Effects of substrate shock on extracellular polymeric substance (EPS) excretion and characteristics of attached biofilm anammox granules†
Yanlong Zhanga,
Haiyuan Maa,
Qigui Niua,
Rong Chenab,
Toshimasa Hojoa and
Yu-You Li*ac
aDepartment of Civil and Environmental Engineering, Graduate School of Engineering, Tohoku University, 6-6-06 Aoba, Aramaki, Aoba-ku, Sendai, 980-8579, Japan. E-mail: Gyokuyu.ri.a5@tohoku.ac.jp
bKey Lab of Northwest Water Resource, Environment and Ecology, MOE, Xi'an University of Architecture and Technology, No.13 Yanta Road, Xi'an 710055, China
cGraduate School of Environmental Studies, Tohoku University, 6-6-06 Aoba, Aramaki, Aoba-ku, Sendai, 980-8579, Japan
First published on 28th November 2016
Abstract
Environmental stresses are assumed to significantly impact the content and concentration of produced extracellular polymeric substances (EPS) and therefore influence the performance of an ananmmox attached film expanded bed (AAFEB) reactor. In this study, a transient high substrate concentration of 2500 mg N L−1 (calculated as the sum of NN4+–N and NO2−–N) for 24 hours stimulated abundant EPS excretion as well as deterioration of anammox granules. The results indicated that a high EPS concentration of 89.6 ± 48.3 mg g−1 VSS resulted in 35.0 ± 0.8% decrease in the granule settling properties and 30.5 ± 0.9% reduction in the total VSS amount. The production of EPS was reasonably attributed to the impact of utilization-associated and stress-associated effects by the substrate. The results of a series of batch experiments indicated that a rapid increase of loosely-bound EPS (LB-EPS) from 41.2 to 114.6 mg g−1 VSS occurred when the substrate concentration steadily increased from 400 to 1000 mg N L−1, in contrast, the tightly-bound EPS (TB-EPS) remained stable at 32.5 ± 2.8 mg g−1 VSS. Thus, the LB-EPS was considered the key factor for the deterioration of granule stability and the substrate concentration should be controlled below 400 mg N L−1 to avoid triggering the accumulation of LB-EPS. Furthermore, the formation and disintegration mechanisms of attached film anammox granules were also elucidated in this study.
1. Introduction
Over the past decade, the anammox (anaerobic ammonium oxidation) process has been widely accepted as an economical and sustainable bioprocess to treat nitrogen rich wastewater with low ratios of carbon![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrogen (C
nitrogen (C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N), such as sludge digestion water and industrial wastewater.1,2 Compared with the conventional nutrient removal process,3 the anammox process has clear advantages over the nitrification–denitrification process in terms of reduced electric consumption and lower organic carbon demand.4,5 However, the physiological properties of anammox, including a very long doubling time (10–14 days), low-cell yield (0.11 g VSS per g NH4+–N) and high sensitivity to environmental stress, have prevented the anammox process from widespread application.5–7 As such, in order to maintain the stable operation of the anammox-based reactor, it is essential to enhance its ability to retain biomass and improve its resistance to environmental stresses. The anammox attached film expanded bed (AAFEB) reactor, which was developed as a new reactor configuration with a high nitrogen removal ability and operational stability has potential to popularize anammox process. The anammox attached film granules demonstrate high settling velocities and good resistance to environmental stresses.8 However, further research on the operation features of the AAFEB reactor is necessary.
N), such as sludge digestion water and industrial wastewater.1,2 Compared with the conventional nutrient removal process,3 the anammox process has clear advantages over the nitrification–denitrification process in terms of reduced electric consumption and lower organic carbon demand.4,5 However, the physiological properties of anammox, including a very long doubling time (10–14 days), low-cell yield (0.11 g VSS per g NH4+–N) and high sensitivity to environmental stress, have prevented the anammox process from widespread application.5–7 As such, in order to maintain the stable operation of the anammox-based reactor, it is essential to enhance its ability to retain biomass and improve its resistance to environmental stresses. The anammox attached film expanded bed (AAFEB) reactor, which was developed as a new reactor configuration with a high nitrogen removal ability and operational stability has potential to popularize anammox process. The anammox attached film granules demonstrate high settling velocities and good resistance to environmental stresses.8 However, further research on the operation features of the AAFEB reactor is necessary.
It has been widely reported that both the content and concentration of extracellular polymeric substances (EPS) have significant impact on the structure, particle size, shear strength, filterability, and settling behavior of granular sludge in biological wastewater treatment processes and thus influence treatment performance.9,10 From this perspective, determining the optimal EPS content has the potential to improve the granulation and stability of aggregates and to avoid cell dewatering and damage from toxic substances through a vast net-like structure with plenty of water.11 However, excessive EPS may decrease the mass transfer efficiency in biofilm and granules and accordingly result in the deterioration of reactor performance.12 Furthermore, since the EPS is always negatively charged, it can bind with positively charged organic pollutants and metals ions through electrostatic interaction,13 and thus inhibit anammox activity since the anammox bacteria is very sensitive to organic pollutants and heavy metals.14–16 According to the above description, the content and concentration of EPS in the anammox biofilm and granules may also exert influence on reactor performance, and an excessively high or low concentration or an unsuitable composition may significantly compromise treatment performance, and even result in the loss of the stable operation of the anammox process.
