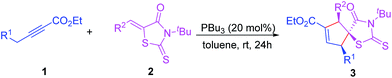Diastereoselective synthesis of cyclopentene spiro-rhodanines containing three contiguous stereocenters via phosphine-catalyzed [3 + 2] cycloaddition or one-pot sequential [3 + 2]/[3 + 2] cycloaddition†
Jiayong Zhanga,
Minxuan Zhanga,
Yuming Lia,
Shang Liua and
Zhiwei Miao *ab
*ab
aState Key Laboratory and Institute of Elemento-Organic Chemistry, Nankai University, Weijin Road 94, Tianjin 300071, People's Republic of China. E-mail: miaozhiwei@nankai.edu.cn
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300071, People's Republic of China
First published on 7th November 2016
Abstract
Two different diastereoselective phosphine-catalyzed cascade reactions to form cyclopentene spiro-rhodanine scaffolds are described. In the first approach, alkynoate derivatives and 5-arylidene-3-(tert-butyl)-2-thioxothiazolidin-4-one react in the presence of PBu3 through a [3 + 2] cycloaddition to afford 5-spiro-cyclopentene-rhodanines in high yields (up to 99%) and with excellent diastereoselectivities [20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric ratio (dr)]. In the second approach, the sequential [3 + 2]/[3 + 2] annulation reaction of ethyl 5-phenylpent-2-ynoate, substituted arylethylpropiolates, amines, and carbon disulfide with phosphine catalysis produces the corresponding monospirocyclic rhodanine products in good yields (up to 92%) and with excellent diastereoselectivities (up to 20
1 diastereomeric ratio (dr)]. In the second approach, the sequential [3 + 2]/[3 + 2] annulation reaction of ethyl 5-phenylpent-2-ynoate, substituted arylethylpropiolates, amines, and carbon disulfide with phosphine catalysis produces the corresponding monospirocyclic rhodanine products in good yields (up to 92%) and with excellent diastereoselectivities (up to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).
Introduction
Spirocyclic compounds are structural motifs frequently found in a large number of natural or synthetic products displaying interesting biological properties.1 Accordingly, the search for developing concise approaches to asymmetrically synthesize spirocyclic molecules, which feature the intriguing combination of two perpendicular rings connected through one tetrasubstituted stereogenic center, has attracted much attention among synthetic and medicinal chemists.2 Some of these approaches have focused on the stereoselective integration of five-membered multiheterocyclic rings into spirocyclic scaffolds.3 Recently, 2-thioxo-1,3-thiazolidin-4-one (rhodanine) derivative epalrestat, thiazolidine-2,4-dione derivatives, glitazones (Avandia, Rezulin, and Actos) and the thiohydantoin derivative (TH2) have been introduced to clinical use for treatment of diabetic complications, type II diabetes, mellitus and epilepsy, respectively (Fig. 1).4 Among the thiazolidinone derivatives, rhodamine is one of the most promising molecules in the drug discovery process. | ||
| Fig. 1 Representative examples of biologically active thiazolidinones and thiohydantoin derivatives. | ||
In 2013, Ye and co-workers described a new synthetic route to cyclohexene-fused spiro-rhodanines by exploiting aminocatalyzed asymmetric Diels–Alder cycloaddition of 2,4-dienals and rhodanine derivatives with high yields and excellent diastereo- and enantioselectivities (Scheme 1a).5 Narayanan also reported the regio- and stereocontrolled synthesis of dispiropyrrolidine derivatives of thiazolidinedione and rhodanine through [3 + 2] cycloaddition reactions (Scheme 1b).6
In contrast to the few examples of asymmetric catalytic synthesis of cyclohexene-fused spiro-rhodanines, the construction of cyclopentene-fused spiro-rhodanines has not been reported to the best of our knowledge. Recently, a nucleophilic-phosphine-catalyzed annulation of electron-deficient alkenes and alkynes has emerged as a powerful tool for the construction of various biologically active carbocycles and heterocycles.7 Typically, they have been used in phosphine-catalyzed [2 + 3] cycloaddition,8 [2 + 4] cycloaddition,9 [3 + 2]/[3 + 2] annulation10 and [4 + 2]/[4 + 2] annulation.11 Encouraged by these elegant studies as well as our recently successes in preparing diverse spiroheterocyclic compounds,12 we wish to report the production of cyclopentene-fused spiro-rhodanine derivatives through phosphine-catalyzed [3 + 2] cycloaddition (Scheme 1c) or a one-pot four-component sequential [3 + 2]/[3 + 2] annulation reaction (Scheme 1d).
Results and discussion
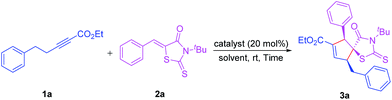
In the initial studies, the reaction of ethyl 5-phenylpent-2-ynoate 1a13 with 5-phenylidene-3-(tert-butyl)-2-thioxothiazolidin-4-one 2a14 was performed in toluene at room temperature in the presence of 20 mol% catalyst. We first examined the reaction by utilizing 1a (0.15 mmol, 1.5 equiv.) and 2a (0.10 mmol) as the substrates and triphenylphosphine (PPh3) (20 mol%) as the catalyst. No product was obtained after 12 h, and 1a was completely recovered (Table 1, entry 1). We assumed that the lack of reaction was due to steric hindrance and the weak nucleophilic ability of the phosphine. Therefore, triphenylphosphine (PPh3) was changed to more strongly nucleophilic phosphines, including methyl(diphenyl)phosphine (PPh2Me), tributylphosphine (PBu3), and various of bisphosphines such as 1,3-bis(diphenylphosphino)ethane (DPPE), 1,3-bis(diphenylphosphino)propane (DPPP), and 1,3-bis(diphenylphosphino)butane (DPPB) as nucleophilic catalysts (Table 1, entries 2–6). Among several phosphine catalysts tested, PBu3 emerged as the preferred catalyst in terms of the yield and diastereoselectivity. With tributylphosphine (PBu3) as the catalyst, the desired cycloaddition product cyclopentene 5-spiro-rhodanine 3a was obtained in 87% yield with excellent diastereoselectivity (dr: >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 1, entry 3).
1) (Table 1, entry 3).
| Entry | Catalyst | Solvent | Time (h) | drb | Yieldc (%) |
|---|---|---|---|---|---|
| a Unless otherwise specified, all reactions were carried out using ethyl 5-phenylpent-2-ynoate 1a (0.15 mmol, 1.5 equiv.) and 5-phenylidene-3-(tert-butyl)-2-thioxothiazolidin-4-one 2a (0.10 mmol) in 1 mL solvent with 20 mol% of catalyst at room temperature.b Diastereomeric ratio (dr) values were determined by 1H NMR of crude products.c Yield of isolated product of 3a after column chromatography.d MTBE = methyl tert-butyl ether.e 10 mol% of catalyst was used. | |||||
| 1 | PPh3 | Toluene | 12 | — | — |
| 2 | PPh2Me | Toluene | 12 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
53 |
| 3 | PBu3 | Toluene | 12 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
| 4 | DPPE | Toluene | 12 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
67 |
| 5 | DPPP | Toluene | 12 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
62 |
| 6 | DPPB | Toluene | 12 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
65 |
| 7 | PBu3 | DCM | 12 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78 |
| 8 | PBu3 | THF | 12 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75 |
| 9 | PBu3 | CHCl3 | 12 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
64 |
| 10 | PBu3 | MeCN | 12 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
61 |
| 11 | PBu3 | MTBEd | 12 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
39 |
| 12 | PBu3 | Toluene | 24 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
| 13e | PBu3 | Toluene | 24 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78 |
With PBu3 chosen as the catalyst, other parameters for the reaction conditions were further examined. A solvent screening was subsequently performed, and toluene was identified to be the best solvent for the reaction, whereas other solvent such as DCM, THF, CHCl3, MeCN, and methyl tert-butyl ether (MTBE), could readily afford the desired product 3a in inferior yields (Table 1, entries 7–11). Further studies suggested that prolong reaction time favoured this reaction, when the reaction time extended to 24 h, the reaction yield increased to 95% (Table 1, entry 12). The loading of catalysts has some influences on the yield. Lower yield was observed when the catalyst loading was decreased to 10 mol% (Table 1, entry 13). It should be noted that the cycloaddition reaction is completely regioselective and highly diastereoselective (only one isomer was detected in all reactions). Thus, the optimal reaction conditions for this transformation were determined to be ethyl 5-phenylpent-2-ynoate (1a; 0.15 mmol), 5-phenylidene-3-(tert-butyl)-2-thioxothiazolidin-4-one (2a; 0.10 mmol), and PBu3 (20 mol%) as catalyst in toluene (1 mL) as a solvent at room temperature.
