Layer-by-layer inkjet printing SPS:PEDOT NP/RGO composite film for flexible humidity sensors
Yan Yuan*,
Bo Peng,
Hang Chi,
Cong Li,
Ren Liu and
Xiaoya Liu
The Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, P. R. China. E-mail: yuanyan@jiangnan.edu.cn
First published on 28th November 2016
Abstract
We report the preparation of poly(3,4-ethylenedioxythiophene) (PEDOT) nanoparticles (NPs) and graphene oxide (GO) ink-based layer-by-layer inkjet printing humidity sensors. Sulfonate polystyrene:PEDOT NPs (SPS:PEDOT NPs) were prepared with hard template SPS NPs and GO was synthesized through a modified Hummers' method. Next, the SPS:PEDOT NP/GO multilayer films were printed layer by layer, with one layer of GO followed by one layer of SPS:PEDOT NP ink on the flexible poly-ethylene terephthalate (PET) substrate by using a home inkjet printer. The morphology and structure of the SPS:PEDOT NPs and GO were characterized through TEM and Raman spectroscopy and those of the film were investigated through optical microscopy. SPS:PEDOT NP-reduced GO (SPS:PEDOT NP/RGO) composite sensors were obtained after thermal annealing. To assess their humidity sensing properties, the sensors were exposed to a wide range of relative humidities (RH 0–98%) at room temperature. The SPS:PEDOT NP/RGO PET-based sensor exhibited superior humidity sensing properties in a wide response range (0.13–68.46%), short response and recovery time (39 and 57 s, respectively), and excellent repeatability and flexibility. The resistance of sensors was almost invariable even after folding 200 times. Overall, the SPS:PEDOT NP/RGO PET-based sensors can be used as humidity sensors.
1 Introduction
Humidity sensors, which convert variations in water vapor in air to electric signals, have been widely used for monitoring, humidity in industrial, agricultural, meteorological, medical, and smart packaging applications, among others.1 In recent years, capacitance,2 resistance, quartz crystal microbalance (QCM),3 and surface acoustic wave (SAW)4 characteristics of humidity sensors have been widely investigated, and various methods—such as spin coating,5 drop casting,6 solution dripping,7 lay by lay self-assembly,8 and dry/solution-based transfer have been employed for fabricating humidity sensors. However, these methods have a few disadvantages such as film inhomogeneity, uncontrollable shape and low efficiency. In addition, they are unsuitable for large scale production and for use in flexible devices. Therefore, developing an alternative method is essential. Several inkjet printing humidity sensors in which small droplets can be directly and continuously printed on to a substrate material to form patterns through computer-controlled inkjet printing have recently been reported.9–15 A wide range resistive humidity sensor, based on graphene/methyl-red composite9 has been reported with 5–90% RH sensing range by inkjet-printing. Silver nanoparticles13 based capacitive humidity sensor by inkjet-printing was reported to sense low humidity range from 30% to 90% RH. Such sensors can greatly reduce production costs and time. Moreover, inkjet printing integrated with roll-to-roll technology can be used to produce large and flexible electronic devices.Functional inks are vital components in inkjet printing and contain ceramics,16 nano-metal particles,17 conductive polymers,18 and carbon materials19,20 among these, the conductive polymer poly(3,4-ethylenedioxythiophene) (PEDOT) and graphene are the most potential materials.
Because of its high conductivity and environmental stability, PEDOT synthesized by the Bayer Corporation in 1988 has been widely used in devices such as sensors, memory cells, solar cells, and electrochromic devices.21 However, the insolubility and infusibility of PEDOT limits its applications. To overcome this limitation, water-soluble polymers (soft template method) were used to disperse PEDOT in water and to dope PEDOT. Yuan et al.11 used a photosensitive alternating copolymer poly(7-(4-vinylbenzyloxyl)-4-methylcoumarin-alt-maleic anhydride) (PVMA) to dope PEDOT in order to produce PEDOT aqueous dispersion with high stability and improved water resistance through photo-dimerization of PVMA. However, PEDOT:PVMA particles synthesized using the soft template method aggregate easily, and therefore the particle size should be limited to less than 200 nm for passing through the printer nozzle. Alternatively, the hard template method can be used to fabricate core–shell PEDOT structures by using monodisperse template particles. The PEDOT colloidal particle is suitable for inkjet printing because of its controllable particle size, large specific surface area, high electro-conductibility, and stable dispersion.22–24 However, they lack flexibility and thus cannot form a flexible film.