Protein and polysaccharides are the major components of EPS, and typically account for 1–60% and 40–95% of content, respectively.17 It is known that EPS can be subdivided into soluble EPS forms and bound EPS forms: bound EPS is closely bound to cells, and are connected with the bio-aggregate characteristics such as shear strength, settling velocity, surface charge, dewaterability and hydrophilicity/hydrophobicity characteristics.9,18 Bound EPS can be further subdivided into tightly-bound EPS (TB-EPS) and loosely-bound EPS (LB-EPS) according to their binding force to cells.9
The production behavior of EPS in anammox biofilm and granules is highly related to the reactor configurations, the type of biomass, and the operational conditions.9,19 A number of investigations have been reported to determine the distribution of EPS in anammox granules and their important roles during anammox granulation process.18,20,21 In addition, the protection effect of EPS to environmental stresses has been also widely reported.22,23 Few studies have been focused on the variation in EPS content and composition under toxic substance stress and the adverse impact on reactor performance. To gain a better understanding of the key characteristics of the EPS in the attached biofilm anammox granules, with the final purpose of optimizing the conditions of the anammox process, this study evaluated the variation of the content and concentration of EPS in a long-term experiment under stable operation conditions and transient substrate shock condition. Furthermore, the shear strength of the granule and the EPS production characteristics under various substrate stresses were studied in a series of batch experiments.
2. Materials and methods
2.1. Reactor operation
The reactor configuration and biomass characteristics were the same as the previous study.8 There were five operation phases in the continuous experiment, during the stable operation period (phase I and II), the substrate TN concentration increased from 600–1250 mg N L−1 with a constant HRT of 3 hours to increase the nitrogen loading rate (NLR) from 4.8–10.0 g N L−1 d−1. Then a transient high substrate concentration of 2500 mg N L−1 was employed for 24 hours to evaluate the shock effects on the reactor performance as well as on EPS production. After that, the substrate concentration was decreased to 1250 mg N L−1 (in phase III), 625 mg N L−1 (in phase IV) and 313 mg N L−1 (in phase V) step by step to recover the treatment performance. The variation of EPS content and concentration and its distribution along with reactor height were evaluated during the experiment.2.2. Batch tests
The EPS production and related characteristics in the attached biofilm anammox granules were evaluated under different TN concentrations by batch tests. The TN concentration gradient was set as 100, 200, 400, 800 and 1000 mg N L−1 using a mixture of (NH4)2SO4, and NaNO2 solution, the ratio of NO2−–N/NH4+–N was set as the reported anammox stoichiometric ratio of 1.32.6 The tests were performed in serum flasks and incubated in a thermostatic water bath shaker (110 rpm) in the dark at 35 °C. Each serum flake was inoculated with 5 g wet anammox granules, which were taken from the bottom of the AAFEB reactor. In order to ensure the sludge loading rate (SLR) was the same as that in the AAFEB reactor, the TN amount injected into each flask was kept at 30 mg. The produced N2 gas was accumulated in the headspace and measured throughout the tests for subsequent processing. Specific anammox activity (SAA) was calculated based on the gas production rate.8,24 Since the SAA is related to the substrate concentration, when the TN concentration is lower than the toxic threshold, the SAA raises as the substrate concentration increases, however, when the substrate concentration is higher than the toxic threshold concentration, the SAA is inhibited. The inhibition rate can be expressed as:
 | (1) |
Where I is the inhibition rate (%), SAA is the specific anammox activity under a certain substrate concentration (g N per g VSS per d), and MSAA is the maximum SAA obtained at the optimum concentration in the gradient range that set above.
The anammox granules were taken out from the serum flasks to analyze the content and concentration of EPS as well as the granular shear strength when the accumulated gas production reached 21 mL, which is the theoretical value calculated by the anammox reaction formula.6 All the tests were performed in parallel samples to ensure reliability.
