibited enhanced biocompatibility and no signs of cytotoxicity even at highest concentration of 1 mg mL−1. Cellular uptake studies illustrated rapid internalization of the C-dots based nanohybrids in the SW-480 cells with bright green fluorescence. Thus, C-dots-based nanohybrids synthesized using microwave-assisted technique exhibit intense potential as eco-friendly nano-bioprobes for cancer cellular imaging applications.
Acknowledgements
We gratefully acknowledge the financial support provided by UGC-Maulana Azad National Fellowship (2012), Ministry of Minority Affairs, Government of India. We sincerely thank the Central Instrumentation Facility (CIF), Pondicherry University for carrying out material characterization. We are grateful to Dr Agnishwar Girigoswamy (Chettinad Health City, Chennai) for arranging facilities to carry out FTIR Analysis.Notes and references
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726 CrossRef CAS PubMed.
- Z. Yang, Z. Li, M. Xu, Y. Ma, J. Zhang, Y. Su, F. Gao, H. Wei and L. Zhang, Nano-Micro Lett., 2013, 5(4), 247 CrossRef.
- W. Lu, X. Qin, S. Liu, G. Chang, Y. Zhang, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Anal. Chem., 2012, 84, 5351 CrossRef CAS PubMed.
- H. Huang, C. Li, S. Zhu, H. Wang, C. Chen, Z. Wang, T. Bai, Z. Shi and S. Feng, Langmuir, 2014, 30, 13542 CrossRef CAS PubMed.
- Y. Kang, Y. Li, Y. Fang, Y. Xu, X. Wei and X. Yin, Sci. Rep., 2015, 5, 11835 CrossRef PubMed.
- Z. C. Yang, M. Wang, A. M. Yong, S. Y. Wong, X. H. Zhang, H. Tan, A. Y. Chang, X. Li and J. Wang, Chem. Commun., 2011, 47, 11615 RSC.
- Y. Zhang, D. Ma, Y. Zhuang, X. Zhang, W. Chen, L. Hong, Q. Yan, K. Yu and S. Huang, J. Mater. Chem., 2012, 22, 16714 RSC.
- E. J. Goh, K. S. Kim, Y. R. Kim, H. S. Jung, S. Beack, W. H. Kong, G. Scarcelli, S. H. Yun and S. K. Hahn, Biomacromolecules, 2012, 13, 2554 CrossRef CAS PubMed.
- Y. Yang, J. Cui, M. Zheng, C. Hu, S. Tan, Y. Xiao, Q. Yang and Y. Liu, Chem. Commun., 2012, 48, 380 RSC.
- P. Luo, C. Li and G. Shi, Phys. Chem. Chem. Phys., 2012, 14, 7360 RSC.
- H. Zhang, Y. Chen, M. Liang, L. Xu, S. Qi, H. Chen and X. Chen, Anal. Chem., 2014, 86, 9846 CrossRef CAS PubMed.
- L. Shen, M. Chen, L. Hu, X. Chen and J. Wang, Langmuir, 2013, 29, 16135 CrossRef CAS PubMed.
- N. Na, T. T. Liu, S. H. Xu, Y. Zhang, D. C. He, L. Y. Huang and J. Ouyang, J. Mater. Chem. B, 2013, 1(6), 787 RSC.
- S. Zhao, M. Lan, X. Zhu, H. Xue, T. W. Ng, X. Meng, C. S. Lee, P. Weng and W. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 17054 CAS.
- J. B. Essner, C. H. Laber and G. N. Baker, J. Mater. Chem. A, 2015, 3, 16354 CAS.
- S. Saha, A. Pal, S. Kundu, S. Basu and T. Pal, Langmuir, 2009, 26, 2885 CrossRef PubMed.
- J. Qi, X. Dang, P. T. Hammond and A. M. Belcher, ACS Nano, 2011, 5, 7108 CrossRef CAS PubMed.
- S. Chernousova and M. Epple, Angew. Chem., Int. Ed., 2013, 52, 1636 CrossRef CAS PubMed.
- Z. Mu, X. W. Zhao, Z. Xie, Y. Zhao, Q. F. Zhong, L. Bo and Z. Z. Gu, J. Mater. Chem. B, 2013, 1, 1607 RSC.
- H. Choi, S. J. Ko, Y. Choi, P. Joo, T. Kim, B. R. Lee, J. W. Jung, H. J. Choi, M. Cha, J. R. Jeong, I. W. Hwang, M. H. Song, B. S. Kim and J. Y. Kim, Nat. Photonics, 2013, 2013, 181, DOI:10.1038/NPHOTON.
- M. Amjadi, Z. Abolghasemi-Fakhri and T. Hallaj, J. Photochem. Photobiol., A, 2015, 309, 8 CrossRef CAS.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. F. Wang, P. G. Luo, H. Yang, M. E. Kose, B. L. Chen, L. M. Veca and S. Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756 CrossRef CAS PubMed.