Graphene—a carbon allotrope with a single two-dimensional layer of atoms—is ultrathin and ultralight and has high strength, flexibility, electrical conductivity, charge carrier mobility of charge carriers (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1), and thermal conductivity (5000 W m−1 K−1).25 Several researchers have fabricated graphene flexible device through inkjet printing. Vineet dua et al.26 printed reduced graphene (RGO) ink on a polyethylene terephthalate (PET) film and obtained a rugged and relatively flexible sensor with a strong sensitivity for Cl2. Secor et al.27 printed graphene (GO) ink for flexible printed electronics by using a professional printer. Despite being bent several times, the flexible electronic circuit retained its excellent electrical properties. Therefore, graphene is a suitable carbon-based printer ink. However, its monitoring range for sensing humidity is narrow.28 Nevertheless, a flexible film can be fabricated by combining graphene with PEDOT colloidal particles.
000 cm2 V−1 s−1), and thermal conductivity (5000 W m−1 K−1).25 Several researchers have fabricated graphene flexible device through inkjet printing. Vineet dua et al.26 printed reduced graphene (RGO) ink on a polyethylene terephthalate (PET) film and obtained a rugged and relatively flexible sensor with a strong sensitivity for Cl2. Secor et al.27 printed graphene (GO) ink for flexible printed electronics by using a professional printer. Despite being bent several times, the flexible electronic circuit retained its excellent electrical properties. Therefore, graphene is a suitable carbon-based printer ink. However, its monitoring range for sensing humidity is narrow.28 Nevertheless, a flexible film can be fabricated by combining graphene with PEDOT colloidal particles.
In the present study, SPS:PEDOT NPs was synthesized to overcome the PEDOT shortcomings, which was not easily passed through the printer nozzle of home printer. In this process, PEDOT polymerized on the surface of the hard template SPS NPs to form the SPS:PEDOT NPs. GO as conductive template was prepared for connecting the SPS:PEDOT NPs. Owing to the instability of the mixture of SPS:PEDOT NPs and GO, layer by layer inkjet printing SPS:PEDOT NPs and GO was designed to fabricate the SPS:PEDOT NP/GO composite films. At the same time, SPS:PEDOT NP ink and GO ink in which several organic solvents were added to adjust their surface tension and viscosity and so on were prepared to print the multilayered films, respectively. SPS:PEDOT NP/RGO composite films which could be used for humidity sensors were obtained after 80 seconds thermal annealing at 180 °C for recovering the electro-conductibility of GO.
2 Materials and methods
2.1 Materials
EDOT (99% pure) was purchased from Suzhou Yacoo Chemical Reagent Co., LLC. Styrene (St) (purified through distillation) and sodium styrene sulfonate (SSS) were purchased from Sigma-Aldrich Co., LLC. Graphite powder (D50 < 600 nm, 99.95% pure) and polyethyleneimine (PEI, MW 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 50% aqueous solution) was purchased from Aladdin Co., LLC. Sodium nitrate (NaNO3), hydrogen peroxide (H2O2), potassium hypermanganate (KMnO4), sulfuric acid (H2SO4), hydrochloric acid (HCl), ferric sulfate [Fe2(SO4)3], ammonium persulfate [(NH4)2S2O8], phosphorus pentoxide (P2O5), lithium chloride (LiCl), potassium acetate (CH3COOH), magnesium chloride (MgCl2), sodium chloride (NaCl), copper sulfate (CuSO4), ethanol, butyl cello solve (BCS), isopropyl alcohol (IPA), dimethyl sulfoxide (DMSO), and polyvinylidene fluoride (PVDF) microporous membranes (pore size, 220 nm and 800 nm) were purchased from Sinopharm Chemical Reagent Shanghai Co., Ltd. PET substrates (4R) were purchased from Minnesota Mining and Manufacturing Co. Deionized (DI) water was used in our experiment. Regenerated cellulose dialysis membrane (MD12) with a molecular weight cut-off of 10
000 50% aqueous solution) was purchased from Aladdin Co., LLC. Sodium nitrate (NaNO3), hydrogen peroxide (H2O2), potassium hypermanganate (KMnO4), sulfuric acid (H2SO4), hydrochloric acid (HCl), ferric sulfate [Fe2(SO4)3], ammonium persulfate [(NH4)2S2O8], phosphorus pentoxide (P2O5), lithium chloride (LiCl), potassium acetate (CH3COOH), magnesium chloride (MgCl2), sodium chloride (NaCl), copper sulfate (CuSO4), ethanol, butyl cello solve (BCS), isopropyl alcohol (IPA), dimethyl sulfoxide (DMSO), and polyvinylidene fluoride (PVDF) microporous membranes (pore size, 220 nm and 800 nm) were purchased from Sinopharm Chemical Reagent Shanghai Co., Ltd. PET substrates (4R) were purchased from Minnesota Mining and Manufacturing Co. Deionized (DI) water was used in our experiment. Regenerated cellulose dialysis membrane (MD12) with a molecular weight cut-off of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MWCO was purchased from Spectrum, Inc.