2.3. EPS extraction and analysis
Attached biofilm anammox granules were taken from three sampling points along with different reactor height (top, middle and bottom) to analyze the respective EPS production during the whole experiment. Because the cation exchange resin (CER) method (Dowes Marathon C, 20–50 mesh, sodium form) has been found effective in avoiding cell lysis,25 the method was modified to extract the EPS. In order to study the functions of the LB-EPS and TB-EPS, they were extracted separately. The attached film anammox granules were washed three times with a buffer solution (Na3PO4 of 2 mmol L−1, NaH2PO4 of 4 mmol L−1, NaCl of 9 mmol L−1 and KCl of 1 mmol L−1). Afterwards the granules were resuspended in 30 mL of the buffer solution and centrifuged at 8000G for 15 min. Then the supernatant was filtered through a 0.45 μm syringe filter: the filtrate was regarded as the LB-EPS. For the extraction of TB-EPS, the granules after the first centrifugation were resuspended in the phosphate buffer solution to its original volume of 30 mL, followed by the addition of CER, with a dosage of 60 g per g VSS. The mixed liquor was stirred by a magnetic stirrer at 900 rpm for 1 h, and then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000G for 15 min. The supernatant which was collected and filtered was regarded as the TB-EPS of the anammox granules.
000G for 15 min. The supernatant which was collected and filtered was regarded as the TB-EPS of the anammox granules.
The content of protein and polysaccharides was analyzed for both the LB-EPS and TB-EPS. Protein was measured using the Lowry method with bovine serum albumin as the standard,26 and the polysaccharide content was measured using the phenol–sulfuric acid method with glucose as the standard.27
2.4. Granular analysis
In the assessment of anammox granules, the size distribution, settling velocity and shear strength were evaluated. The size distribution of the granules was determined by image analysis of a photograph using the image analysis software ImageJ. (1.48v, USA) and was divided into five groups according to the particle size (mm): <1.0, 1.0–2.0, 2.0–3.0, 3.0–4.0 and >4.0. The granule settling velocity of each group was measured during the middle 1 m through a 2 m water column.28 For each group, the granule settling velocity was measured in 10 replicates to ensure the reliability. The shear strength was presented in integrity coefficient according to the previous study,20 2 g of wet granules were added to 100 mL of the buffer solution. The mixed liquor was sheared in a thermostatic water bath shaker for 20 min at 200 rpm and 35 °C. After settlement for 1 min in a 1000 mL cylinder, the suspended solid (SS) of the supernatant and sediment were determined. The integrity coefficient can be used to quantify the shear strength, as follows:
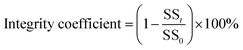 | (2) |
Where, SS0 is the total amount of the attached biofilm anammox granule, g, and SSt is amount of the sludge in the supernatant after 1 min sedimentation, g.
The SEM observation of the anammox granule was also performed. The granules were fixed in a 2.5% glutaraldehyde solution for 4 hours and then dehydrated in a graded series of ethanol (50, 60, 70, 80, 90, 100%, 10 min per step). When dried, the samples were glued to an adhesive coated tab (EMS, Washington, USA), sputter coated with a palladium/gold alloy, and analyzed with a field emission scanning electron microscope (JEOL, JSM-7800F, Japan) at 15 kV.
2.5. Water quality analysis
Samples of the influent and effluent were collected every 2 days and filtered through a 0.45 μm syringe filter. The nitrogen concentration of NH4+–N, NO2−–N and NO3−–N was analyzed by capillary electrophoresis (Agilent 7100), and the TN concentration was calculated as the sum of the three kinds of nitrogen compounds. All other analyses were carried out according to the standard method.293. Results and discussion
3.1. Long-term performance of the AAFEB reactor
Previous study have indicated that varying the NLR and influent substrate concentration resulted in different nitrogen removal performance, SAA and substrate tolerance ability.8 The EPS production and biomass-effluent separation ability were also affected by variations in substrate. During the stable operation phases I and II, the substrate TN concentration increased from 600 mg N L−1 to 1250 mg N L−1 with a constant HRT of 3 hours (Fig. S1A†). Due to the effects of effluent recirculation, the diluted substrate concentration (SNinf, calculated as the sum of the diluted NH4+–N and NO2−–N concentration) increased from 160 ± 9 to 297 ± 29 mg L−1 (Fig. S1A†). Correspondingly, the NLR increased from 4.8 to 10.0 g N L−1 d−1 (Table 1) with a stable TN removal efficiency of 85–92% (Fig. 1B). Along with the change in NLR and substrate concentration, the average EPS concentration (average value of the three sampling points, calculated as the sum of proteins and polysaccharides) and effluent suspended solids slightly increased (Fig. 1A and B).| Phase I | Phase II | Substrate shock | Phase III | Phase IV | Phase V | |