- X. Zhai, P. Zhang, C. Liu, T. Bai, W. Li, L. Dai and W. Liu, Chem. Commun., 2012, 48, 7955 RSC.
- S. Han, H. Zhang, Y. Xie, L. Liu, C. Shan, X. Li, W. Liu and Y. Tang, Appl. Surf. Sci., 2015, 328, 368 CrossRef CAS.
- L. Zhang, D. Wang, H. Huang, L. Liu, Y. Zhou, X. Xia, K. Deng and X. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 6646 CAS.
- X. Wang, L. Cao, S. Yang, F. Lu, M. J. Meziani, L. Tian, K. Sun, M. A. Bloodgood and Y. Sun, Angew. Chem., Int. Ed., 2010, 49(31), 5310 CrossRef CAS PubMed.
- F. Wang, Z. Xie, H. Zhang, C. Liu and Y. Zhang, Adv. Funct. Mater., 2011, 21, 1027 CrossRef CAS.
- R. Liu, D. Wu, S. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., Int. Ed., 2009, 48, 4598 CrossRef CAS PubMed.
- Z. Yan, J. Shu, Y. Yu, Z. Zhang, Z. Liu and J. Chen, Luminescence, 2015, 30, 388 CrossRef CAS PubMed.
- Q. Wang, X. Liu, L. C. Zhang and Y. Lv, Analyst, 2012, 137(22), 5392 RSC.
- P. C. Hsu, Z. Y. Shih, C. H. Lee and H. T. Chang, Green Chem., 2012, 14, 917 RSC.
- B. Das, P. Dadhich, P. Pal, P. K. Srivas, K. Bankoti and S. Dhara, J. Mater. Chem. B, 2014, 2, 6839 RSC.
- S. Sahu, B. Behera, T. K. Maiti and S. Mohapatra, Chem. Commun., 2012, 48, 8835 RSC.
- T. C. Prathna, N. Chandrasekharan, A. M. Raichur and A. Mukherjee, Colloids Surf., B, 2011, 82, 152 CrossRef CAS PubMed.
- M. V. Sujitha and S. Kannan, Spectrochim. Acta, Part A, 2013, 102, 15 CrossRef CAS PubMed.
- S. Shashank Chetty, S. Praneetha, S. Basu, C. Sachidanandan and A. Vadivel Murugan, Sci. Rep., 2016, 6, 26078 CrossRef PubMed.
- S. Praneetha and A. Vadivel Murugan, RSC Adv., 2013, 3, 25403 RSC.
- S. Praneetha and A. Vadivel Murugan, ACS Sustainable Chem. Eng., 2015, 3, 224 CrossRef CAS.
- R. Krishnapriya, S. Praneetha and A. Vadivel Murugan, CrystEngComm, 2015, 17, 8353 RSC.
- United States Department of Agriculture (USDA) Food Composition Database, NDB No. 09150.
- V. V. Makarov, A. J. Love, O. V. Sinitsyna, S. S. Makarova, I. V. Yaminsky, M. E. Taliansky and N. O. Kalinina, Acta Nat., 2014, 6(1), 35 CAS.
- W. Songping and M. Shuyuan, Mater. Chem. Phys., 2005, 89, 423 CrossRef.
- M. K. H. Bhuiyan and M. Emran Quayum, Dhaka Univ. J. Pharm. Sci., 2013, 12(1), 29 Search PubMed.
- H. N. Verma, P. Singh and R. M. Chavan, Vet. World, 2014, 7(2), 72 CrossRef CAS.
- B. Chen, H. Zhong, W. Zhang, Z. Tan, Y. Li, C. Yu, T. Zhai, Y. Bando, S. Yang and B. Zou, Adv. Funct. Mater., 2012, 22, 2081 CrossRef CAS.
- L. Wang and H. Susan Zhou, Anal. Chem., 2014, 86, 8902 CrossRef CAS PubMed.
- P. Rai, T. D. Cole, E. Thompson, D. P. Millar and S. Linn, Nucleic Acids Res., 2003, 31(9), 2323 CrossRef CAS PubMed.
- A. Grinvald and I. Z. Steinberg, Anal. Biochem., 1974, 59, 583 CrossRef CAS PubMed.
- T. Mossman, J. Immunol. Methods, 1983, 65, 55 CrossRef.
- M. Nasrollahzadeh, S. Mohammad Sajadi and M. Khalaj, RSC Adv., 2014, 4, 47313 RSC.
- S. Y. Park, H. U. Lee, Y. C. Lee, S. Chol, D. H. Cho, H. S. Kim, S. Bang, S. Seo, S. C. Lee, J. Won, B. C. Son, M. Yang and J. Lee, Sci. Rep., 2015, 5, 12420 CrossRef PubMed.
- P. Hsu, P. Chen, C. Ou, H. Chang and H. Chang, J. Mater. Chem. B, 2013, 1, 1774 RSC.
| This journal is © The Royal Society of Chemistry 2016 |