000 MWCO was purchased from Spectrum, Inc.
2.2 Preparation
The preparation of the SPS:PEDOT NPs is illustrated in Scheme 1(b). Different solutions of SPS and EDOT in different molar concentrations were prepared to assess the dispersibility of PEDOT in SPS aqueous dispersion. The details of the reactant molar ratio are listed in Table 1. First, SPS and EDOT were dissolved in DI water and added into a 100 mL three-necked round flask. The solid content of the mixed solution was 4 wt%. The pH value of the mixture was adjusted to approximately 3.0 by adding HCl. APS and Fe2(SO4)3 were dissolved in 10 mL of DI water and quickly added to the solution. The reaction temperature was maintained at 30 °C and the mixture was stirred at 350 rpm for 20 h. Then, the mixture turned from white to dark blue. Next, the SPS:PEDOT NPs aqueous dispersion was dialyzed in DI water for 3 days. Finally, the best ratio of SPS and EDOT was selected using as the basic aqueous dispersion to fabricate the SPS:PEDOT NP ink. The solid concentration of the basic aqueous solution was 1.2 wt%.
| Sample | SPSa (mol) | EDOT (mol) | APS (mol) | Fe2(SO4)3 (mol) |
|---|---|---|---|---|
| a The dosage of SPS was calculated on the basis of the sulfonic acid (–SO3H) content. | ||||
| SPS:PEDOT NPs-1 | 1 | 0 | 1.5 | 0.002 |
| SPS:PEDOT NPs-2 | 5 | 1 | 1.5 | 0.002 |
| SPS:PEDOT NPs-3 | 3 | 1 | 1.5 | 0.002 |
| SPS:PEDOT NPs-4 | 2 | 1 | 1.5 | 0.002 |
| SPS:PEDOT NPs-5 | 1.5 | 1 | 1.5 | 0.002 |
| SPS:PEDOT NPs-6 | 1 | 1 | 1.5 | 0.002 |
| Sample | SPS:PEDOT NPs ink | GO ink |
|---|---|---|
| The basic aqueous dispersion | 5 g | 5 g |
| Ethanol | 0.15 g | 0.15 g |
| BCS | 0.35 g | 0.35 g |
| IPA | 0.15 g | — |
| DMSO | 0.25 g | — |
2.3 Instrumentation and resistance measurements of the sensor
Attenuated total reflectance Fourier transform infrared (ATR-FTIR) analysis was performed using a Nicolet 6700 spectrometer (Thermo Fisher Scientific Inc.). Raman experiments were performed using an inVia Reflex (intensity = 1%, λ = 532 nm, Renishaw Company). Particle size was determined using the Brookhaven ZetaPALS instrument (Brookhaven Instrument Company) at 25 °C in DI water at an angle of 90°. The S element mass percent was measured using the Vario EL Elementar (Elementar Analysensysteme GmbH). The morphology of the SPS NPs and SPS:PEDOT NPs were observed using transmission electron microscope (TEM JEOL JEM-2100 microscope operating at 200 kV). Optical microscopy images of patterns were investigated using a KH-8700 ultradepth three-dimensional microscope (Japan HiROX Co., Ltd). The surface tension were measured through OCA 40 contact angle measurement (Data Physics Co.), and viscosity was measured using Brookfield DV-S rotational viscometer (Brookfield Residential Co.). The electrode patterns were designed using CorelDRAW software (Corel Co.). Inkjet printing was performed using an Epson 1390 inkjet printer (Epson (China) Co., Ltd) at a resolution of 8230 and 5760 × 1440 dpi and printing speed of 15 ppm. The SPS:PEDOT NP/GO multilayer films were thermally annealed by DZF-6021 heat oven (Wuxi Jianyi Instrument Machinery CO., Ltd). The RTS-9 four-point probe instrument (Guangzhou Four-Point Probe Technology Co., Ltd) was used to measure the surface resistance of the thin-film electrode.The resistance of different layers of the SPS:PEDOT NP/RGO PET-based films were compared. A SPS:PEDOT NP/RGO PET-based film was connected to an Agilent 34401A 6½ digital multimeter (Agilent Technologies Inc.) with A 2.54 mm female header connector (Shenzhen Goxconn Co., Ltd) and the 4R resistance mode was used for data acquisition. The humidity sensing measurements are presented in Scheme 2. A PET-based SPS:PEDOT NP/RGO sensor was folded a lot