|---|---|---|---|---|---|---|
| Periods (d) | 0–25 | 26–59 | 60 | 61–88 | 89–159 | 160–213 |
| TN (mg N L−1) | 600 | 1250 | 2500 | 1250 | 625 | 313 |
| SNinf (mg N L−1) | 160 ± 9 | 297 ± 29 | 1048 | 542 ± 129 | 288 ± 50 | 98 ± 27 |
| HRT (h) | 3 | 3 | 3 | 3 | 3 | 1.5 |
| NLR (g N L−1 d−1) | 4.8 | 10.0 | 20.0 | 10.0 | 5.0 | 5.0 |
| NRR (g N L−1 d−1) | 4.20 ± 0.11 | 8.93 ± 0.16 | 14.77 | 6.99 ± 1.58 | 3.11 ± 0.51 | 3.90 ± 0.47 |
| Free ammonia (mg L−1) | 6.68–23.22 | 18.34–48.13 | 131.98 | 33.00–74.00 | 15.27–61.48 | 6.12–27.21 |
| Free nitrous acid (μg L−1) | 1.94–7.55 | 2.73–8.99 | 13.06 | 4.84–16.28 | 1.04–8.74 | 0.26–6.38 |
| Average VSS (g L−1) | 56.3 ± 1.2 | 67.2 ± 1.8 | 67.2 ± 1.8 | 56.3 ± 2.1 | 48.1 ± 1.2 | 48 ± 1.1 |
| Sludge blanket height (cm) | 65 | 68 | 68 | 64 | 66 | 69 |
| Total VSS (g) | 229.8 ± 4.2 | 287.0 ± 7.6 | 287.0 ± 7.6 | 226.3 ± 7.4 | 199.4 ± 3.6 | 208.0 ± 3.3 |
 | ||
| Fig. 1 Variation of the average EPS concentration (A) and effluent suspended solids concentration (B) during the experiment. | ||
A substrate shock of 2500 mg N L−1 over a period of 24 hours was performed on day 60, with operation conditions immediately recovered to the levels applied in phase II. As shown in Fig. S1† and 1, the transient high substrate concentration profoundly inhibited the anammox reaction. In phase III, the TN removal efficiency gradually decreased to 40%, resulting in an increase in SNinf from 320 to 721 mg N L−1 and the continuous inhibition of the anammox reaction. The average EPS and effluent suspended solids (as SS) rapidly increased to peak values of 89.6 ± 48.3 mg g−1 VSS and 0.083 g L−1, respectively.
In order to recover anammox activity and investigate the effects of substrate concentration on EPS production, the substrate concentration was decreased to 625 mg N L−1 from the 89th day with a constant HRT of 3 hours. Accordingly, the SNinf and NLR decreased to 294 mg N L−1 and 5.0 g N L−1 d−1, respectively. As shown in Fig. S1,† even though the TN removal efficiency recovered to around 80% as soon as the operation conditions were changed, anammox activity remained inhibited due to the high substrate concentration in phase IV. This resulted in a gradual decrease in TN removal efficiency to 40%. Even though the anammox reaction remained inhibited, the average EPS concentration and effluent SS decreased gradually until they reached the level observed in stable operation phase II (Fig. 1). Following the further decrease in substrate concentration to 313 mg N L−1 in phase V, the SNinf immediately decreased to 200 mg N L−1, and the TN removal efficiency gradually increased to around 85% and remained stable (Fig. S1†). The results obtained from the long-term experiment indicate that besides significantly inhibiting the anammox reaction, the transient high substrate concentration of 2500 mg N L−1 (SNinf of 1048 mg N L−1) led to a considerable increase in the EPS concentration and effluent SS. It should also be noted that although the anammox reaction was in the inhibition condition during phase III and IV, EPS excretion proceeded more efficiently when the SNinf was at a high level of 542 ± 129 mg N L−1 (phase III). This had dramatically reduced during phase IV when the SNinf was at a lower level of 288 ± 50 mg N L−1.
3.2. Changes in biomass and EPS distribution
| Aggregate type | Reactor configuration | Protein | Polysaccharide | PN/PS | Extraction methods | Reference |
|---|---|---|---|---|---|---|
| a Dry wet.b The sum of TB-EPS and LB-EPS. | ||||||
| Methanogenic granules | UASB | 87 mg per g DWa | 68 mg per g DWa | 1.28 | Sonication/centrifugation | 37 |
| Methanogenic granules | UASB | 41 mg per g TS | 14 mg per g TS | 2.93 | CER | 38 |
| Methanogenic granules | SRUSB | 100–130 mg per g VSS | 100–130 mg per g VSS | 1.0–1.16 | Formaldehyde NaOH | 39 |
| Aerobic granules | SBR | 253.8 mg per g VSS | 20.8 mg per g VSS | 12.20 | EDTA | 40 |
| Aerobic granules | SBR | 101.1 mg per g VSS | 15.8 mg per g VSS | 6.40 | Heating | 41 |
| Aerobic granules | SBR | 30.12–47.86 mg per g VSSb | 26.52–25.70 mg per g VSSb | 1.14–1.86 | CER | 42 |
| Anammox granules | UCR | 63.8–82.9 mg per g VSS | 151–310 mg per g VSS | 0.34–0.52 | CER | 43 |
| Anammox granules | Three-bio-electrode reactor | 63.8–307 mg per g VSS | 151–287 mg per g VSS | 0.42–1.07 | CER | 43 |
| Anammox granules | UASB | 55.6 ± 3.2 mg per g VSS | 70.8 ± 6.5 mg per g VSS | 0.8 | CER | 20 |