Using these optimized reaction conditions, we then examined the scope and limitations of the PBu3-catalyzed [3 + 2] cycloaddition reaction between alkynoate derivatives 1 and 5-arylidene-3-(tert-butyl)-2-thioxothiazolidin-4-one 2, and the results are shown in Table 2. Significant structural variation in compound 2 could be carried out. Aryl units containing electron-donating or electron-withdrawing substitutents in different position were readily tolerated, thus giving preferentially the corresponding cyclopentene 5-spiro-rhodanines 3 in moderate to good yields with excellent diastereoselectivities (Table 2, entries 1–17). Notably, the electronic properties of the substituent on 2 have a certain influence on this reaction, and electron-donating substituents resulted in moderate yields (Table 2, entries 10–17).
| Entry | Product | R1 | R2 | drb | Yieldc (%) |
|---|---|---|---|---|---|
| a Reaction conditions: ethyl 5-arylpent-2-ynoate 1 (0.15 mmol), 5-arylidene-3-(tert-butyl)-2-thioxothiazolidin-4-one 2 (0.10 mmol), in 1 mL of toluene at room temperature in the presence of 20 mol% of PBu3.b Diastereomeric ratio (dr) values were determined by 1H NMR of crude products.c Isolated yield after silica gel chromatography of 3. | |||||
| 1 | 3a | Bn | C6H5 (2a) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
| 2 | 3b | Bn | 2-Br-C6H4 (2b) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| 3 | 3c | Bn | 3-NO2-C6H4 (2c) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 |
| 4 | 3d | Bn | 3-CF3-C6H4 (2d) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 |
| 5 | 3e | Bn | 2-Cl-C6H4 (2e) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
| 6 | 3f | Bn | 2,4-(Cl)2-C6H3 (2f) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 7 | 3g | Bn | 4-Br-C6H4 (2g) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 |
| 8 | 3h | Bn | 4-Cl-C6H4 (2h) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
| 9 | 3i | Bn | 4-F-C6H4 (2i) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
| 10 | 3j | Bn | 4-MeO-C6H4 (2j) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
79 |
| 11 | 3k | Bn | 4-iPr-C6H4 (2k) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
| 12 | 3l | Bn | 3-Me-C6H4 (2l) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 |
| 13 | 3m | Bn | 4-Me-C6H4 (2m) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
| 14 | 3n | Bn | 4-N,N-(Me)2-C6H4 (2n) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
82 |
| 15 | 3o | Bn | 2-Me-C6H4 (2o) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
89 |
| 16 | 3p | Bn | 3,4-(Me)2-C6H3 (2p) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
86 |
| 17 | 3q | Bn | 2-MeO-C6H4 (2q) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84 |
| 18 | 3r | Bn | 1-Naphthyl (2r) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
| 19 | 3s | Bn | 2-Thienyl (2s) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 20 | 3t | Ph | C6H5 (2a) | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 |
| 21 | 3u | Ph | 2-Br-C6H4 (2b) | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
| 22 | 3v | Ph | 4-iPr-C6H4 (2k) | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81 |
We were delighted to find that the 5-arylidene rhodanine derivatives 2r and 2s, bearing naphthyl and thienyl groups, respectively, underwent smooth sequential annulations with 1a, to give the corresponding products (i.e., 3r and 3s) in good yields with excellent diastereoselectivities (Table 2, entries 18 and 19). It appears that the benzyl group in the alkynoates 1 is critical for achieving a high diastereoselectivity. When ethyl-4-phenylbut-2-ynoate 1b was employed as the substrate the reaction proceeded in high yields, but with poor diastereoselectivities (Table 2, entries 20–22). The diastereomeric ratio of product was determined by 1H NMR spectroscopy of the crude product. In order to determine the structure of the products, a single crystal X-ray diffraction study of 3l was performed.15 The molecular structure of 3l is shown in Fig. 2, and the structure showed that the relative configuration of the product was assigned as erythro.
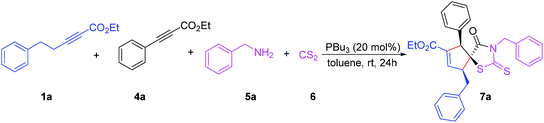
Encouraged by these results, we envisioned that the cycloaddition reaction could proceed by multi-component coupling reactions (MCCRs) in one pot. MCCRs are useful tool to synthesize complex molecular architectures.16 Owing to their exceptional synthetic efficiency and high atom economy, the design of MCCRs for the synthesis of diverse biologically active compounds, have gained great attention in organic synthesis.17 These advantages of MCCRs prompted us to design synthesis of cyclopentene 5-spiro-rhodanine derivatives via phosphine-catalyzed one-pot sequential [3 + 2]/[3 + 2] cycloaddition. First, we found optimized conditions by studying a model reaction between ethyl 5-phenylpent-2-ynoate 1a (0.15 mmol), phenylethylpropiolate 4a (0.10 mmol),18 benzylamine 5a (0.22 mmol), and carbon disulfide 6 (0.11 mmol) with 20 mol% of phosphine catalyst in 1.0 mL of toluene at room temperature. In the absence of catalyst, the reaction did not proceed even after a prolonged reaction time (Table 3, entry 1). Gratifyingly, the optimized conditions were simple, in which 20 mol% PBu3 at room temperature in toluene as solvent were required for the formation of the four-component coupling product 7a in 85% yield with excellent diastereoselectivity (dr: >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 3, entry 2). Other phosphine and bisphosphine catalysts, such as PPh2Me, PPh2Et, DPPP, and DPPB, also gave the desired product 7a in similar yields (Table 3, entries 3–6). A low product yield was obtained when the reaction was performed with 10 mol% of PBu3 (Table 3, entry 7).
1) (Table 3, entry 2). Other phosphine and bisphosphine catalysts, such as PPh2Me, PPh2Et, DPPP, and DPPB, also gave the desired product 7a in similar yields (Table 3, entries 3–6). A low product yield was obtained when the reaction was performed with 10 mol% of PBu3 (Table 3, entry 7).
| Entry | Catalyst | Solvent | drb | Yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: ethyl 5-phenylpent-2-ynoate 1a (0.15 mmol), phenylethylpropiolate 4a (0.10 mmol), benzylamine 5a (0.22 mmol), carbon disulfide 6 (0.11 mmol) in 1 mL of solvent at room temperature in the presence of 20 mol% of catalyst.b Diastereomeric ratio (dr) values were determined by 1H NMR of crude products.c Isolated yield after silica gel chromatography of 7.d 10 mol% of catalyst was used. | ||||
| 1 | — | Toluene | — | — |
| 2 | PBu3 | Toluene | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 |
| 3 | PPh2Me | Toluene | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
46 |
| 4 | PPh2Et | Toluene | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
42 |
| 5 | DPPP | Toluene | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
55 |
| 6 | DPPB | Toluene | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
62 |
| 7d | PBu3 | Toluene | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
52 |
| 8 | PBu3 | DCM | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
63 |
| 9 | PBu3 | THF | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
68 |
| 10 | PBu3 | MeCN | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
64 |
We then examined the effect of the solvent on the yield and diastereoselectivity. Compared with DCM, THF, and MeCN, the use of toluene as a solvent gave a better yield and diastereoselectivity (Table 3, entries 8–10). Thus, the optimal reaction conditions for this transformation were determined to be ethylalkynoate 1a (0.15 mmol), phenylethylpropiolate 4a (0.10 mmol), benzylamine 5a (0.22 mmol), carbon disulfide 6 (0.11 mmol), and PBu3 (20 mol%) as a catalyst in toluene (1 mL) as a solvent at room temperature.