of angles (greater than 90°) by ourselves to measure the stability, which repeated 200 times. Keysight BenchVue software recorded the real-time resistance of the folds. And the resistance values were record when the values stabilised. Saturated saline solutions in airtight container (humidity bottles laboratory equipment) provide stable and controllable RH at a stable temperature. Different saturated solutions were assessed by mixing LiCl, CH3COOK, MgCl2, K2CO3, NaCl, and CuSO4 with water in a closed vessel, which were used to attain approximately 11%, 23%, 33%, 43%, 75%, and 98% RH levels at 25 °C, respectively. Phosphorus pentoxide (P2O5) powder was used as a desiccant in a closed vessel to achieve 0% RH levels at 25 °C.8,30 The humidity sensing properties were assessed by placing the SPS:PEDOT NP/RGO PET-based sensors within various RH ranges in airtight containers and measuring the response of the RH sensor. The resistance response (R′) was measured the resistance change in each RH environment by transferring the sensor from one RH container to the zero RH container and was evaluated using the following formula: R′ = (RRH − R0)/R0 × 100%, where RRH and R0 are the resistance of the sensor in the corresponding RH and 0% RH (dry air), respectively.11 The environment of the experiments was at constant temperature (25 ± 0.3 °C) and humidity (40 ± 2%) facilities. And at least 20 devices were fabricated in the tested.
3 Results and discussions
3.1 Characterization of SPS:PEDOT NPs and GO
The ATR-FTIR spectra of PS and SPS NPs are shown in Fig. 1. Compared with PS NPs, the ATR-FTIR spectra of SPS show the hydroxyl stretching vibration of the –SO3H at approximately 3500 cm−1. The symmetric stretching vibration and the asymmetric stretching band of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O were observed at 1035 cm−1 and 1176 cm−1, respectively.31 These bands indicate that the –SO3H functional groups were introduced into the SPS NPs. Furthermore, the TEM images of smooth SPS NPs (diameter, 100 nm) are presented in Fig. 1. Elemental analysis indicates that the S element mass percent of SPS NPs was 4.9 wt%. The mole fractions of –SO3H was 18.9% in SPS NPs.
O were observed at 1035 cm−1 and 1176 cm−1, respectively.31 These bands indicate that the –SO3H functional groups were introduced into the SPS NPs. Furthermore, the TEM images of smooth SPS NPs (diameter, 100 nm) are presented in Fig. 1. Elemental analysis indicates that the S element mass percent of SPS NPs was 4.9 wt%. The mole fractions of –SO3H was 18.9% in SPS NPs.
Raman spectrum of the SPS:PEDOT NPs are shown in Fig. 2. In this figure, the main peak at 1438 cm−1 can be assigned to symmetric Cα![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cβ (–O) stretching and those at 1575 and 1511 cm−1 to the asymmetric Cα
Cβ (–O) stretching and those at 1575 and 1511 cm−1 to the asymmetric Cα![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cβ stretching. The peaks at 1375 cm−1, 1255 cm−1, 1005 cm−1, 577 cm−1, 700 cm−1, and 445 cm−1 can be assigned to the Cβ
Cβ stretching. The peaks at 1375 cm−1, 1255 cm−1, 1005 cm−1, 577 cm−1, 700 cm−1, and 445 cm−1 can be assigned to the Cβ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cβ stretching, Cα–Cα inter-ring stretching, C–O–C deformation, symmetric C–S–C deformation, oxyethylene ring deformation, and SO2 bending, respectively.32 The TEM image of SPS:PEDOT NPs (Fig. 2) shows that the edges of SPS:PEDOT NPs are more rough than that of the SPS (Fig. 1), which demonstrates that PEDOT is polymerized on the surface of SPS. Raman spectra and TEM images indicate that PEDOT was successfully polymerized using SPS NPs as the hard template, resulting in SPS:PEDOT NPs.
