Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantioselective Diels–Alder reaction in the confined space of homochiral metal–organic frameworks†
Koichi
Tanaka
*a,
Shohei
Nagase
a,
Taku
Anami
a,
Michał
Wierzbicki
b and
Zofia
Urbanczyk-Lipkowska
b
aDepartment of Chemistry and Materials Engineering, Faculty of Chemistry, Materials and Bioengineering, Kansai University, Suita, Osaka 564-8680, Japan. E-mail: ktanaka@kansai-u.ac.jp; Fax: +81-06-6368-0861; Tel: +81-06-6368-0861
bInstitute of Organic Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warszawa, Poland. E-mail: ocryst@icho.edu.pl; Fax: +48 22 6326681; Tel: +48 22 3432207
First published on 17th November 2016
Abstract
A novel homochiral porous metal–organic framework (MOF) has been synthesized using (R)-2,2′-dihydroxy-1,1′-binaphthyl-4,4′-dibenzoic acid as the chiral ligand. This MOF acts as an effective heterogeneous catalyst for the enantioselective Diels–Alder reaction between isoprene and N-ethyl maleimide.
Porous metal–organic frameworks (MOFs) with high surface areas and thermal stabilities have attracted much attention recently owing to their versatile applications in storage,1 separation,2 sensing,3 and catalysis.4 In particular, chiral MOFs, which are assembled using chiral organic ligands and metal ions, are of interest for applications in enantioselective separation and catalysis that are important for the pharmaceutical industry. Some homochiral MOFs and their applications in enantioselective reactions and separation have been described in the literature,5 but there has been limited success until now. We have previously reported the synthesis of homochiral (R)-MOF-1 using chiral ligand 1, as well as its application in the asymmetric aminolysis6 and alcoholysis7 of epoxides, and in the asymmetric sulfoxidation8 of sulfides using aqueous H2O2. We have also reported the efficient HPLC enantioseparation of several racemates using homochiral (R)-MOF-2 (prepared from chiral ligand 2) as the chiral stationary phase.9
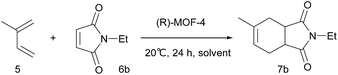
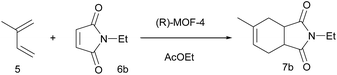
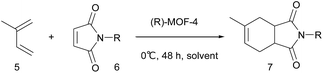
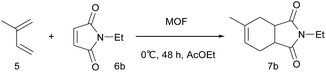
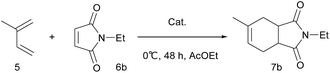
The [4 + 2] cycloaddition reaction between a diene and a dienophile, known as the Diels–Alder reaction, is one of the most powerful methods for C–C bond construction in synthetic organic chemistry.10 In particular, asymmetric catalytic variants of this reaction have received much attention, owing to their ability to rapidly provide enantioenriched carbocycles from simple substrates.11 Chiral MOFs are promising candidates for these asymmetric Diels–Alder reactions because they can encapsulate the reactants and organize them in a confined chiral space that contains Lewis acidic metal sites. However, there has been only two reports on MOF-catalyzed heterogeneous Diels–Alder reactions; however, these were not enantioselective.12 Herein, we report the synthesis of novel homochiral (R)-MOF-4 and its successful application as a heterogeneous catalyst in the enantioselective Diels–Alder reaction between isoprene and N-ethyl maleimide.
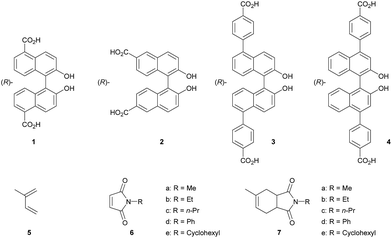
Chiral organic ligand 4 was synthesized by Suzuki cross-coupling of 4-methoxycarbonyl phenylboronic acid and (R)-4,4′-dibromo-2,2′-diacetyl-1,1′-binaphthyl (prepared from (R)-4,4′-dibromo-2,2′-dihydroxy-1,1′-binaphthyl),13 followed by hydrolysis and acidification. (R)-MOF-4 was obtained as green prisms after a solvothermal reaction of chiral organic linker 4 and Cu(NO3)2 3H2O in a mixed solvent (DMF–H2O) at 55 °C for 4 days. The structure was characterized by IR spectroscopy, thermogravimetric analysis, and single crystal X-ray diffraction. The structure was characterized by IR spectroscopy (Fig. S1†), thermogravimetric analysis (Fig. S2†), solid CD spectroscopy (Fig. S3†), powder (Fig. S4†) and single crystal X-ray diffraction. X-ray diffraction analysis reveals that (R)-MOF-4 crystallizes in the trigonal space group R32 (hexagonal axes).‡ Asymmetric unit of (R)-MOF-4 consists of di-copper(II) cations, two anionic ligands 4, three molecules of N-methylformamide (including two disordered between two positions with ca. 0.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