Having determined the optimal conditions, we investigated the scope of the one-pot four-component protocol for the reaction of ethyl 5-phenylpent-2-ynoate 1a, substituted arylethylpropiolates 4a–d, and amines 5a–c, with carbon disulfide 6 as presented in Table 4. Substituted arylethylpropiolates 4 containing electron-donating or electron-withdrawing groups at the para position of the benzene ring reacted smoothly to give 7a–e in moderate to good yields with excellent diastereoselectivities (Table 4, entries 1–5). We next examined various aliphatic amines using phenylethylpropiolate 4a, and carbon disulfide 6 as the reaction partners. Aliphatic amines 5b–c smoothly participated in the [3 + 2]/[3 + 2] cycloaddition to afford the corresponding products 7 in moderate yields with excellent diastereoselectivities (Table 4, entries 2–7). The aromatic ring of arylethylpropiolates 4 bearing an electron-donating or -withdrawing group were all compatible, had little influence on the reaction outcome and resulted in the formation of the corresponding products 7 in good to excellent yields (74–92%) (Table 4, entries 2–5).
| Entry | Product | R3 | R4 | drb | Yieldc (%) |
|---|---|---|---|---|---|
| a Reaction conditions: ethyl 5-phenylpent-2-ynoate 1a (0.15 mmol), arylethylpropiolate 4 (0.10 mmol), amine 5 (0.22 mmol), carbon disulfide 6 (0.11 mmol) in 1 mL of solvent at room temperature in the presence of 20 mol% of catalyst.b Diastereomeric ratio (dr) values were determined by 1H NMR of crude products.c Isolated yield after silica gel chromatography of 7. | |||||
| 1 | 7a | H (4a) | Bn (5a) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 |
| 2 | 7b | 4-Me (4b) | nPr (5b) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
79 |
| 3 | 7c | 4-Me (4b) | nBu (5c) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
76 |
| 4 | 7d | 4 Et (4c) | nBu (5c) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
74 |
| 5 | 7e | 4 F (4d) | nPr (5b) | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| 6 | 7f | H (4a) | nPr (5b) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
89 |
| 7 | 7g | H (4a) | nBu (5c) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
A mechanism for this one-pot sequential [3 + 2]/[3 + 2] annulation cycloaddition is proposed on the basis of previous literature reports,10a,19 and is shown in Scheme 2. The first cycle involves the nucleophilic attack of the phosphine on phenylethylpropiolate 4a to yield the phosphonium salt A (zwitterionic intermediate). The process is continued by the addition of RNH2 to CS2 and subsequent undergoes α addition to intermediate A to give the intermediate B.
Followed by a hydrogen shift to get the intermediate B′ by a reverse equilibrium, which undergoes another intramolecular nucleophilic addition to give intermediate C. Proton transfer and subsequent β-elimination of the catalyst phosphine leads to the formation of the corresponding adduct 2.
In the second cycle, the nucleophilic attack of the regenerated catalyst PBu3 on ethyl alkynoate 1a to give monozwitterion D, which can isomerize to intermediate E. Intermediate E is assumed to undergo δ-addition to the carbon–carbon double bond of substrate 2 to product intermediate F, which further undergoes an intramolecular cyclization to furnish the phosphorane G. Charge migration followed by tributyl phosphine elimination of intermediate G produces the final product 7. The reaction rate and diastereoselective product distribution of 7 is attributed to the steric hindrance effects and electronic repulsions between benzyl group and tributyl-phosphonium.
Conclusions
In conclusion, we have developed an efficient method for the diastereoselective synthesis of 5-spiro-cyclopentene-rhodanine skeletons through phosphine-catalyzed [3 + 2] cycloaddition of alkynoate derivatives 1 with 5-arylidene-3-(tert-butyl)-2-thioxothiazolidin-4-one 2. As an extension, we also developed a phosphine-catalyzed one-pot four-component sequential [3 + 2]/[3 + 2] cycloaddition reaction of ethyl 5-phenylpent-2-ynoate, substituted arylethylpropiolates, amines, with carbon disulfide. The two protocols proceeded well under mild conditions to furnish a series of 5-spiro-cyclopentene-rhodanines containing three contiguous stereocenters, including one spiro quateruary chiral center in excellent diastereoselectivities (only one isomer), and good to excellent yields. Further investigations on the mechanism and synthetic application of this new approach to complex molecule synthesis are currently underway in our laboratory and will be reported in due course.Experimental
General section
All reactions were performed under N2 atmosphere in oven-dried glassware with magnetic stirring. Solvents were dried and distilled prior to use according to the standard methods. Unless otherwise indicated, all materials were obtained from commercial sources, and used as purchased without dehydration. Flash column chromatography was performed on silica gel (particle size 10–40 μm, Ocean Chemical Factory of Qingdao, China). Nitrogen gas (99.999%) was purchased from Boc Gas Inc. 1H NMR, 13C NMR and 19F NMR spectra were recorded in CDCl3 at Bruker 400 MHz spectrometers, TMS served as internal standard (δ = 0 ppm) for 1H NMR and 13C NMR. HR-MS were recorded on APEXII and ZAB-HS spectrometer.Experimental section
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/EtOAc) to afford the product 3.
1 petroleum ether/EtOAc) to afford the product 3.
Ethyl 9-benzyl-3-(tert-butyl)-4-oxo-6-phenyl-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3a). Yellow oil (45.6 mg, 95% yield); 1H NMR (400 MHz, CDCl3): δ 7.34–7.26 (m, 5H), 7.22 (d, J = 6.8 Hz, 3H), 7.01 (dd, J = 7.4, 1.9 Hz, 2H), 6.77 (t, J = 2.3 Hz, 1H), 4.76 (t, J = 2.1 Hz, 1H), 4.12–3.95 (m, 2H), 3.95–3.85 (m, 1H), 2.99 (dd, J = 13.8, 8.3 Hz, 1H), 2.90 (dd, J = 13.8, 8.3 Hz, 1H), 1.61 (s, 9H), 1.05 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.5, 179.0, 163.7, 144.4, 137.6, 136.8, 135.8, 129.2, 128.8, 128.7, 128.3, 128.0, 126.8, 73.1, 65.1, 60.7, 60.3, 54.5, 38.0, 28.8, 13.9; HRMS (ESI): m/z calcd for C27H30NO3S2 [M + H]+ 480.1662, found 480.1664.
Ethyl 9-benzyl-6-(2-bromophenyl)-3-(tert-butyl)-4-oxo-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3b). Yellow oil (51.4 mg, 92% yield); 1H NMR (400 MHz, CDCl3): δ 7.59 (dd, J = 8.0, 1.2 Hz, 1H), 7.36–7.26 (m, 3H), 7.26–7.19 (m, 3H), 7.16 (dd, J = 7.8, 1.6 Hz, 1H), 6.98 (dd, J = 7.7, 1.6 Hz, 1H), 6.85 (t, J = 2.3 Hz, 1H), 5.26 (d, J = 0.9 Hz, 1H), 4.11–3.93 (m, 2H), 3.83–3.71 (m, 1H), 3.08 (dd, J = 13.5, 6.8 Hz, 1H), 2.63 (dd, J = 13.5, 10.2 Hz, 1H), 1.76 (s, 9H), 1.03 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 201.0, 180.4, 163.1, 145.2, 138.3, 137.7, 135.4, 133.1, 129.4, 129.0, 128.9, 128.7, 127.6, 126.8, 126.7, 69.5, 65.4, 60.7, 58.4, 56.0, 39.4, 28.8, 13.9; HRMS (ESI): m/z calcd for C27H29BrNO3S2 [M + H]+ 558.0767, found 558.0765.
Ethyl 9-benzyl-3-(tert-butyl)-6-(3-nitrophenyl)-4-oxo-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3c). Yellow oil (50.4 mg, 96% yield); 1H NMR (400 MHz, CDCl3): δ 8.21–8.12 (m, 1H), 7.89 (t, J = 1.9 Hz, 1H), 7.50 (t, J = 7.9 Hz, 1H), 7.35–7.27 (m, 3H), 7.22 (d, J = 7.1 Hz, 3H), 6.87 (t, J = 2.3 Hz, 1H), 4.90 (t, J = 2.3 Hz, 1H), 4.08–3.97 (m, 2H), 2.99 (d, J = 8.6 Hz, 2H), 1.55 (s, 9H), 1.10 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 199.3, 178.4, 163.2, 147.9, 146.1, 138.8, 137.0, 135.1, 134.3, 129.2, 128.9, 126.9, 123.5, 123.1, 73.3, 65.6, 61.0, 60.2, 54.5, 37.5, 28.8, 14.0; HRMS (ESI): m/z calcd for C27H29N2O5S2 [M + H]+: 525.1512, found 525.1511.
Ethyl 9-benzyl-3-(tert-butyl)-4-oxo-2-thioxo-6-(3-(trifluoromethyl)phenyl)-1-thia-3-azaspiro[4.4]-non-7-ene-7-carboxylate (3d). Yellow oil (53.7 mg, 98% yield); 1H NMR (400 MHz, CDCl3): δ 7.55 (d, J = 7.7 Hz, 1H), 7.43 (t, J = 7.7 Hz, 1H), 7.33–7.26 (m, 3H), 7.24–7.14 (m, 4H), 6.82 (s, 1H), 4.85 (s, 1H), 4.08 (d, J = 7.1 Hz, 1H), 4.02 (dd, J = 7.1 Hz, 6.9 Hz, 2H), 2.97 (d, J = 8.2 Hz, 2H), 1.55 (s, 9H), 1.05 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 199.7, 178.6, 163.4, 145.4, 137.7, 137.2, 134.9, 132.4, 129.2, 128.8, 128.7, 126.9, 125.4, 124.9, 124.9, 60.8, 73.5, 65.4, 60.8, 60.4, 54.5, 37.6, 28.7, 13.9; 19F NMR (376 MHz, CDCl3): δ −62.59; HRMS (ESI): m/z calcd for C28H29F3NO3S2 [M + H]+: 548.1535, found 548.1535.