Cβ stretching, Cα–Cα inter-ring stretching, C–O–C deformation, symmetric C–S–C deformation, oxyethylene ring deformation, and SO2 bending, respectively.32 The TEM image of SPS:PEDOT NPs (Fig. 2) shows that the edges of SPS:PEDOT NPs are more rough than that of the SPS (Fig. 1), which demonstrates that PEDOT is polymerized on the surface of SPS. Raman spectra and TEM images indicate that PEDOT was successfully polymerized using SPS NPs as the hard template, resulting in SPS:PEDOT NPs.
Zeta particle diameter was measured to investigate the dispersibility of SPS:PEDOT NPs to PEDOT in aqueous solution [Fig. 3(a)]. As EDOT content increases, more EDOT aggregates on the surface of SPS particles, leading to a gradual increase in the zeta particle size of the SPS:PEDOT NPs. The effective diameters of SPS:PEDOT NPs dispersions at different feed ratios are listed in Table 3, and their images are presented in Fig. 3(b). The images show that SPS:PEDOT NPs 1–5 can form stable dispersion. However, when PEDOT concentration is excessive, SPS:PEDOT NPs-6 precipitates in aqueous solution. Simultaneously, an increase in PEDOT concentration is used as the basic aqueous dispersion for inkjet printing because SPS:PEDOT NPs-5 has high PEDOT concentration and fine aqueous dispersion.
 | ||
| Fig. 3 (a) Zeta particle size distribution curves of SPS:PEDOT NPs dispersion with different feed ratio. (b) Digital image of SPS:PEDOT NP dispersion at different feed ratios. | ||
| Sample | Effective diameter (nm) |
|---|---|
| SPS:PEDOT NPs-1 | 165.6 |
| SPS:PEDOT NPs-2 | 170.4 |
| SPS:PEDOT NPs-3 | 209.9 |
| SPS:PEDOT NPs-4 | 340.0 |
| SPS:PEDOT NPs-5 | 551.5 |
| SPS:PEDOT NPs-6 | 693.4 |
Raman spectra of graphite powder, GO and RGO (GO after thermally annealed for 80 s at 180 °C) are compared in Fig. 4. The typical Raman peaks around 1600 cm−1 and 1350 cm−1 are represented by G and D bands, respectively. The D band is correlated to the second order of zone boundary phonons activated by defects. The G band associated with the optical E2g phonons at the Brillouin zone center is generated by the bond stretching of sp2 carbon pairs.33,34 ID/IG of GO and graphite powder is 1.36 and 0.42, respectively. Therefore, the disorder degree of GO is more activated than that of graphite powder, which evidences the successful synthesis of GO. ID/IG of RGO is 1.06. That indicate a lot of GO is reduced after thermally annealed for 80 s at 180 °C because the G band of RGO is recovered partially.
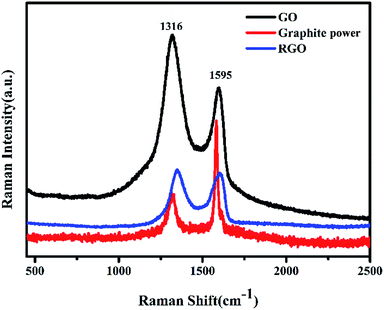 | ||
| Fig. 4 Raman spectra of graphite powder, GO and RGO (GO after thermally annealed for 80 s at 180 °C). | ||
3.2 Microstructure and conductive properties of the inkjet-printing patterns
The digital photos of the printed logo and sensor device of SPS:PEDOT NP/RGO on PET is presented in Fig. 5(a) and (b), the printed patterns were well-defined in different shapes. Patterned drop array [Fig. 5(c)] and lines [Fig. 5(d)] with an average resolution of 51.1 ± 1.6 μm and 47.6 ± 0.3 μm are clear printed, respectively. The clear pattern could be attributed to a rapid inkjet printer process and the surface energy between the substrate and ink.35The sheet resistance of SPS:PEDOT NP/RGO at different holding times with 20 printed layers and a heating temperature of 180 °C is shown in Fig. 6(a). The square resistance of patterns decreases with rise in holding time and stabilizes at 80 s (784.3 kΩ sq−1). This is because a part of the sp2 carbon pairs of GO can gradually recovery during the holding time at heating temperature of 180 °C. The RGO films form the conductive net to connect the SPS:PEDOT NPs. The synergistic effect of RGO and SPS:PEDOT NPs improves the electrical property of the films. The sheet square resistance of devices is compared with those of different printed layers at heating temperature of 180 °C and holding time of 80 s [Fig. 6(b)]. The square resistance of patterns decreases with the increase in the number of the printed layers and stabilizes when there are 25 printed layers (117.6 kΩ sq−1). This stability is attributed to the rising density of the electric pattern with the increase in the number of printed layers. Finally, the prerequisites of the inkjet printing sensors are confirmed at 25 printed layers and 80 s holding time.