| Anammox granules | UASB | 164.4 ± 9.3 mg per g VSS | 71.8 ± 2.3 mg per g VSS | 2.29 | EDTA | 33 |
| Biofilm | MBR | 79.51 ± 3.61 mg per g VSS | 30.12 ± 1.52 mg per g VSS | 2.64 ± 0.12 | Heating | 44 |
| Attached biofilm anammox granule | AAFEB | 21.1 ± 8.7 mg per g VSS (stable operation stage) | 10.2 ± 0.3 mg per g VSS (stable operation stage) | 2.6 ± 1.4 (stable operation stage) | CER | This study |
| 46.2 ± 24.9 mg per g VSS (non-stable operation stage) | 10.8 ± 2.6 mg per g VSS (non-stable operation stage) | 5.4 ± 2.1 (non-stable operation stage) | ||||
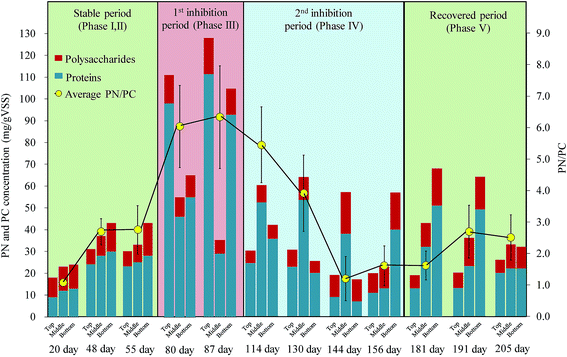 | ||
| Fig. 3 Variation of EPS composition along with reactor height during different operation stages (stable operation period, 1st inhibition period, 2nd inhibition period, recovered period). | ||
Because appropriate EPS production benefits the biomass granulation and flocculation process, it enhances the effluent–solids separation and biomass retention in biological wastewater treatment processes.17 However, excessive EPS has been shown to weaken biomass retention ability.30 In this study, transient substrate shock led to extreme variations in EPS production and distribution, which further compromised the reactor performance. As shown in Fig. 3, in the first inhibition period, protein concentration sharply increased to 77.2 ± 33.1 mg g−1 VSS with a stable polysaccharides concentration of 10.8 ± 2.6 mg g−1 VSS. The highest concentration of EPS had accumulated in the top part of the reactor, whereas it was at the bottom of the reactor during the stable operation period. In the second inhibition period, even though the anammox reaction remained inhibited, after the sudden decrease of substrate concentration in phase IV, the protein concentration in EPS gradually decreased. This continued until the protein concentration was at the level of the stable operation stage. It should be noted that concentration of polysaccharides remained stable. In addition, the distribution characteristic of EPS along with reactor height recovered to conditions similar to those noted in previous stable operation phases. Yang et al. (2009)31 proposed that excessive EPS had adverse effects on the sludge settleability and cell attaching ability due to the bound water brought into the aggregates by the excessive EPS, which results in the production of highly porous flocs with a low density. In this study, the high substrate concentration led to the excessive excretion of EPS and also a decrease in the density of anammox granules, followed by a biomass wash out from the reactor that aggravated the reactor performance. Corresponding to the variation in the EPS content and concentration, the average proteins/polysaccharides (PN/PC) ratio also sharply increased from 2.6 ± 1.4 (stable period) to 5.4 ± 2.1 after the transient substrate shock and then gradually recovered (Fig. 3). As an important parameter to assess the granule characteristics, the PN/PC ratio played a significant role in granule settleability, shear strength and substrate transfer. This is further discussed in the following sections. The variation in EPS content and concentration during the inhibitory phases indicated that EPS production was also substrate stress-associated.
 | ||
| Fig. 4 The anammox particle size distribution (A) and granule setting velocity of each particle size group (B) under different operation conditions (before inhibition, inhibited and recovered). | ||
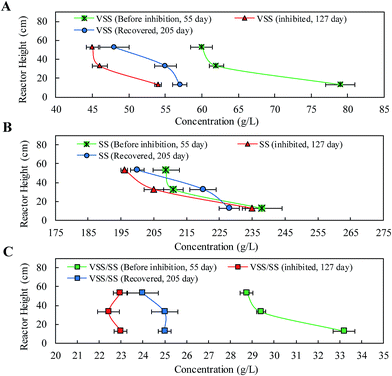
The correlation between granule settleability and particle size, density, geometrical shape, surface charge and hydrophilicity/hydrophobicity has been noted in previous studies.9,32 As shown in Fig. 4B, the settling velocity of the granules in the stable phase was proportional to particle size and in the range of 185–560 m h−1. It should be noted that this is considerably faster than the velocities reported in previous studies.33 It is apparent that the ideal settling velocity would significantly enhance biomass retention ability. Transient substrate shock led to a marked reduction in the settling velocity, reducing it by approximately one third to 121–359 m h−1. Many studies have confirmed that the negative charge of EPS has an adverse effect on settleability.34 Furthermore, Tang et al. (2011)33 indicated that a higher PN/PC resulted in lower shear strength and higher fluid viscosity of anammox granules, which weakened the settleability and raised the risk of biomass washout. Under the multi-functions of the increase in small granules (particle size <1 mm) and the decrease in settleability, obvious biomass washout accounting for 30.5 ± 0.9% of the total amount of VSS washed out from the reactor, which further deteriorated the reactor performance.