Ethyl 9-benzyl-3-(tert-butyl)-6-(2-chlorophenyl)-4-oxo-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3e). Yellow oil (48.8 mg, 95% yield); 1H NMR (400 MHz, CDCl3): δ 7.40 (dd, J = 5.7, 3.6 Hz, 1H), 7.32 (t, J = 7.3 Hz, 2H), 7.22 (dd, J = 8.3, 4.7 Hz, 5H), 6.98 (dd, J = 5.8, 3.5 Hz, 1H), 6.85 (t, J = 2.1 Hz, 1H), 5.27 (s, 1H), 4.12–4.02 (m, 2H), 3.83 (dd, J = 12.1, 4.6 Hz, 1H), 3.06 (dd, J = 13.5, 7.1 Hz, 1H), 2.66 (dd, J = 13.5, 9.9 Hz, 1H), 1.74 (s, 9H), 1.03 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.8, 180.1, 163.2, 145.4, 137.7, 136.4, 135.3, 135.2, 129.8, 129.2, 129.0, 128.8, 128.8, 126.9, 126.8, 69.9, 65.3, 60.7, 56.0, 55.6, 39.1, 28.7, 13.9: HRMS (ESI): m/z calcd for C27H29ClNO3S2 [M + H]+: 514.1272, found 514.1268.
Ethyl 9-benzyl-3-(tert-butyl)-6-(2,4-dichlorophenyl)-4-oxo-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3f). Yellow oil (54.3 mg, 99% yield); 1H NMR (400 MHz, CDCl3): δ 7.42 (d, J = 2.1 Hz, 1H), 7.32 (t, J = 7.3 Hz, 2H), 7.21 (dd, J = 13.5, 7.1 Hz, 4H), 6.87 (t, J = 5.5 Hz, 2H), 5.21 (s, 1H), 4.12–3.97 (m, 2H), 3.84 (dd, J = 8.2, 7.3 Hz, 1H), 3.05 (dd, J = 13.6, 7.2 Hz, 1H), 2.66 (dd, J = 13.5, 9.6 Hz, 1H), 1.72 (s, 9H), 1.08 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.3, 180.0, 163.0, 145.8, 137.5, 135.8, 135.1, 134.6, 134.3, 129.6, 129.6, 129.1, 128.9, 127.3, 126.9, 69.8, 65.5, 60.9, 55.7, 55.6, 39.0, 28.7, 14.0. HRMS (ESI): m/z calcd for C27H28Cl2NO3S2 [M + H]+: 548.0882, found 548.0873.
Ethyl 9-benzyl-6-(4-bromophenyl)-3-(tert-butyl)-4-oxo-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3g). Yellow oil (53.6 mg, 96% yield); 1H NMR (400 MHz, CDCl3): δ 7.42 (d, J = 8.4 Hz, 2H), 7.35–7.27 (m, 2H), 7.21 (t, J = 6.7 Hz, 3H), 6.88 (d, J = 8.4 Hz, 2H), 6.78 (t, J = 2.3 Hz, 1H), 4.73 (s, 1H), 4.10–4.05 (m, 2H), 3.92–3.87 (ddd, J = 21.3, 12.2, 5.1 Hz, 1H), 3.00–2.86 (ddd, J = 21.3, 4.9 Hz, 2H), 1.59 (s, 9H), 1.08 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 199.9, 178.8, 163.4, 144.9, 137.3, 136.0, 135.3, 131.5, 130.4, 129.2, 128.8, 126.8, 122.0, 72.9, 65.2, 60.8, 59.7, 54.6, 37.9, 28.8, 14.0; HRMS (ESI): m/z calcd for C27H29BrNO3S2 [M + H]+: 558.0767, found 558.0757.
Ethyl 9-benzyl-3-(tert-butyl)-6-(4-chlorophenyl)-4-oxo-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3h). Yellow oil (48.8 mg, 95% yield); 1H NMR (400 MHz, CDCl3): δ 7.28 (dd, J = 10.2, 4.7 Hz, 3H), 7.25 (s, 1H), 7.20 (t, J = 6.4 Hz, 3H), 6.93 (d, J = 8.4 Hz, 2H), 6.77 (t, J = 2.3 Hz, 1H), 4.73 (t, J = 2.1 Hz, 1H), 4.13–3.95 (m, 2H), 3.90 (tt, J = 13.8, 2.0 Hz, 1H), 2.99–2.85 (ddd, J = 33.1, 13.8, 8.3 Hz, 2H), 1.58 (d, J = 7.6 Hz, 9H), 1.07 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 199.9, 178.9, 163.4, 144.8, 137.4, 135.5, 135.3, 133.9, 130.1, 129.1, 128.8, 128.5, 126.8, 73.0, 65.2, 60.8, 59.7, 54.6, 37.9, 28.8, 14.0; HRMS (ESI): m/z calcd for C27H29ClNO3S2 [M + H]+: 514.1272, found 514.1266.
Ethyl 9-benzyl-3-(tert-butyl)-6-(4-fluorophenyl)-4-oxo-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3i). Yellow oil (46.3 mg, 93% yield); 1H NMR (400 MHz, CDCl3): δ 7.55 (dd, J = 12.8, 6.1 Hz, 2H), 7.48 (d, J = 6.8 Hz, 3H), 7.25 (d, J = 6.6 Hz, 4H), 7.04 (d, J = 1.4 Hz, 1H), 5.02 (s, 1H), 4.41–4.24 (m, 2H), 4.18 (t, J = 7.7 Hz, 1H), 3.31–3.06 (m, 2H), 1.86 (s, 9H), 1.34 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.1, 178.9, 163.5, 144.6, 137.4, 135.5, 130.4, 130.4, 129.2, 128.8, 126.8, 115.3, 115.1, 73.3, 65.2, 60.7, 59.7, 54.4, 37.9, 28.8, 14.0; 19F NMR (376 MHz, CDCl3): δ −113.87; HRMS (ESI): m/z calcd for C27H29FNO3S2 [M + H]+: 498.1567, found 498.1565.
Ethyl 9-benzyl-3-(tert-butyl)-6-(4-methoxyphenyl)-4-oxo-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3j). Yellow oil (40.3 mg, 79% yield); 1H NMR (400 MHz, CDCl3): δ 7.28 (d, J = 6.6 Hz, 2H), 7.22 (s, 3H), 6.93 (d, J = 7.3 Hz, 2H), 6.82 (d, J = 7.8 Hz, 2H), 6.74 (s, 1H), 4.70 (s, 1H), 4.12–3.99 (m, 2H), 3.86 (s, 1H), 3.78 (s, 3H), 2.93 (dt, J = 13.4 Hz, 2H), 1.62 (s, 9H), 1.08 (t, J = 6.7 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 199.5, 178.1, 162.7, 158.2, 143.0, 136.6, 134.9, 128.8, 128.1, 128.0, 127.7, 125.7, 112.6, 72.3, 64.0, 59.6, 58.5, 54.2, 53.3, 37.1, 27.8, 13.0; HRMS (ESI): m/z calcd for C28H32NO4S2 [M + H]+: 510.1767, found 510.1760.
Ethyl 9-benzyl-3-(tert-butyl)-6-(4-isopropylphenyl)-4-oxo-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3k). Yellow oil (45.4 mg, 87% yield); 1H NMR (400 MHz, CDCl3): δ 7.30 (t, J = 7.2 Hz, 2H), 7.22 (d, J = 7.2 Hz, 3H), 7.13 (d, J = 7.5 Hz, 2H), 6.93 (d, J = 7.5 Hz, 2H), 6.75 (s, 1H), 4.71 (s, 1H), 4.16–3.94 (m, 2H), 3.84 (t, J = 8.0 Hz, 1H), 2.99 (dd, J = 8.0 Hz, 1H), 2.93–2.79 (m, 2H), 1.62 (s, 9H), 1.22 (s, 3H), 1.20 (s, 3H), 1.05 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.6, 179.0, 163.7, 148.6, 144.0, 137.7, 136.0, 134.1, 129.1, 128.7, 128.7, 126.7, 126.3, 73.1, 64.9, 60.6, 59.8, 54.4, 38.2, 33.7, 28.7, 23.9, 13.9; HRMS (ESI): m/z calcd for C30H36NO3S2 [M + H]+: 522.2131, found 522.2130.