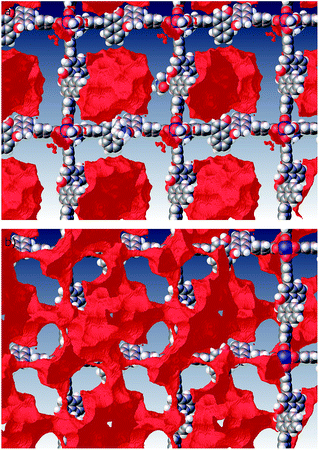
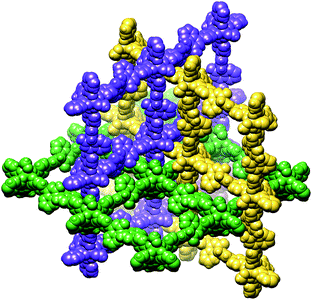
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.36 occupancy) and one molecule of methanol. As expected, metal–organic framework is organized around di-copper(II) ions as quasi square-planar network (Fig. 1). It contains one well localized DMF molecule that is H-bonded to one of the hydroxy groups of the ligand and two disordered DMF molecules that are coordinated in axial positions of both Cu(II) atoms. In contrast to previously reported relatively porous interpenetrating homochiral (R)-MOF-3,12b in the present case ca. 88% of crystal volume is occupied by already localized (R)-MOF-4 and DMF molecules (Fig. 2). As seen in Fig. 1, crystals contain discrete voids of approximately 4.6 Å radius that are not arranged in tunnels pointing across the whole crystals. However, when all solvent molecules present in crystal are removed another channel like of a 2.4 Å radius are formed around catalytic centres that may afford crystal penetration by relatively small linear organic molecules (Fig. 1, bottom part). In this case about 50.1% of the crystal volume is accessible. Therefore, one might assume that enantioselection may take place inside of an internal empty larger chiral cavity generated by evacuation of included DMF solvents. Thus the reaction of relatively small reactant may occur in the cavity.
0.36 occupancy) and one molecule of methanol. As expected, metal–organic framework is organized around di-copper(II) ions as quasi square-planar network (Fig. 1). It contains one well localized DMF molecule that is H-bonded to one of the hydroxy groups of the ligand and two disordered DMF molecules that are coordinated in axial positions of both Cu(II) atoms. In contrast to previously reported relatively porous interpenetrating homochiral (R)-MOF-3,12b in the present case ca. 88% of crystal volume is occupied by already localized (R)-MOF-4 and DMF molecules (Fig. 2). As seen in Fig. 1, crystals contain discrete voids of approximately 4.6 Å radius that are not arranged in tunnels pointing across the whole crystals. However, when all solvent molecules present in crystal are removed another channel like of a 2.4 Å radius are formed around catalytic centres that may afford crystal penetration by relatively small linear organic molecules (Fig. 1, bottom part). In this case about 50.1% of the crystal volume is accessible. Therefore, one might assume that enantioselection may take place inside of an internal empty larger chiral cavity generated by evacuation of included DMF solvents. Thus the reaction of relatively small reactant may occur in the cavity.
Crystalline evacuated (R)-MOF-4 exhibited excellent catalytic activity in the asymmetric Diels–Alder reaction between isoprene and N-ethyl maleimide when used as a heterogeneous catalyst. When isoprene 5 and N-ethyl maleimide 6b were stirred in various solvents in the presence of evacuated (R)-MOF-4 at 20 °C for 24 h, optically active adduct 7b was formed in the yields shown in Table 1. When the reaction was carried out in AcOEt or cyclohexane, relatively higher enantioselectivities (71% ee and 63% ee, respectively) were obtained (Table 1, entries 4 and 7). In contrast, reactions in EtOH, i-PrOH, CHCl3, toluene, and n-hexane resulted in lower enantioselectivities (Table 1, entries 2, 3, 5–8), and only the racemic product was isolated when the reaction was performed in MeOH (Table 1, entry 1).
Next, we examined the effect of reaction temperature (Table 2), and the best result (81% yield, 75% ee) was obtained when the reaction was carried out at 0 °C for 48 h (Table 2, entry 2). The enantioselectivity of the reaction decreased at elevated temperatures (entries 4–6).
To explore this catalytic activity further, we used a variety of N-substituted maleimide derivatives (Table 3) as dienophiles. N-methyl maleimide 6a showed lower reactivity and enantioselectivity than 6b (Table 3, entry 1). Sterically bulky N-substituted maleimides 6c–6e resulted in poor catalytic efficiency in terms of both product yield and enantioselectivity. This may be due to the weaker encapsulation of sterically bulky dienophiles in the chiral channels of (R)-MOF-4.