3.3. Batch tests for EPS production under different substrate concentrations
Excessive EPS is usually excreted under extreme environmental stress to protect bacteria from damage.9,35 While this self-defense behavior serves to maintain bacteria activity, it also results in unstable reactor operation performance. The stimulation effects of substrate on anammox activity, EPS production and granule shear strength are shown in Fig. 5. Accompanying the increase in TN concentration, the SAA increased to the maximum value of 0.63 g N per g VSS per d when the TN concentration was 200 mg N L−1, and was 97% inhibited when the TN concentration increased to 1000 mg N L−1, the half maximal inhibitory concentration of TN (IC50) was calculated to be 620 mg N L−1 (Fig. 5A). At the end of the batch experiment, a considerable quantity of EPS had been produced and had accumulated on the surface of the granules (see Fig. S4†). Further analysis showed that with the increase in TN concentration, the content and concentration of TB-EPS remained stable at 32.5 ± 2.8 mg g−1 VSS (Fig. 5B). However, the LB-EPS slightly increased when the TN concentration increased to 400 mg N L−1, and a further rapid increase to 114.6 mg g−1 VSS occurred when the TN concentration steadily increased from 400 to 1000 mg N L−1 (Fig. 5C). In addition, with increasing LB-EPS concentration, the polysaccharide concentration remained stable at 9.4 ± 1.7 mg g−1 VSS while the protein concentration increased rapidly from 20.4 mg g−1 VSS to 103.2 mg g−1 VSS. Corresponding to the variation of TB-EPS and LB-EPS, the PN/PC ratio of TB-EPS remained stable at 2.14 ± 0.31, but that of LB-EPS increased rapidly from 2.17 to 11.58.The shear strength tests indicate an inverse correlation between LB-EPS and granule shear strength (Fig. 5D). As a parameter to describe granule shear strength, the integrity coefficient decreased from 98.8 to 88.6% with an increase in the LB-EPS concentration from 26.2 to 103.3 mg g−1 VSS. The results indicate that excessive LB-EPS plays a negative role in granule stability. Li et al. (2007)30 indicated a positive correlation between LB-EPS and bioflocculation ability when the LB-EPS was low. In this study, a deterioration of attachment ability between cells and the aggregates was observed when the LB-EPS was high, but there was insufficient evidence to conclude the existence of any relationship between TB-EPS and granule stability. The LB-EPS concentration was considerably higher than TB-EPS when the anammox granules were affected by excessively high TN concentrations, which resulted in an obvious negative effect on granule stability. Zhang et al. (2016)8 indicated an safe substrate concentration of lower than 320 mg N L−1 should be maintained in the reactor, in this study, a TN concentration of higher than 400 mg N L−1 resulted in the deterioration of anammox granules and the further damage of reactor performance.
3.4. Mechanisms for granule formation and disintegration
The formation of the anammox granules suggests the self-immobilization of anammox bacteria on the surface of the carriers, resulting in a compact structure and a super-high settling velocity. However, transient substrate shock led to the disintegration of the attached anammox biofilm from the granules, which led to a further deterioration in reactor performance. The proposed mechanisms for granule formation and disintegration are shown in Fig. 6. The formation process of the anammox granules is in good agreement with the model provided by Liu et al.36 for granulation mechanisms. Firstly, initial cell-to-cell (e.g. anammox and filamentous bacteria) and cell-to-carrier movement, which involves physical forces and cell mobility, attracts the cells to each other, then the cellular clusters attach to the surface of carriers though hydrogen bonds and filamentous bridging. Following the effects of the polymeric chain of EPS, biomass accumulation and hydrodynamic shear force, the granules become stable and compact. In this process, physical, chemical and biological factors are associated with the granule formation. The anammox granule has a multiple-layer structure with two distinct regions. The plane structure is shown in Fig. 6B, the outer region is the attached anammox biofilm bound by EPS. The inner region is a stable carrier formed in the reactor. The image from the SEM observations supports the view that LB-EPS (Fig. 6F), TB-EPS (Fig. 6G) and filamentous bacteria (Fig. 6H) coexist in the granule and play a role in the granulation process.It has been reported that the attached film is structured with communities of cells enclosed in an EPS matrix and adhered to the surface of the carriers.9,30 Therefore, EPS is viewed as an important mediator in the adhesion process. However, the adverse effects of excessive EPS on granule stability have rarely been reported. In this study, along with the increase in EPS concentration after transient substrate shock, the amount of SS in the effluent increased sharply. According to the results, some of the excessive EPS peeled off from the carrier, removing an abundance of anammox cells, as can be seen in the washed out sludge shown in Fig. 6C. Moreover, because the granules were encased in the excessive EPS (Fig. 6D) and were bonded to each other (Fig. 6E), the mass transfer efficiency was extremely reduced since the substrate must pass through the EPS layer to reach the anammox cells. In addition, because the produced bubbles accumulated in the granules agglomeration rather than being immediately discharged from the reactor, the density of the aggregates was reduced and the up-floating of granules occurred.
4. Conclusions
While appropriate EPS production benefits the biomass granulation and flocculation process, the adverse effect of excessive EPS caused by substrate chock was investigated in this study. A transient substrate shock of 2500 mg N L−1 for 24 hours significantly decreased the treatment performance of the AAFEB reactor and destroyed the structure of the formed anammox granules. Accompanying with the transient substrate shock, excessive EPS of 89.6 ± 48.3 mg g−1 VSS accumulated in the reactor and led to 35 ± 0.8% reduction in the granule settling velocity and 30.5 ± 0.9% reduction in the amount of total VSS, which led to the further deterioration of the reactor performance. EPS production was shown to be both substrate utilization-associated and substrate stress-associated. Depend on the results of batch experiment, the influent TN concentrate shock of higher than 400 mg N L−1 should be avoided in case of the accumulation of LB-EPS. The batch experiment also indicated that the excreted LB-EPS under various substrate concentrations was responsible for variations in the granule settleability and hydrodynamic shear strength.Acknowledgements
The authors wish to thank the Japan Society for the Promotion of Science (JSPS) for financial supporting this study.References
- B. Kartal, J. G. Kuenen and M. C. M. van Loosdrecht, Science, 2010, 328, 702–703 CrossRef CAS PubMed.
- E. Rikmann, I. Zekker, M. Tomingas, P. Vabamae, K. Kroon, A. Saluste, T. Tenno, A. Menert, L. Loorits, S. S. C. D. Rubin and T. Tenno, J. Biosci. Bioeng., 2014, 118, 426–433 CrossRef CAS PubMed.
- L. Daija, A. Selberg, E. Rikmann, I. Zekker, T. Tenno and T. Tenno, Desalin. Water Treat., 2016, 57, 18679–18687 CrossRef CAS.
- I. Zekker, E. Rikmann, A. Mandel, K. Kroon, A. Seiman, J. Mihkelson, T. Tenno and T. Tenno, Environ. Technol., 2016, 37, 1933–1946 CrossRef CAS PubMed.
- W. R. L. van der Star, W. R. Abma, D. Blommers, J. W. Mulder, T. Tokutomi, M. Strous, C. Picioreanu and M. C. M. Van Loosdrecht, Water Res., 2007, 41, 4149–4163 CrossRef CAS PubMed.
- M. Strous, J. J. Heijnen, J. G. Kuenen and M. S. M. Jetten, Appl. Microbiol. Biotechnol., 1998, 50, 589–596 CrossRef CAS.
- Y. N. Gao, Z. J. Liu, F. X. Liu and K. Furukawa, Biodegradation, 2012, 23, 363–372 CrossRef CAS PubMed.
- Y. L. Zhang, Q. G. Niu, H. Y. Ma, S. L. He, K. Kubota and Y.-Y. Li, Bioresour. Technol., 2016, 211, 31–40 CrossRef CAS PubMed.
- G. P. Sheng, H. Q. Yu and X. Y. Li, Biotechnol. Adv., 2010, 28, 882–894 CrossRef CAS PubMed.
- X. W. Liu, G. P. Sheng and H. Q. Yu, Biotechnol. Adv., 2009, 27, 1061–1070 CrossRef CAS PubMed.
- I. W. Sutherland, Trends Microbiol., 2001, 9, 222–227 CrossRef CAS PubMed.
- Y. M. Zheng and H. Q. Yu, Water Res., 2007, 41, 39–46 CrossRef CAS PubMed.
- M. Esparza-Soto and P. Westerhoff, Water Res., 2003, 37, 2301–2310 CrossRef CAS PubMed.
- Z. Bi, S. Qiao, J. T. Zhou, X. Tang and Y. J. Cheng, Chem. Eng. J., 2014, 244, 89–96 CrossRef CAS.
- Y. Li, Z. X. Huang, W. Q. Ruan, H. Y. Ren and M. X. Zhao, J. Chem. Technol. Biotechnol., 2015, 90, 139–148 CrossRef CAS.
- Y. L. Zhang, H. Y. Ma, Q. G. Niu, R. Chen, T. Hojo and Y. Y. Li, Bioresour. Technol., 2016, 222, 261–269 CrossRef CAS PubMed.
