High-pressure circular dichroism spectroscopy up to 400 MPa using polycrystalline yttrium aluminum garnet (YAG) as pressure-resistant optical windows†
Abstract
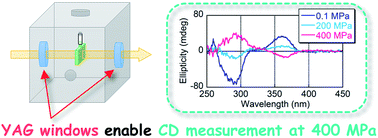
Circular dichroism (CD) spectroscopy at high pressure (≤400 MPa) was accomplished by using polycrystalline yttrium aluminum garnet (Y3Al5O12, YAG) as pressure-resistant optical windows. Conformational changes, including main-chain helix inversions of poly(quinoxaline-2,3-diyl)s (PQXs), in organic solvents at high hydrostatic pressure (>200 MPa) using a newly developed high-pressure CD cell with polycrystalline YAG windows were demonstrated.

 Please wait while we load your content...
Please wait while we load your content...