The catalytic performances of (R)-MOF-1, (R)-MOF-2, and (R)-MOF-3 in the asymmetric Diels–Alder reaction between isoprene and N-ethyl maleimide were compared with that of (R)-MOF-4. (R)-MOF-1 and (R)-MOF-2 afforded the optically active products with 5% ee and 25% ee, respectively (Table 4, entries 1 and 2), whereas interpenetrating (R)-MOF-3 gave low product yields and did not show enantioselectivity (Table 4, entry 3).
We also performed control experiments to study the confinement effect of (R)-MOF-4 on the catalyst. When the reaction was performed using Cu(OAc)2 as the catalyst, cycloaddition product rac-7b was obtained in 25% yield (Table 5, entry 2). When the reaction was performed using (R)-1,1′-bi-2-naphthol (BINOL), rac-7b was obtained in 21% yield (Table 5, entry 3). With a combination of (R)-BINOL and Cu(OAc)2 as the catalyst, the reaction proceeded with similar efficiency, affording rac-7b in 10% yield (Table 4, entry 4), which is comparable to that for the reaction without any catalyst (Table 4, entry 1). The catalyst was separated by filtration and washed several times with methanol. The recovered catalyst can be used for further catalytic cycles.
In summary, the synthesis and structural elucidation of a novel extended homochiral MOF, (R)-MOF-4 with a non-interpenetrating framework were successfully achieved. With the free void space of homochiral (R)-MOF-4, it is reasonable to assume that the reactions occur inside its chiral pores. Obtained (R)-MOF-4 exhibited excellent catalytic activity as a heterogeneous catalyst in the asymmetric Diels–Alder reaction between isoprene and N-ethyl maleimide. Balky reactants have been shown to have lower reactivity and enantioselectivity due to their weaker interaction with the chiral MOF. Further study of the mechanism and scope of this reaction is now underway.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (C) (No. 26410126) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT). We thank the PETRA III synchrotron facility in Hamburg for the allocation of synchrotron radiation beam time.Notes and references
- (a) K. S. Suslick, P. Bhyrappa, J. H. Chou, M. E. Kosal, S. Nakagaki, D. W. Smithenry and S. R. Wilson, Acc. Chem. Res., 2005, 38, 283–291 CrossRef CAS PubMed; (b) L. J. Murray, M. Dinca and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294–1314 RSC; (c) A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2010, 43, 58–67 CrossRef CAS PubMed; (d) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed; (e) B. Van de Voorde, B. Bueken, J. Denayer and D. De Vos, Chem. Soc. Rev., 2014, 43, 5766–5788 RSC.
- (a) B. Van de Voorde, B. Bueken, J. Denayer and D. De Vos, Chem. Soc. Rev., 2014, 43, 5766–5788 RSC; (b) S. R. Venna and M. A. Carreon, Chem. Eng. Sci., 2015, 124, 3–19 CrossRef CAS; (c) W. Li, Y. Zhang, Q. Li and G. Zhang, Chem. Eng. Sci., 2015, 135, 232–257 CrossRef CAS; (d) D. Banerjee, A. J. Cairns, J. Liu, R. K. Motkuri, S. K. Nune, C. A. Fernandez, R. Krishna, D. M. Strachan and P. K. Thallapally, Acc. Chem. Res., 2015, 48, 211–219 CrossRef CAS PubMed; (e) L. Heinke, M. Tu, S. Wannapaiboon, R. Fischer and C. Woell, Microporous Mesoporous Mater., 2015, 216, 200–215 CrossRef CAS.
- (a) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed; (b) D. Liu, K. Lu, C. Poon and W. Lin, Inorg. Chem., 2014, 53, 1916–1924 CrossRef CAS PubMed.