Ethyl 9-benzyl-3-(tert-butyl)-4-oxo-2-thioxo-6-(m-tolyl)-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3l). Yellow oil (41.9 mg, 85% yield); 1H NMR (400 MHz, CDCl3): δ 7.29 (dd, J = 9.5, 5.5 Hz, 2H), 7.24–7.14 (m, 4H), 7.06 (d, J = 7.5 Hz, 1H), 6.79 (d, J = 6.3 Hz, 2H), 6.74 (t, J = 2.2 Hz, 1H), 4.73 (d, J = 1.9 Hz, 1H), 4.17–3.97 (m, 2H), 3.94–3.85 (m, 1H), 2.94 (dd, J = 13.8, 8.3 Hz, 2H), 2.30 (s, 3H), 1.61 (s, 9H), 1.06 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.7, 179.1, 163.8, 144.1, 137.7, 137.6, 136.8, 135.9, 129.4, 129.2, 128.8, 128.2, 126.7, 125.8, 73.1, 65.0, 60.7, 60.3, 54.5, 38.0, 28.8, 21.5, 14.0; HRMS (ESI): m/z calcd for C28H32NO3S2 [M + H]+: 494.1818, found 494.1823.
Ethyl 9-benzyl-3-(tert-butyl)-4-oxo-2-thioxo-6-(p-tolyl)-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3m). Yellow oil (42.9 mg, 87% yield); 1H NMR (400 MHz, CDCl3): δ 7.28 (d, J = 7.1 Hz, 2H), 7.22 (d, J = 6.1 Hz, 3H), 7.09 (d, J = 7.7 Hz, 2H), 6.89 (d, J = 7.9 Hz, 2H), 6.74 (d, J = 2.1 Hz, 1H), 4.71 (s, 1H), 4.15–3.97 (m, 2H), 3.86 (dd, J = 11.4, 5.0 Hz, 1H), 2.98 (dd, J = 13.6, 8.2 Hz, 1H), 2.87 (dd, J = 13.7, 8.5 Hz, 1H), 2.31 (s, 3H), 1.62 (s, 9H), 1.07 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.6, 179.2, 163.7, 144.0, 137.7, 135.9, 134.0, 129.2, 129.1, 128.8, 128.6, 126.7, 73.0, 65.0, 60.7, 59.9, 54.5, 38.2, 28.8, 21.2, 14.0; HRMS (ESI): m/z calcd for C28H32NO3S2 [M + H]+: 494.1818, found 494.1820.
Ethyl 9-benzyl-3-(tert-butyl)-6-(4-(dimethylamino)phenyl)-4-oxo-2-thioxo-1-thia-3-azaspiro-[4.4]-non-7-ene-7-carboxylate (3n). Yellow oil (42.9 mg, 82% yield); 1H NMR (400 MHz, CDCl3): δ 7.33–7.27 (m, 2H), 7.25–7.19 (m, 3H), 6.88 (d, J = 8.6 Hz, 2H), 6.71 (t, J = 2.2 Hz, 1H), 6.62 (d, J = 8.6 Hz, 2H), 4.63 (s, 1H), 4.12–3.97 (m, 2H), 3.78 (t, J = 8.3 Hz, 1H), 2.99 (dd, J = 13.6, 7.7 Hz, 1H), 2.92 (d, J = 10.1 Hz, 6H), 2.82 (dd, J = 13.6, 9.0 Hz, 1H), 1.65 (s, 9H), 1.10 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.8, 179.4, 157.0, 150.1, 143.4, 137.9, 136.4, 129.4, 129.1, 128.8, 126.7, 124.8, 112.1, 73.3, 64.8, 60.6, 59.3, 54.3, 40.5, 38.5, 28.8, 14.0; HRMS (ESI): m/z calcd for C29H35N2O3S2 [M + H]+: 523.2084, found 523.2090.
Ethyl 9-benzyl-3-(tert-butyl)-4-oxo-2-thioxo-6-(o-tolyl)-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3o). Yellow oil (43.9 mg, 89% yield); 1H NMR (400 MHz, CDCl3): δ 7.33 (t, J = 7.3 Hz, 2H), 7.21 (dd, J = 8.5, 4.3 Hz, 6H), 6.99–6.90 (m, 1H), 6.83–6.71 (m, 1H), 4.98 (d, J = 1.1 Hz, 1H), 4.12–3.91 (m, 2H), 3.83–3.47 (m, 1H), 3.06 (dd, J = 13.4, 6.6 Hz, 1H), 2.69 (dd, J = 13.4, 10.4 Hz, 1H), 2.30 (s, 3H), 1.74 (s, 9H), 1.03 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.7, 180.4, 163.4, 143.6, 138.0, 137.1, 137.1, 136.5, 130.8, 129.0, 128.9, 127.9, 126.9, 126.8, 126.3, 70.0, 65.0, 60.6, 55.9, 54.4, 39.4, 28.8, 20.1, 13.9; HRMS (ESI): m/z calcd for C28H32NO3S2 [M + H]+: 494.1818, found 494.1819.
Ethyl 9-benzyl-3-(tert-butyl)-6-(3,4-dimethylphenyl)-4-oxo-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3p). Yellow oil (43.7 mg, 86% yield); 1H NMR (400 MHz, CDCl3): δ 7.29 (d, J = 7.0 Hz, 2H), 7.22 (d, J = 6.9 Hz, 3H), 7.04 (d, J = 7.6 Hz, 1H), 6.81–6.63 (m, 3H), 4.69 (s, 1H), 4.00–4.10 (m, 2H), 3.85 (t, J = 8.1 Hz, 1H), 2.99 (dd, J = 13.6, 8.1 Hz, 1H), 2.87 (dd, J = 13.6, 8.7 Hz, 1H), 2.21 (s, 6H), 1.63 (s, 9H), 1.09 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.8, 179.2, 163.8, 143.7, 137.7, 136.3, 136.3, 136.0, 134.4, 129.9, 129.6, 129.1, 128.8, 126.7, 126.0, 72.9, 64.9, 60.6, 59.8, 54.6, 38.2, 28.8, 19.9, 19.6, 14.0; HRMS (ESI): m/z calcd for C29H34NO3S2 [M + H]+: 508.1975, found 508.1981.
Ethyl 9-benzyl-3-(tert-butyl)-6-(2-methoxyphenyl)-4-oxo-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3q). Yellow oil (42.8 mg, 84% yield); 1H NMR (400 MHz, CDCl3): δ 7.43–7.26 (m, 3H), 7.21 (t, J = 7.4 Hz, 4H), 6.91 (d, J = 3.8 Hz, 2H), 6.80 (s, 1H), 5.14 (s, 1H), 4.09–3.99 (m, 2H), 3.79 (s, 3H), 3.72 (s, 1H), 3.00 (dd, J = 13.1, 6.4 Hz, 1H), 2.66–2.48 (m, 1H), 1.77 (s, 9H), 1.08 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 201.5, 180.3, 163.6, 157.5, 145.1, 138.0, 135.1, 129.2, 129.0, 128.8, 127.7, 126.9, 126.7, 120.4, 110.6, 69.9, 64.8, 60.6, 55.7, 54.7, 52.4, 39.1, 28.9, 14.0; HRMS (ESI): m/z calcd for C28H32NO4S2 [M + H]+: 510.1767, found 510.1771.
Ethyl 9-benzyl-3-(tert-butyl)-6-(naphthalen-1-yl)-4-oxo-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3r). Yellow oil (46.1 mg, 87% yield); 1H NMR (400 MHz, CDCl3): δ 7.97–7.89 (m, 1H), 7.84 (dd, J = 5.9, 3.5 Hz, 1H), 7.78 (d, J = 8.0 Hz, 1H), 7.52–7.44 (m, 2H), 7.44–7.39 (m, 1H), 7.33–7.27 (m, 2H), 7.23 (d, J = 8.0 Hz, 3H), 7.16–7.12 (m, 1H), 6.85 (t, J = 2.3 Hz, 1H), 5.68 (s, 1H), 4.06–3.94 (m, 2H), 3.94–3.73 (m, 1H), 3.02 (dd, J = 13.7, 7.8 Hz, 1H), 2.87 (dd, J = 13.7, 8.9 Hz, 1H), 1.57 (s, 9H), 0.92 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.9, 180.2, 163.7, 144.2, 137.7, 136.3, 134.4, 133.9, 132.2, 129.3, 129.1, 128.8, 128.7, 126.8, 126.6, 126.0, 125.8, 125.0, 122.8, 72.2, 65.2, 60.6, 55.9, 54.4, 38.5, 28.6, 13.9; HRMS (ESI): m/z calcd for C31H32NO3S2 [M + H]+: 530.1818, found 530.1818.