The resistance of the SPS:PEDOT NP/RGO thin-film electrode as a function of the number of folding cycles was showed in Fig. 7. The resistance of the electrode was almost invariable after 200 folding cycles, demonstrating the flexibility of the SPS:PEDOT NP/RGO PET-based electrode.
 | ||
| Fig. 7 Resistance of SPS:PEDOT NP/RGO thin-film electrode as a function of the number of folding cycles. | ||
3.3 Humidity sensor measurement
The resistance response measurement of the SPS:PEDOT NP/RGO thin-film sensors exposed to different RH levels (0%, 11%, 23%, 33%, 43%, 75%, and 98% RH) are presented in Fig. 8(a). A specific pattern of SPS:PEDOT NP/RGO thin-film sensors were prepared. The response/recovery cycles of RH tests were performed in different RH environments each cycle lasted approximately 500 s (an exposure time of approximately 250 s and a recovery time of approximately 250 s) under 0% RH. The sensor resistance response decreased in humid environments when RH increased from 0% to 98%, the corresponding sensor resistance response decreased proportionally. A linear fitted curve of the average resistance response in the RH range from 0% to 98% is show in Fig. 8(b). And the error bars was calculated according to the repeated experiments data about the same sensors in different cycles. The linear fitted curve equation is Y = −0.18178 × −0.66391, Y is resistance response and X is RH (%) the linear regression coefficient (R2) was 0.993, which evidences the favourable RH linearity of the SPS:PEDOT NP/RGO PET-based sensor. This is attributed to the synergistic effect of RGO and SPS:PEDOT NPs, which widened the humidity monitoring range and enhanced the stability. | ||
| Fig. 8 (a) Resistance response measurement of SPS:PEDOT NP/RGO thin-film sensors under switching RH (b) resistance response of SPS:PEDOT NP/RGO thin-film sensors as a function of RH. | ||
The stability of SPS:PEDOT NP/RGO PET-based sensors for different RH at room temperature is illustrated in Fig. 9. The SPS:PEDOT NP/RGO PET-based sensors were stable after 200 cycles, indicating the repeatability of the sensors under 0% to 98% RH. Table 4 presented the averages and standard deviations of resistance responses with different test cycles.