- (a) J. Y. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, B. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC; (b) L. Ma and W. Lin, Top. Curr. Chem., 2010, 293, 175–205 CrossRef CAS PubMed; (c) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196–1231 CrossRef CAS PubMed; (d) J. M. Falkowski, S. Liu and W. Lin, RSC Catal. Ser., 2013, 12, 344–364 CAS; (e) A. Dhakshinamoorthy, M. Opanasenko, J. Cejka and H. Garcia, Catal. Sci. Technol., 2013, 3, 2509–2540 RSC; (f) J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C. Su, Chem. Soc. Rev., 2014, 43, 6011–6061 RSC; (g) A. H. Chughtai, N. Ahmad, H. A. Younus, A. Laypkov and F. Verpoort, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- (a) Y. Liu, W. Xuan and Y. Cui, Adv. Mater., 2010, 22, 4112–4135 CrossRef CAS PubMed; (b) C. Wang, M. Zheng and W. Lin, J. Phys. Chem. Lett., 2011, 2, 1701–1709 CrossRef CAS; (c) G. Nickerl, A. Henschel, R. Gruenker, K. Gedrich and S. Kaskel, Chem. Ing. Tech., 2011, 83, 90–103 CrossRef CAS; (d) Z. Gu, C. Yang, N. Chang and X. Yan, Acc. Chem. Res., 2012, 45, 734–745 CrossRef CAS PubMed; (e) J. M. Falkowski, S. Liu and W. Lin, Isr. J. Chem., 2012, 52, 591–603 CrossRef CAS; (f) S. Regati, Y. He, M. Thimmaiaha, P. Lia, S. Xiangb, B. Chen and J. C. Zhao, Chem. Commun., 2013, 49, 9836–9838 RSC; (g) P. Peluso, V. Mamane and S. Cossu, J. Chromatogr. A, 2014, 1363, 11–26 CrossRef CAS PubMed; (h) T. Duerinck and J. F. M. Denayer, Chem. Eng. Sci., 2015, 124, 179–187 CrossRef CAS; (i) Y. Cui, B. Li, H. He, W. Zhou, B. Chen and G. Qian, Acc. Chem. Res., 2016, 49, 483–493 CrossRef CAS PubMed.
- K. Tanaka, S. Oda and M. Shiro, Chem. Commun., 2008, 820–822 RSC.
- (a) K. Tanaka and K. Otani, New J. Chem., 2010, 34, 2389–2391 RSC; (b) K. Tanaka, K. Otani, T. Murase, S. Nishihote and Z. Urbanczyk-Lipkowska, Bull. Chem. Soc. Jpn., 2012, 85, 709–714 CrossRef CAS.
- K. Tanaka, K. Kubo, K. Iida, K. Otani, T. Murase, D. Yanamoto and M. Shiro, Asian J. Org. Chem., 2013, 2, 1055–1060 CrossRef CAS.
- (a) K. Tanaka, T. Muraoka, D. Hirayama and A. Ohnish, Chem. Commun., 2012, 48, 8577–8579 RSC; (b) K. Tanaka, Y. Kikumoto, N. Hota and H. Takahashi, New J. Chem., 2014, 38, 880–883 RSC; (c) K. Tanaka, N. Hotta, S. Nagasea and K. Yoza, New J. Chem., 2016, 40, 4891–4894 RSC; (d) K. Tanaka, T. Muraoka, Y. Otubo, H. Takahashi and A. Ohnishi, RSC Adv., 2016, 6, 21293–21301 RSC.
- J. Funel and S. Abele, Angew. Chem., Int. Ed., 2013, 52, 3822–3863 CrossRef CAS PubMed.
- (a) E. J. Corey, Angew. Chem., Int. Ed., 2002, 41, 1650–1667 CrossRef CAS; (b) M. Hatano and K. Ishihara, Chem. Commun., 2012, 48, 4273–4283 RSC.
- (a) B. Gole, A. K. Bar, A. Mallick, R. Banerjee and P. S. Mukherjee, Chem. Commun., 2013, 49, 7439–7441 RSC; (b) K. Tanaka, D. Yanamoto, K. Yoshimura, T. Anami and Z. Urbanczyk-Lipkowska, CrystEngComm, 2015, 17, 1291–1295 RSC.
- M. W. A. MacLean, T. K. Wood, G. Wu, R. P. Lemieux and C. M. Crudden, Chem. Mater., 2014, 26, 5852–5859 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis of (R)-MOF-4, catalytic Diels–Alder reactions, IR, CD, TG spectra and HPLC chromatographic data, XRD data. CCDC 1483410. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra23740a |
‡ Crystal data for (R)-MOF-4: C78H64Cu2N3O16, MW = 1426.40, a = b = 33.835(5), c = 39.859(8) Å, β = 120.0°, V = 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 517(14) Å3, F (000) = 13 517(14) Å3, F (000) = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 302, dexp = 1.328(4) and dcalc = 1.079 Mg m−3, trigonal; space group R32 (No. 155), Z = 18, μ (MoKα) = 0.541 cm−1, λ = 0.6888 Å, T = 100(2) K, 137 302, dexp = 1.328(4) and dcalc = 1.079 Mg m−3, trigonal; space group R32 (No. 155), Z = 18, μ (MoKα) = 0.541 cm−1, λ = 0.6888 Å, T = 100(2) K, 137![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923 reflections measured, 17 923 reflections measured, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 unique (Rint = 0.0292), final R1 = 0.0732, wR2 = 0.2230, for 16 540 unique (Rint = 0.0292), final R1 = 0.0732, wR2 = 0.2230, for 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 757 observed reflections with I > 2σ(I); GOF = 1.041. CCDC: 1483410. 757 observed reflections with I > 2σ(I); GOF = 1.041. CCDC: 1483410. |
| This journal is © The Royal Society of Chemistry 2016 |