Ethyl 9-benzyl-3-(tert-butyl)-4-oxo-6-(thiophen-2-yl)-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3s). Yellow oil (48.1 mg, 99% yield); 1H NMR (400 MHz, CDCl3): δ 7.31 (t, J = 7.2 Hz, 2H), 7.22 (t, J = 8.6 Hz, 4H), 6.97 (t, J = 3.5 Hz, 1H), 6.83 (s, 1H), 6.71 (s, 1H), 4.95 (s, 1H), 4.18–4.04 (m, 2H), 3.74 (t, J = 8.3 Hz, 1H), 3.06 (dd, J = 13.4, 7.5 Hz, 1H), 2.91–2.81 (m, 1H), 1.68 (s, 9H), 1.12 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.3, 179.0, 163.3, 144.0, 140.9, 137.7, 136.0, 129.1, 128.8, 127.4, 127.1, 126.8, 125.4, 71.9, 65.0, 60.8, 54.5, 54.4, 39.1, 28.8, 14.0; HRMS (ESI): m/z calcd for C25H28NO3S3 [M + H]+: 486.1226, found 486.1224.
Ethyl 3-(tert-butyl)-4-oxo-6,9-diphenyl-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3t). Yellow oil (42.4 mg, 91% yield); 1H NMR (400 MHz, CDCl3): δ 7.34 (d, J = 7.0 Hz, 2H), 7.27 (d, J = 7.0 Hz, 3H), 7.23 (s, 1H), 7.17–6.97 (m, 5H), 4.93 (s, 1H), 4.86 (s, 1H), 4.15–4.03 (m, 2H), 1.78 (s, 9H), 1.08 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.2, 179.6, 163.6, 143.0, 137.5, 137.4, 137.1, 129.0, 128.9, 128.8, 128.7, 128.6, 128.5, 128.4, 128.3, 127.9, 126.9, 74.8, 65.0, 60.8, 59.6, 58.8, 29.0, 13.9; HRMS (ESI): m/z calcd for C26H28NO3S2 [M + H]+: 466.1505, found 466.1502.
Ethyl 6-(2-bromophenyl)-3-(tert-butyl)-4-oxo-9-phenyl-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3u). Yellow oil (50.6 mg, 93% yield); 1H NMR (400 MHz, CDCl3): δ 7.55 (d, J = 7.9 Hz, 1H), 7.32 (dd, J = 14.5, 8.6 Hz, 4H), 7.13–6.99 (m, 5H), 5.45 (s, 1H), 4.73 (s, 1H), 4.17–4.00 (m, 2H), 1.86 (s, 9H), 1.07 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 201.2, 181.1, 163.1, 143.2, 138.6, 137.5, 133.1, 129.3, 129.0, 128.9, 128.8, 128.6, 128.4, 127.5, 126.4, 71.5, 65.4, 61.6, 60.8, 58.2, 29.0, 13.9; HRMS (ESI): m/z calcd for C26H27BrNO3S2 [M + H]+: 544.0610, found 544.0646.
Ethyl 3-(tert-butyl)-6-(4-isopropylphenyl)-4-oxo-9-phenyl-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (3v). Yellow oil (41.1 mg, 81% yield); 1H NMR (400 MHz, CDCl3): δ 7.33 (ddd, J = 15.1, 14.1, 9.1 Hz, 4H), 7.14 (ddd, J = 15.0, 8.0, 3.3 Hz, 4H), 7.07–6.88 (m, 2H), 4.89 (t, J = 2.2 Hz, 1H), 4.83 (t, J = 2.1 Hz, 1H), 4.26–3.95 (m, 2H), 2.87 (dd, J = 14.0, 7.0 Hz, 1H), 1.89–1.39 (m, 9H), 1.22 (ddd, J = 7.6, 7.0, 1.1 Hz, 6H), 1.08 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 200.4, 179.6, 163.6, 148.5, 142.5, 137.7, 137.3, 134.7, 128.9, 128.8, 128.6, 128.3, 127.6, 127.0, 126.4, 74.7, 65.0, 60.7, 59.2, 58.7, 33.8, 28.9, 23.9, 13.9; HRMS (ESI): m/z calcd for C29H34NO3S2 [M + H]+: 508.1975, found 508.1982.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/EtOAc) to afford the product 7.
1 petroleum ether/EtOAc) to afford the product 7.
Ethyl 3,9-dibenzyl-4-oxo-6-phenyl-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (7a). Yellow oil (43.6 mg, 85% yield); 1H NMR (400 MHz, CDCl3): δ 7.26 (d, J = 3.2 Hz, 5H), 7.18 (dd, J = 12.7, 6.4 Hz, 6H), 7.11 (d, J = 7.3 Hz, 2H), 6.76 (dd, J = 13.7, 4.5 Hz, 3H), 4.88 (d, J = 14.6 Hz, 2H), 4.47 (d, J = 14.3 Hz, 1H), 4.14–4.05 (m, 1H), 4.04–3.95 (m, 2H), 3.08 (dd, J = 13.8, 7.2 Hz, 1H), 2.99–2.86 (m, 1H), 1.03 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 198.7, 177.8, 163.8, 144.1, 136.8, 136.2, 136.1, 134.7, 129.1, 128.6, 128.5, 128.4, 128.3, 127.9, 126.9, 76.1, 60.8, 60.7, 55.6, 47.2, 37.1, 13.9; HRMS (ESI): m/z calcd for C30H28NO3S2 [M + H]+: 514.1505, found 514.1514.
Ethyl 9-benzyl-4-oxo-3-propyl-2-thioxo-6-(p-tolyl)-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (7b). Yellow oil (37.9 mg, 79% yield); 1H NMR (400 MHz, CDCl3): δ 7.23 (d, J = 7.0 Hz, 2H), 7.17 (d, J = 7.5 Hz, 3H), 7.03 (d, J = 7.8 Hz, 2H), 6.81 (d, J = 7.9 Hz, 2H), 6.76 (d, J = 2.1 Hz, 1H), 4.95 (d, J = 41.2 Hz, 1H), 4.17–4.02 (m, 2H), 4.01–3.94 (m, 1H), 3.57 (ddd, J = 13.1, 9.1, 6.1 Hz, 1H), 3.45–3.30 (m, 1H), 3.05 (dd, J = 13.9, 7.4 Hz, 1H), 2.95 (dd, J = 13.9, 9.5 Hz, 1H), 2.28 (s, 3H), 1.44–1.32 (m, 2H), 1.08 (t, J = 7.1 Hz, 3H), 0.79 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 199.2, 178.1, 163.8, 144.0, 137.6, 137.0, 136.4, 133.3, 129.0, 129.0, 128.6, 128.3, 126.8, 76.0, 60.7, 60.6, 55.5, 45.9, 37.3, 21.2, 20.2, 14.0, 11.1; HRMS (ESI): m/z calcd for C27H30NO3S2 [M + H]+: 480.1662, found 480.1665.
Ethyl 9-benzyl-3-butyl-4-oxo-2-thioxo-6-(p-tolyl)-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (7c). Yellow oil (37.5 mg, 76% yield); 1H NMR (400 MHz, CDCl3): δ 7.23 (d, J = 7.0 Hz, 2H), 7.19–7.12 (m, 3H), 7.03 (d, J = 7.9 Hz, 2H), 6.80 (d, J = 8.0 Hz, 2H), 6.75 (t, J = 2.3 Hz, 1H), 4.88 (t, J = 2.4 Hz, 1H), 4.15–4.01 (m, 2H), 3.98 (ddd, J = 9.5, 6.3, 2.6 Hz, 1H), 3.61 (ddd, J = 13.2, 9.0, 5.9 Hz, 1H), 3.49–3.37 (m, 1H), 3.04 (dd, J = 13.9, 7.4 Hz, 1H), 2.94 (dd, J = 13.9, 9.5 Hz, 1H), 2.28 (s, 3H), 1.35–1.26 (m, 2H), 1.23–1.13 (m, 2H), 1.07 (t, J = 7.1 Hz, 3H), 0.87 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 199.1, 178.0, 163.8, 144.0, 137.6, 137.0, 136.4, 133.3, 129.0, 129.0, 128.6, 128.4, 126.8, 75.9, 60.7, 60.6, 55.5, 44.3, 37.3, 28.8, 21.2, 19.9, 14.0, 13.7; HRMS (ESI): m/z calcd for C28H32NO3S2 [M + H]+: 494.1818, found 494.1823.
Ethyl 9-benzyl-3-butyl-6-(4-ethylphenyl)-4-oxo-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (7d). Yellow oil (37.5 mg, 74% yield); 1H NMR (400 MHz, CDCl3): δ 7.23 (s, 1H), 7.17 (dd, J = 5.0, 2.5 Hz, 4H), 7.04 (t, J = 8.9 Hz, 2H), 6.83 (d, J = 8.0 Hz, 2H), 6.76 (t, J = 2.3 Hz, 1H), 4.91 (dd, J = 18.3, 16.0 Hz, 1H), 4.18 (dd, J = 14.3, 7.2 Hz, 1H), 4.07–3.96 (m, 2H), 3.62 (ddd, J = 13.1, 8.9, 6.0 Hz, 1H), 3.51–3.32 (m, 2H), 3.05 (dd, J = 13.9, 7.4 Hz, 1H), 2.99–2.89 (m, 2H), 2.59 (q, J = 7.6 Hz, 2H), 1.25 (d, J = 7.1 Hz, 2H), 1.17 (d, J = 7.6 Hz, 3H), 1.06 (t, J = 7.1 Hz, 3H), 0.92–0.82 (m, 3H); 13C NMR (101 MHz, CDCl3): δ 199.1, 178.0, 163.8, 144.0, 137.0, 133.5, 129.0, 128.6, 128.5, 128.3, 127.7, 126.8, 126.3, 76.0, 60.7, 60.6, 55.5, 49.5, 44.2, 37.3, 28.8, 19.9, 15.3, 13.9, 13.7; HRMS (ESI): m/z calcd for C29H34NO3S2 [M + H]+: 508.1975, found 508.1976.