| RH (%) | 0 | 11 | 23 | 33 | 43 | 75 | 98 |
| Average (−) | 0.28 | 7.27 | 13.87 | 22.37 | 9.19 | 42.33 | 69.28 |
| STDEV | 0.094 | 0.341 | 1.201 | 0.502 | 0.221 | 0.378 | 0.525 |
To consider the real-time response and recovery times of the sensors, the time-dependent response and recovery curves of the sensor to 98% RH were plotted (Fig. 10). The time taken by a sensor to achieve 98% RH of the total resistance change is defined as the response or recovery time. The response time and recovery time of sensor is approximately 39 s and 57 s, respectively. Table 5 compared the different characteristics such as response/recovery time, fabrication method, range and the sensitivity of the sensors. From the overall comparison as shown in Table 5, it can be observed that our proposed sensors had high sensitivity range and rapid response and recovery rates. From the Table 5, it can be observed that the response/recovery time is lower than Smith humidity senor. However, the sensing range is higher than Smith as it has 1–96% RH while our proposed sensor has 0–98% RH.36
| Reference | Material | Fabrication method | Sensing range | Response/recovery time |
|---|---|---|---|---|
| Yuan11 | PEDOT:PVMA | Inkjet printing | 11–98% | Not given |
| Smith36 | Graphene | CVD | 1–96% | 0.6 s/0.4 s |
| Ghosh37 | Graphene | Dropping coating | 4–84% | 180 s/180 s |
| Chen38 | Graphene | CVD | 35–98% | Not given |
| Kuang39 | SnO2 | CVD | 5–85% | 120 s/60 s |
| Sappat10 | PEDOT/PSS | Inkjet printing | 20–66% | Not given |
| Gomes40 | PANI | Inkjet printing | 13, 40, 90% | Not given |
| This work | SPS:PEDOT NP/RGO | Inkjet printing | 0–98% | 39 s/57 s |
4 Conclusions
In this paper, SPS:PEDOT NPs which can decrease the use of the expensive EDOT was synthesized by in situ polymerization. The SPS:PEDOT NP/GO multilayer films were printed with one layer of GO followed by one layer of SPS:PEDOT NP ink on the flexible polyethylene terephthalate (PET) substrate by using a home inkjet printer, which can solve the instability of the mixture of SPS:PEDOT NPs and GO. In addition, the synergistic effect of RGO and SPS:PEDOT NP improved the humidity sensing properties, such as wide response range (0–98%), short response and recovery time (39 and 57 s, respectively), excellent repeatability, and flexibility. The resistance of sensors was almost invariable even after folding 200 times. And the all-printed sensors fabrication process is simple, low-cost and printing on the flexible PET substrate easily. The inkjet printed SPS:PEDOT NP/RGO sensing layers has a promising candidate for the wearable electronic devices.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51403082), the Enterprise university-research prospective program Jiangsu Province (No. BY2015019-06), the Fundamental Research Funds for the Central Universities (No. JUSRP51507 and JUSRP51513).References
- J. Virtanen, L. Ukkonen, T. Bjorninen, A. Z. Elsherbeni and L. Sydänheimo, IEEE Trans. Instrum. Meas., 2011, 60, 2768–2777 CrossRef CAS.
- D. Wang, Y. Huang, W. Cai, M. Tian, P. Liu and Y. Zhang, J. Appl. Polym. Sci., 2014, 131, 169–172 Search PubMed.
- Z. Yuan, H. Tai, Z. Ye, C. Liu, G. Xie, X. Du and Y. Jiang, Sens. Actuators, B, 2016, 234, 145–154 CrossRef CAS.
- S. M. Balashov, O. V. Balachova, A. V. U. Braga, A. P. Filho and S. Moshkalev, Sens. Actuators, B, 2015, 217, 88–91 CrossRef CAS.
- Y. Yao, X. Chen, H. Guo, Z. Wu and X. Li, Sens. Actuators, B, 2012, 161, 1053–1058 CrossRef CAS.
- H. Bi, K. Yin, X. Xie, J. Ji, S. Wan, L. Sun, M. Terrones and M. S. Dresselhaus, Sci. Rep., 2013, 3, 2714 Search PubMed.
- C. L. Zhao, M. Qin, W. H. Li and Q. A. Huang, Actuators and Microsystems Conference (TRANSDUCERS), 2011 16th International, 2011, pp. 1954–1957 Search PubMed.
- D. Zhang, J. Tong, B. Xia and Q. Xue, Sens. Actuators, B, 2014, 203, 263–270 CrossRef CAS.
- S. Ali, A. Hassan, G. Hassan, J. Bae and C. H. Lee, Carbon, 2016, 105, 23–32 CrossRef CAS.
- A. Sappat and A. Wisitsoraat, Telecommunications & Information Technology, 2011 8th International Conference, IEEE, 2011, pp. 34–37 Search PubMed.
- Y. Yuan, Y. Zhang, R. Liu, J. Liu, Z. Li and X. Liu, RSC Adv., 2016, 6, 47498–47508 RSC.
- I. Nikolaou, H. Hallil, V. Conedera, G. Deligeorgis, C. Dejous and D. Rebiere, IEEE Sens. J., 2016, 16, 7620–7627 CrossRef.
- A. Rivadeneyra, J. Fernández-Salmerón, M. Agudo, J. A. López-Villanueva, L. F. Capitan-Vallvey and A. J. Palma, Sens. Actuators, B, 2014, 195, 123–131 CrossRef CAS.
- G. Tarapata, M. Marzęcki, R. Selma, D. Paczesny and R. Jachowicz, Sens. Actuators, A, 2016, 247, 641–646 CrossRef CAS.
