Synthesis and biological evaluation of heterocyclic hydrazone transition metal complexes as potential anticancer agents†
Abstract
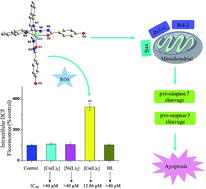
In an effort to better understand the anticancer activity of the tridentate heterocyclic hydrazone metal complexes, three new transition metal complexes with (E)-4-hydroxy-N′-(quinolin-2-ylmethylene)benzohydrazide (HL), [Co(L)2] (C1), [Ni(L)2] (C2) and [Cu(L)2] (C3) were synthesized and characterized. The Cu(II) complex C3 had significantly higher intracellular reactive oxygen species (ROS) level and antitumor efficacy than C1 and C2. Further apoptosis, mitochondrial membrane potential and western blot studies showed that C3 can effectively induce caspase-mediated apoptosis in A549 cells through the activation of the mitochondrial signaling pathway. Moreover, the affinity of C3 towards human serum albumin (HSA) was investigated by fluorescence spectroscopy and molecular docking, which revealed hydrophobic interaction and hydrogen bonds in the subdomain IIA of HSA.

 Please wait while we load your content...
Please wait while we load your content...