- T. T. More, J. S. S. Yadav, S. Yan, R. D. Tyagi and R. Y. Surampalli, J. Environ. Manage., 2014, 144, 1–25 CrossRef CAS PubMed.
- X. L. Hou, S. T. Liu and Z. T. Zhang, Water Res., 2015, 75, 51–62 CrossRef CAS PubMed.
- Z. J. Ding, I. Bourven, G. Guibaud, E. D. van Hullebusch, A. Panico, F. Pirozzi and G. Esposito, Appl. Microbiol. Biotechnol., 2015, 99, 9883–9905 CrossRef CAS PubMed.
- S. Q. Ni, N. Sun, H. L. Yang, J. Zhang and H. H. Ngo, Biochem. Eng. J., 2015, 101, 126–133 CrossRef CAS.
- D. Wang, G. W. Wang, G. Q. Zhang, X. C. Xu and F. L. Yang, Bioresour. Technol., 2013, 131, 527–530 CrossRef CAS PubMed.
- Q. Guo, B. S. Xing, P. Li, J. L. Xu, C. C. Yang and R. C. Jin, Bioresour. Technol., 2015, 192, 765–773 CrossRef CAS PubMed.
- Q. Q. Zhang, Z. Z. Zhang, Q. Guo, Q. Q. Chen, R. C. Jin and X. Y. Jia, Sep. Purif. Technol., 2015, 142, 108–115 CrossRef CAS.
- A. Dapena-Mora, I. Fernandez, J. L. Campos, A. Mosquera-Corral, R. Mendez and M. S. M. Jetten, Enzyme Microb. Technol., 2007, 40, 859–865 CrossRef CAS.
- B. Frolund, R. Palmgren, K. Keiding and P. H. Nielsen, Water Res., 1996, 30, 1749–1758 CrossRef.
- B. Frolund, T. Griebe and P. H. Nielsen, Appl. Microbiol. Biotechnol., 1995, 43, 755–761 CrossRef CAS PubMed.
- G. S. Mcknight, Anal. Biochem., 1977, 78, 86–92 CrossRef CAS PubMed.
- A. Franco, E. Roca and J. M. Lema, Water Res., 2006, 40, 871–880 CrossRef CAS PubMed.
- APHA, 2005.
- X. Y. Li and S. F. Yang, Water Res., 2007, 41, 1022–1030 CrossRef CAS PubMed.
- S. F. Yang and X. Y. Li, Process Biochem., 2009, 44, 91–96 CrossRef CAS.
- M. S. de Graaff, H. Temmink, G. Zeeman, M. C. M. van Loosdrecht and C. J. N. Buisman, Water Res., 2011, 45, 63–74 CrossRef CAS PubMed.
- C. J. Tang, P. Zheng, C. H. Wang, Q. Mahmood, J. Q. Zhang, X. G. Chen, L. Zhang and J. W. Chen, Water Res., 2011, 45, 135–144 CrossRef CAS PubMed.
- J. W. Morgan, C. F. Forster and L. Evison, Water Res., 1990, 24, 743–750 CrossRef CAS.
- L. Zhu, M. L. Lv, X. Dai, Y. W. Yu, H. Y. Qi and X. Y. Xu, Bioresour. Technol., 2012, 107, 46–54 CrossRef CAS PubMed.
- Y. Liu, H. L. Xu, S. F. Yang and J. H. Tay, Water Res., 2003, 37, 661–673 CrossRef CAS PubMed.
- R. Metivier, I. Bourven, J. Labanowski and G. Guibaud, Environ. Sci. Pollut. Res., 2013, 20, 7275–7285 CrossRef CAS PubMed.
- D. J. Batstone and J. Keller, Water Res., 2001, 35, 1723–1729 CrossRef CAS PubMed.
- T. W. Hao, J. H. Luo, L. Wei, H. R. Mackey, R. L. Liu, G. R. Morito and G. H. Chen, Water Res., 2015, 71, 74–84 CrossRef CAS PubMed.
- J. Li, L. B. Ding, A. Cai, G. X. Huang and H. Horn, BioMed Res. Int., 2014, 1–12 Search PubMed.
- D. Wei, L. Shi, T. Yan, G. Zhang, Y. F. Wang and B. Du, Bioresour. Technol., 2014, 171, 211–216 CrossRef CAS PubMed.
- Y. J. Liu, Z. Liu, F. K. Wang, Y. P. Chen, P. Kuschk and X. C. Wang, Bioresour. Technol., 2014, 158, 201–208 CrossRef CAS PubMed.
- X. Yin, S. Qiao, J. T. Zhou and X. Quan, Bioresour. Technol., 2015, 196, 376–382 CrossRef CAS PubMed.
- C. Q. Yin, F. G. Meng and G. H. Chen, Water Res., 2015, 68, 740–749 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20097d |
| This journal is © The Royal Society of Chemistry 2016 |