Ethyl 9-benzyl-6-(4-fluorophenyl)-4-oxo-3-propyl-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (7e). Yellow oil (44.4 mg, 92% yield); 1H NMR (400 MHz, CDCl3): δ 7.26 (d, J = 7.0 Hz, 2H), 7.19 (t, J = 6.1 Hz, 3H), 6.99–6.89 (m, 4H), 6.82 (t, J = 2.4 Hz, 1H), 4.94 (t, J = 2.6 Hz, 1H), 4.18–4.10 (m, 1H), 4.05 (ddd, J = 16.8, 9.6, 6.7 Hz, 2H), 3.56 (ddd, J = 13.0, 9.2, 6.1 Hz, 1H), 3.36 (ddd, J = 13.0, 9.2, 6.0 Hz, 1H), 3.11 (dd, J = 14.0, 7.0 Hz, 1H), 2.97 (dd, J = 14.0, 9.8 Hz, 1H), 1.40–1.31 (m, 2H), 1.10 (t, J = 7.1 Hz, 3H), 0.80 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 198.5, 177.7, 163.5, 144.6, 136.7, 136.0, 130.3, 130.2, 129.0, 128.6, 126.9, 115.3, 115.1, 76.2, 60.8, 60.2, 55.3, 45.9, 37.0, 20.2, 13.9, 11.1; 19F NMR (376 MHz, CDCl3) δ −113.86; HRMS (ESI): m/z calcd for C26H27FNO3S2 [M + H]+: 484.1411, found 484.1408.
Ethyl 9-benzyl-4-oxo-6-phenyl-3-propyl-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (7f). Yellow oil (41.4 mg, 89% yield); 1H NMR (400 MHz, CDCl3): δ 7.25–7.20 (m, 5H), 7.19–7.12 (m, 3H), 6.91 (dd, J = 6.7, 2.8 Hz, 2H), 6.81–6.75 (m, 1H), 4.93 (t, J = 2.6 Hz, 1H), 4.09 (dd, J = 10.8, 7.1 Hz, 1H), 4.05–3.96 (m, 2H), 3.55 (ddd, J = 13.0, 9.2, 6.1 Hz, 1H), 3.44–3.30 (m, 1H), 3.07 (dd, J = 14.0, 7.3 Hz, 1H), 2.94 (dd, J = 13.9, 9.6 Hz, 1H), 1.44–1.30 (m, 2H), 1.04 (t, J = 7.1 Hz, 3H), 0.77 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 199.0, 177.9, 163.8, 144.3, 136.9, 136.3, 129.0, 128.6, 128.5, 128.3, 128.0, 126.8, 76.0, 61.0, 60.7, 55.5, 45.9, 37.2, 20.2, 13.9, 11.1; HRMS (ESI): m/z calcd for C26H28NO3S2 [M + H]+: 466.1505, found 466.1514.
Ethyl 9-benzyl-3-butyl-4-oxo-6-phenyl-2-thioxo-1-thia-3-azaspiro[4.4]non-7-ene-7-carboxylate (7g). Yellow oil (41.7 mg, 87% yield); 1H NMR (400 MHz, CDCl3): δ 7.24 (dd, J = 8.5, 5.0 Hz, 5H), 7.17 (d, J = 6.5 Hz, 3H), 6.92 (dd, J = 6.5, 2.8 Hz, 2H), 6.79 (t, J = 2.3 Hz, 1H), 4.93 (s, 1H), 4.22–4.10 (m, 1H), 4.07–3.94 (m, 2H), 3.60 (ddd, J = 13.3, 9.0, 5.8 Hz, 1H), 3.48–3.38 (m, 1H), 3.07 (dd, J = 14.0, 7.2 Hz, 1H), 2.92 (ddd, J = 15.9, 13.5, 7.8 Hz, 2H), 1.47–1.28 (m, 2H), 1.23–1.14 (m, 2H), 1.05 (t, J = 7.1 Hz, 3H), 0.88 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 198.9, 177.9, 163.8, 144.2, 136.9, 136.3, 129.0, 128.6, 128.5, 128.3, 128.2, 128.0, 126.8, 126.3, 75.8, 61.0, 60.7, 55.4, 44.3, 37.2, 28.8, 19.9, 13.9, 13.6; HRMS (ESI): m/z calcd for C27H30NO3S2 [M + H]+: 480.1662, found 480.1667.
Acknowledgements
We thank the National Natural Science Foundation of China (21072102), the Committee of Science and Technology of Tianjin (15JCYBJC20700) and State Key Laboratory of Elemento-Organic Chemistry in Nankai University for financial support.Notes and references
- For selected reviews on spirocyclic compounds, see: (a) S. Kotha, A. C. Deb, K. Lahiri and E. Manivannan, Synthesis, 2009, 165 CrossRef CAS; (b) R. Pradhan, M. Patra, A. K. Behera, B. K. Mishra and R. K. Behera, Tetrahedron, 2006, 62, 779 CrossRef CAS; (c) M. Sannigrahi, Tetrahedron, 1999, 55, 9007 CrossRef CAS; (d) N. R. Ball-Jones, J. J. Badillo and A. K. Franz, Org. Biomol. Chem., 2012, 10, 5165 RSC; (e) R. Rios, Chem. Soc. Rev., 2012, 41, 1060 RSC; (f) A. K. Franz, N. V. Hanhan and N. R. Ball-Jones, ACS Catal., 2013, 3, 540 CrossRef CAS; (g) Z.-Y. Cao and J. Zhou, Org. Chem. Front., 2015, 2, 849 RSC.
- For recent reviews on the enantioselective synthesis of spirocyclic compounds, see: (a) R. Rios, Chem. Soc. Rev., 2012, 41, 1060 RSC; (b) A. K. Franz, N. V. Hanhan and N. R. Ball-Jones, ACS Catal., 2013, 3, 540 CrossRef CAS; (c) D. Li, L.-Q. Wang, D.-X. Yang, B.-Z. Zhang and R. Wang, ACS Catal., 2015, 5, 7432 CrossRef CAS; (d) S. Bera, B. Chatterjee and D. Mondal, RSC Adv., 2016, 6, 77212 RSC.
- (a) W.-B. Wu, H.-C. Huang, X.-Q. Yuan, K.-L. Zhu and J.-X. Ye, Chem. Commun., 2012, 48, 9180 RSC; (b) P. Y. Géant, M. Urban, M. Remes and I. Císarová, Eur. J. Org. Chem., 2013, 7979 CrossRef; (c) B. Han, W. Huang, W. Ren, G. He, J.-H. Wang and C. Peng, Adv. Synth. Catal., 2015, 357, 561 CrossRef CAS; (d) H. H. Zhang, Z. Q. Zhu, T. Fan, J. Liang and F. Shi, Adv. Synth. Catal., 2016, 358, 1259 CrossRef CAS; (e) C. S. Wang, R. Y. Zhu, J. Zheng, F. Shi and S. J. Tu, J. Org. Chem., 2015, 80, 512 CrossRef CAS PubMed; (f) Y. M. Wang, H. H. Zhang, C. Li, T. Fan and F. Shi, Chem. Commun., 2016, 52, 1804 RSC; (g) K. Q. Chen, Y. Li, C. L. Zhang, D. Q. Sun and S. Ye, Org. Biomol. Chem., 2016, 14, 2007 RSC; (h) S. R. Yetra, S. Mondal, S. Mukherjee, R. G. Gonnade and A. T. Biju, Angew. Chem., Int. Ed., 2016, 55, 268 CrossRef CAS PubMed.
- (a) C. F. Brown, Chem. Rev., 1961, 61, 463 CrossRef; (b) S. P. Singh, S. S. Parmar, K. Raman and V. I. Steinberg, Chem. Rev., 1981, 81, 175 CrossRef CAS; (c) R. B. Lesyk and B. S. Zimenkovsky, Curr. Org. Chem., 2004, 8, 1547 CrossRef CAS; (d) T. Tomašići and L. P. Mašič, Curr. Org. Chem., 2009, 16, 1596 Search PubMed; (e) M. Ono, S. Hayashi, K. Matsumura, H. Kimura, Y. Okamoto, M. Ihara, R. Takahashi, H. Mori and H. Saji, ACS Chem. Neurosci., 2011, 2, 269 CrossRef CAS PubMed; (f) T. Mendgen, C. Steuer and C. D. Klein, J. Med. Chem., 2012, 55, 743 CrossRef CAS PubMed; (g) M. Ono, S. Hayashi, K. Matsumura, H. Kimura, Y. Okamoto, M. Ihara, R. Takahashi, H. Mori and H. Saji, ACS Chem. Neurosci., 2011, 2, 269 CrossRef CAS PubMed.
- K. Zhu, H. Huang, W. Wu, Y. Wei and J. Ye, Chem. Commun., 2013, 49, 2157 RSC.
- R. Murugan, S. Anbazhagan and S. S. Narayanan, Eur. J. Med. Chem., 2009, 44, 3272 CrossRef CAS PubMed.
- For selected reviews, see: (a) X. Lu, C. Zhang and Z. Xu, Acc. Chem. Res., 2001, 34, 535 CrossRef CAS PubMed; (b) J. L. Methot and W. R. Roush, Adv. Synth. Catal., 2004, 346, 1035 CrossRef CAS; (c) X. Lu, Y. Du and C. Lu, Pure Appl. Chem., 2005, 77, 1985 CrossRef CAS; (d) L.-W. Ye, J. Zhou and Y. Tang, Chem. Soc. Rev., 2008, 37, 1140 RSC; (e) B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102 RSC; (f) A. Marinetti and A. Voituriez, Synlett, 2010, 174 CrossRef CAS; (g) S. X. Wang, X. Han, F. Zhong, Y. Wang and Y. Lu, Synlett, 2011, 2766 CAS; (h) F. López and J. L. Mascarenas, Chem.–Eur. J., 2011, 17, 418 CrossRef PubMed; (i) T. L. Wang, X. Y. Han, F. R. Zhong, W. J. Yao and Y. X. Lu, Acc. Chem. Res., 2016, 49, 1369 CrossRef CAS PubMed; (j) Y. C. Fan and O. Kwon, Chem. Commun., 2013, 49, 11588 RSC; (k) Y. Wei and M. Shi, Chem.–Asian J., 2014, 9, 2720 CrossRef CAS PubMed.
- (a) Y.-S. Du, X.-Y. Lu and Y.-H. Yu, J. Org. Chem., 2002, 67, 8901 CrossRef CAS PubMed; (b) S. Kamijo, C. Kanazawa and Y. Yamamoto, J. Am. Chem. Soc., 2005, 127, 9260 CrossRef CAS PubMed; (c) G.-L. Zhao and M. Shi, J. Org. Chem., 2005, 70, 9975 CrossRef CAS PubMed; (d) C. T. Mbofana and S. J. Miller, ACS Catal., 2014, 4, 3671 CrossRef CAS; (e) S. Gabillet, O. Loreau, S. Specklin and E. Rasalofonjatovo, J. Org. Chem., 2014, 79, 9894 CrossRef CAS PubMed; (f) M. Sampath and T. P. Loh, Chem. Sci., 2010, 1, 739 RSC.
- (a) S. Gabillet, D. Lecercle, O. Loreau, M. Carboni, S. Dezard, J. M. Gomis and F. Taran, Org. Lett., 2007, 9, 3925 CrossRef CAS PubMed; (b) R. Manikandan and M. Jeganmohan, Org. Lett., 2014, 16, 652 CrossRef CAS PubMed.
- (a) L.-J. Yang, W. Wang, J. Nie, S. Li and J.-A. Ma, Org. Lett., 2013, 15, 5214 CrossRef CAS PubMed; (b) J. An, L.-Q. Lu, Q.-Q. Yang, T. Wang and W.-J. Xiao, Org. Lett., 2013, 15, 542 CrossRef CAS PubMed; (c) E.-Q. Li and Y. Huang, Chem. Commun., 2014, 50, 948 RSC.
- (a) Y. Jia, X. Tang, G.-W. Cai, R.-X. Jia, B.-R. Wang and Z.-W. Miao, Eur. J. Org. Chem., 2015, 4720 CrossRef CAS; (b) K. D. Ashtekar, X.-L. Ding, E. Toma, H. Gholami, C. Rahn, P. Reed and B. Borhan, Org. Lett., 2016, 18, 3976 CrossRef CAS PubMed.
- (a) C.-B. Yu, W.-P. Zheng, J.-C. Zhan, Y.-C. Sun and Z.-W. Miao, RSC Adv., 2014, 4, 63246 RSC; (b) C.-B. Yu, H.-R. Lyu, Y. Cai, X.-Y. Miao and Z.-W. Miao, RSC Adv., 2013, 3, 18857 RSC; (c) W.-P. Zheng, J.-Y. Zhang, S. Liu, C.-B. Yu and Z.-W. Miao, RSC Adv., 2015, 5, 91108 RSC; (d) G.-W. Cai, S. Liu, J.-Y. Zhang, Y.-Y. Ren, H. Wang and Z.-W. Miao, Synth. Commun., 2016, 46, 793 CrossRef CAS.
- G. Henrion, T. E. J. Chavas, X. Le Goff and F. Gagosz, Angew. Chem., Int. Ed., 2013, 52, 6277 CrossRef CAS PubMed.
- (a) S. Shah and B. Singh, Bioorg. Med. Chem. Lett., 2012, 22, 5388 CrossRef CAS PubMed; (b) A. Urbanaitė, M. Jonušis and R. Bukšnaitienė, Eur. J. Org. Chem., 2015, 7091 CrossRef.
- Crystallographic data for the structural analysis of compound 3l has been deposited at the Cambridge Crystallographic Data Centre as No. CCDC 1494917.†.
- (a) I. Ugi, Pure Appl. Chem., 2001, 73, 187 CrossRef CAS; (b) N. A. M. Yehia, K. Polborn and T. J. J. Muller, Tetrahedron Lett., 2002, 43, 6907 CrossRef CAS; (c) L. Weber, K. Illgen and M. Almstetter, Synlett, 1999, 366 CrossRef CAS; (d) L. Weber, Drug Discovery Today, 2002, 7, 143 CrossRef CAS PubMed; (e) R. V. Orru and M. de Greef, Synthesis, 2003, 1471 CrossRef CAS; (f) N. Hall, Science, 1994, 266, 32 CAS; (g) M. S. Singh and S. Chowdhury, RSC Adv., 2012, 2, 4547 RSC; (h) L.-J. Xing, T. Lu, W.-L. Fu, M.-M. Lou, B. Chen, Z.-S. Wang, Y. Jin, D. Li and B. Wang, Adv. Synth. Catal., 2015, 357, 3076 CrossRef CAS; (i) G.-S. Cheng, X. He, L.-M. Tian, J.-W. Chen, C.-J. Li, X.-S. Jia and J. Li, J. Org. Chem., 2015, 80, 11100 CrossRef CAS PubMed; (j) S. Ahamad, R. Kant and K. Mohanan, Org. Lett., 2016, 18, 280 CrossRef CAS PubMed.
- (a) A. Nefzi, J. M. Ostresh and R. A. Houghten, Chem. Rev., 1997, 97, 449 CrossRef CAS PubMed; (b) L. A. Thompson, Curr. Opin. Chem. Biol., 2000, 4, 324 CrossRef CAS PubMed; (c) A. Dömling, Curr. Opin. Chem. Biol., 2002, 6, 306 CrossRef; (d) Y.-L. Gu, Green Chem., 2012, 14, 2091 RSC; (e) M. Zangouei, A. A. Esmaeili and J. T. Mague, Synlett, 2016, 27, 1669 CrossRef CAS; (f) J. Zhang, W.-J. Yang, J. Sun and C.-G. Yan, Eur. J. Org. Chem., 2015, 7571 CrossRef CAS; (g) M. Rajeswari, S. Kumari and J. M. Khurana, RSC Adv., 2016, 6, 9297 RSC.
- H. Gao and J. Zhang, Chem.–Eur. J., 2012, 18, 2777 CrossRef CAS PubMed.
- (a) S. Gabillet, D. Lecercle, O. Loreau, M. Carboni, S. Dezard, J. M. Gomis and F. Taran, Org. Lett., 2007, 9, 3925 CrossRef CAS PubMed; (b) S. Gabillet, O. Loreau, S. Specklin, E. Rasalofonjatovo and F. Taran, J. Org. Chem., 2014, 79, 9894 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General information, experimental procedures, characterized data and copies of 1H and 13C NMR spectra for products. CCDC 1494917. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra23399f |
| This journal is © The Royal Society of Chemistry 2016 |