- A. Rivadeneyra, J. Fernández-Salmerón, M. Agudo-Acemel, J. A. López-Villanueva, L. F. Capitan-Vallvey and A. J. Palma, Sens. Actuators, A, 2016, 244, 56–65 CrossRef CAS.
- Z. Pan, Y. Wang, H. Huang, Z. Ling, Y. Dai and S. Ke, Ceram. Int., 2015, 41, 12515–12528 CrossRef CAS.
- A. Kosmala, Q. Zhang, R. Wright and P. Kirby, Mater. Chem. Phys., 2012, 132, 788–795 CrossRef CAS.
- A. Singh, M. Katiyar and A. Garg, RSC Adv., 2015, 5, 78677–78685 RSC.
- O. Kwon, H. Kim, H. Ko, J. Lee, B. Lee, C. Jung, J. Choi and K. Shin, Carbon, 2013, 58, 116–127 CrossRef CAS.
- L. Huang, Y. Huang, J. Liang, X. Wan and Y. Chen, Nano Res., 2011, 4, 675–684 CrossRef CAS.
- Q. Jiang, C. Liu and J. Xu, et al., J. Polym. Sci., Part B: Polym. Phys., 2014, 52, 737–742 CrossRef CAS.
- L. Bazinet, D. Montpetit, D. Ippersiel, J. Amiot and F. Lamarche, J. Colloid Interface Sci., 2001, 237, 62–69 CrossRef CAS PubMed.
- F. Caruso, Adv. Mater., 2001, 13, 11–22 CrossRef CAS.
- M. G. Han and S. H. Foulger, Chem. Commun., 2004, 2154–2155 RSC.
- K. I. Bolotin, K. J. Sikes, Z. Jiang, M. Klima, G. Fudenberg, J. Hone, P. Kim and H. L. Stormer, Solid State Commun., 2008, 146, 351–355 CrossRef CAS.
- V. Dua, S. P. Surwade, S. Ammu, S. R. Agnihotra, S. Jain, K. E. Roberts, S. Park, R. S. Ruoff and S. K. Manohar, Angew. Chem., 2010, 122, 2200–2203 CrossRef.
- E. B. Secor, B. Y. Ahn, T. Z. Gao, J. A. Lewis and M. C. Hersam, Adv. Mater., 2015, 27, 6683 CrossRef CAS PubMed.
- P. G. Su and Z. M. Lu, Sens. Actuators, B, 2015, 211, 157–163 CrossRef CAS.
- S. L. Ting, C. X. Guo, K. C. Leong, D. H. Kim, C. M. Li and P. Chen, Electrochim. Acta, 2013, 111, 441–446 CrossRef CAS.
- J. A. Dean, Lange's Handbook of Chemistry, McGraw-Hill Professional Publishing, 2004 Search PubMed.
- B. Brijmohan Smita and S. Steven, Ind. Eng. Chem. Res., 2005, 44, 8039–8045 CrossRef.
- S. Garreau, G. Louarn, J. P. Buisson, G. A. Froyer and S. Lefrant, Macromolecules, 1999, 32, 6807–6812 CrossRef CAS.
- S. Y. Toh, K. S. Loh, S. K. Kamarudin and R. W. D. Wan, Chem. Eng. J., 2014, 251, 422–434 CrossRef CAS.
- N. H. N. Azman, N. L. Hong and Y. Sulaiman, Electrochim. Acta, 2016, 188, 785–792 CrossRef.
- K. Y. Shin, J. Y. Hong and J. Jang, Adv. Mater., 2011, 23, 2113–2118 CrossRef CAS PubMed.
- A. D. Smith, E. Karim and N. Frank, Nanoscale, 2015, 7, 19099–19109 RSC.
- A. Ghosh, D. J. Late and L. S. Panchakarla, et al., J. Exp. Nanosci., 2009, 4, 313–322 CrossRef CAS.
- M. C. Chen, C. L. Hsu and T. J. Hsueh, IEEE Electron Device Lett., 2014, 35, 590–592 CrossRef CAS.
- Q. Kuang, C. Lao, Z. L. Wang, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2007, 129, 6070–6071 CrossRef CAS PubMed.
- T. C. Gomes, C. J. L. Constantino, E. M. Lopes, A. E. Job and N. Alves, Thin Solid Films, 2012, 520, 7200–7204 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |








