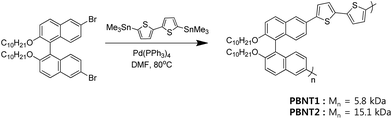
Binaphthyl-incorporated π-conjugated polymer/gold nanoparticle hybrids: a facile size- and shape-tailored synthesis†
Sun Gu Songa,
Seonggyun Haa,
Kyeong-Bae Seob,
Jookyeong Leea,
Tae-Lim Choib,
Thathan Premkumar*ac and
Changsik Song*a
aDepartment of Chemistry, Sungkyunkwan University, Seobu-ro 2066, Jangan-gu, Suwon-si, Gyeonggi-do 16419, Republic of Korea. E-mail: songcs@skku.edu; tprem@skku.edu
bDepartment of Chemistry, Seoul National University, Gwanak-ro 1, Seoul 08826, Republic of Korea
cUniversity College, Sungkyunkwan University, Seobu-ro 2066, Jangan-gu, Suwon-si, Gyeonggi-do 16419, Republic of Korea
First published on 7th November 2016
Abstract
Size- and shape-tailored metal nanoparticles and π-conjugated polymer hybrids are applied in various fields such as surface-enhanced Raman scattering, sensors, heterogeneous catalysis, solar cells, and memory devices. For such applications, it is important to precisely control the morphology of the polymer–metal nanoparticle hybrids. In our study, we utilized binaphthyl-incorporated conjugated polymers to simultaneously reduce auric ions (Au3+) to gold nanoparticles and stabilize them, which resulted in nanoparticle and π-conjugated polymer hybrids. Interestingly, we found that the size and shape of the gold nanoparticles were controlled by the solvents utilized and molecular weights of the binaphthyl-containing π-conjugated polymers. We attributed this to the difference in the coverage of the gold nanoparticle facets by the polymers, which was dictated by the conformation of the polymers and reduction rates in different solvents. In addition, the hybrid materials showed enhanced electrochemical properties compared to the polymers.
Introduction
Over the last few decades, hybridization of π-conjugated polymers or conducting polymers (CPs) and metal nanoparticles has been actively studied for various applications.1–5 The incorporation of metal nanoparticles within the polymers enhances the electronic and photonic properties of the polymers, and influences the catalytic properties of metal nanoparticles. The enhanced electronic structures of polymers help in a better transfer of charges to the catalytic centers.6 CPs with strong electron-donating properties or low oxidation potentials reduce the metal cations by electron transfer to form metal nanoparticles.7 In addition, the metal nanoparticles are stabilized by the CPs, resulting in CP/metal nanoparticle hybrid materials. These hybrid materials exhibit not only interesting properties of the individual components (CP and metal nanoparticles) but also synergistic effects.7 These hybrid nanocomposites have improved chemical and electrical properties over their counterparts. Many researchers have applied them in surface-enhanced Raman scattering,8 sensors,9 heterogeneous catalysis,10 solar cells,11 and memory devices.12Precisely controlled size and shape of the CP/metal nanoparticle hybrids affect their conducting, mechanical, optical, and catalytic properties, which are directly linked to the performance of devices. Thus, many researchers have developed different fabrication methods using appropriate reaction conditions including temperature, pH, ionic strength, surfactants, and solvents for the size and shape control.13 Usually, CP/metal nanoparticle hybrids are synthesized by in situ synthesis or one-pot synthesis. For example, Wan et al. synthesized silver incorporated conductive polypyrrole submicron spheres for supercapacitors.14 They used citrates for stabilizing Ag+ and produced silver citrate micelles that functioned as templates for in situ polymerization. Their method showed improved size distribution of silver nanoparticles. McCullough group synthesized regioregular polythiophene/gold nanoparticle hybrid materials.15 They observed narrow size distribution of gold nanoparticles (AuNPs). However, it is still difficult to precisely control the shape of CP/metal nanoparticle hybrids by in situ or one-pot synthesis, which generally results in spheres.16 Moreover, the aggregation and segregation17 of large, inhomogeneous metal nanoparticles in polymers are difficult to overcome.
Although many CPs have already been utilized in the in situ synthesis of NPs, all the CPs have linear polymer backbones. We previously developed thiophene-based CPs with a binaphthyl moiety that interacted well with nanomaterials such as carbon nanotubes, presumably, due to the tweezer-like structure of the binaphthyls (Scheme 1).18 Thus we anticipated that the ability of well interaction of binaphthyls would help the dispersion of metal nanoparticles (e.g., AuNPs). In addition, bithiophene groups render the polymers sufficiently electron-rich so that they can reduce the auric ions to form gold nanoparticles, which are suitable for in situ synthesis.
Here we developed the size and shape controlled in situ synthesis of CP/AuNPs and observed their enhanced electrochemical properties. We demonstrated that the binaphthyl-containing CPs act not only as reducing agents but also as stabilizing agents in a facile one-pot synthesis of the CP and gold nanoparticle (AuNP) hybrids under ambient conditions. The novelty of this work is to utilize CPs with a tweezer-like backbone, arising from the unique structure of binaphthyls. Unexpectedly we found that during the in situ synthesis, the morphology of AuNPs could be controlled. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) analyses show that the size and shape of the AuNPs are controlled by different hydrodynamic radius of the polymer in different solvents and molecular weight of CPs, indicating that the hydrodynamic radii and reduction rates of the CPs are strongly related to the morphology of AuNPs. We believe that size- and shape-tailored CP/AuNP hybrids might be interesting for a series of applications such as sensing and catalysis.
Experimental section
Materials and characterization
Methods
Results and discussion
Due to the unique features of the dihedral angles in binaphthyl groups, the incorporation of binaphthyl groups in CPs have resulted in interesting properties such as conformation-dependent electroactivity control19 and chiral amine sensing.20 Recently, we showed that binaphthyl-containing CPs selectively disperse carbon nanotubes18 utilizing the tweezer-like structure of the binaphthyl groups. Therefore, we predicted that the binaphthyl moiety might as well disperse nanomaterials other than carbon nanotubes such as inorganic metal nanoparticles. We utilized binaphthyl-incorporated, thiophene-based π-conjugated polymers (PBNTs) to form PBNT/AuNP hybrids. In addition, CPs are known to have reduction potentials appropriate for reducing metal ions.21 Thus, the PBNTs can reduce auric ions to AuNPs (as a reductant) and simultaneously disperse them well in the composites (as a dispersant).PBNTs were synthesized from dibromo-binaphtyl and bis-stannyl-bithiophene via Stille cross coupling reaction as reported.18 In order to observe the effects of the molecular weight of the polymer, we synthesized two polymers: PBNT1 (Mn = 5.8 kDa) and PBNT2 (Mn = 15.1 kDa) by controlling the concentrations of monomers. For the synthesis of PBNT/AuNP hybrid nanocomposites, HAuCl4·3H2O was introduced in a chloroform or toluene solution of PBNT (0.5 mg mL−1) to form AuNPs. The formation of AuNPs was confirmed by TEM, and the AuNPs appear to be stabilized by the oxidized PBNTs, as shown in Fig. 1a. Since we did not utilize any reductant (for example, NaBH4) to form AuNPs, PBNTs might have been the reductant (i.e., they are oxidized during the process). The oxidation of PBNTs was monitored by UV-Vis spectroscopy (Fig. 1b); the initial band-gap (π–π*) transition at around 407 nm was reduced and sub-gap transitions (598 nm and ∼1100 nm, similar to polarons) were developed, which is typical to the oxidation of π-conjugated polymers.22 We observed that the synthesized AuNPs were relatively well dispersed in the solution and wrapped by PBNTs, probably with the help of the tweezer-like structure of the binaphthyl groups.18 The dispersed state of PBNT/AuNP hybrids was maintained only for a few hours, due to returning back to aggregation. However, the shape of AuNPs in the hybrids was preserved for even more than 6 months. It should be highlighted here that our synthesis is a one-step facile method to prepare CP-nanoparticle composites because of the dual role of the PBNTs (reductant and dispersant).
The shape and morphology of the in situ synthesized PBNT/AuNP hybrids could be controlled by the molecular weight of polymers and the type of solvents used. Chloroform dispersions containing PBNT1, which has a lower molecular weight (∼5.8 kDa), had AuNPs of around 33.5 ± 6.1 nm (Fig. 2a). In contrast, PBNT2, which has a higher molecular weight (∼15.1 kDa) gave rise to AuNPs that are approximately five times larger around 157.8 ± 51.0 nm (Fig. 2b). In addition, the shape of AuNPs was slightly different depending on the polymers used; scanning electron microscopy (SEM) images (see ESI, Fig. S1†) showed that the AuNPs prepared using the smaller PBNT1 polymer were mostly sphere-like, while those prepared using the larger PBNT2 polymer were mostly triangular or hexagonal (Fig. S2†).
In toluene, the variation in the morphology of AuNPs was more dramatic (Fig. 2c and d). Instead of sphere-like shapes, the AuNPs synthesized in toluene had branched structures. The smaller PBNT1 polymer formed AuNPs with smaller branches and the whole particles were around 99.4 ± 25.7 nm. On the other hand, the larger PBNT2 polymer produced AuNPs with larger branches and the whole particles were around 246.5 ± 73.0 nm. Thus, the type of the solvent used affects the morphology of nanoparticles more dramatically than the molecular weight of PBNTs. In addition, we observed that the redox reactions (i.e., reduction of Au3+ ions and oxidation of PBNTs) occurred faster in toluene than in chloroform (Fig. S3†). Regarding the reproducibility, we have confirmed that PBNTs with molecular weights of Mn = 4000–7000 g mol−1 produced mostly smaller, sphere-like AuNPs in chloroform, while those with intermediate molecular weights of Mn = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–15
000–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 produced mostly larger, branched shape of AuNPs in toluene.
000 g mol−1 produced mostly larger, branched shape of AuNPs in toluene.
We have tried to measure the surface plasmon resonances of synthesized AuNPs after considerable washing of polymer residues (Fig. 2e and f). Interestingly indeed, we observed what is expected as the surface plasmon bands of AuNPs at around 600 nm for the sphere samples (prepared in chloroform, black lines). In contrast, for the branched samples prepared in toluene (red lines), the peaks >1000 nm were also observed, which can be assigned to transverse resonance in rod-like structures. However, we have to admit that those peaks could be also observed in oxidized thiophene-based polymers (polarons). Some polymers could be adhere so tightly to the surface of AuNPs, resulting in absorption peaks resembling surface plasmonic bands. Thus we cannot completely exclude the possibility of polymer absorptions in the measurements. Even though, it is quite interesting that the absorption peaks >1000 nm were only observed in the samples prepared in toluene, which suggests those peaks would come from transverse longitudinal resonances in branched structures of AuNPs.
We speculate that the nanoparticle formation follows the normal, two-step seeding and growth method.13,23 Since seeding is promoted via the reduction of Au3+ ions by PBNTs, the presence of a larger number of repeat units in the higher-molecular-weight polymers (PBNT2s) leads to a larger number of Au seeds in close proximity, resulting in the bigger Au nanoparticles. The interesting part is the dramatic change in the growth mechanism due to the type of the solvent used. In chloroform, the AuNPs grow according to the step-growth mechanism.23b In toluene, in contrast, they grow by the particle–particle aggregation and fusion, giving rise to branched structures. Our hypothesis is that the variation in the growth mechanism occurs due to CP–AuNP interactions; the PBNTs interact well with the AuNPs in chloroform, covering almost all of the nanoparticle facets. Such interactions seem to be relatively weak in toluene; the PBNTs are loosely bound to the AuNPs, and nanoparticle aggregation and fusion occur via their exposed facets.
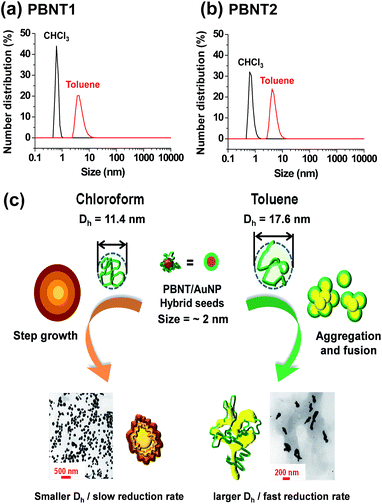
To support our hypothesis, we measured the hydrodynamic diameter (Dh) of PBNTs in chloroform and toluene by dynamic light scattering (DLS). As shown in Fig. 3a and b, PBNTs appear to spread out more in toluene than in chloroform. The Z-average hydrodynamic diameter (Dh) of PBNT2 is 17.6 nm in toluene, while it is 11.4 nm in chloroform. We assume that chloroform makes PBNT2 compact, enabling the polymer to interact strongly with the AuNPs via the nanoparticle facets. In toluene, however, PBNT2 does not cover all the facets of AuNPs, exposing only some of them as fusion sites.
Thus, we propose the growth mechanism of AuNPs generated in situ by PBNTs as follows (Fig. 3c). At first, gold ions (Au3+) are reduced by PBNTs because of the difference in their redox potentials, followed by the absorption of polymers on the gold clusters and further surface reduction of the gold ions happened on the surface. The formed AuNP seeds grow via two pathways. PBNT1 with a small Dh (in chloroform) stabilize the facets of nanoparticles evenly, and almost all of the AuNPs form sphere-like particles via the step-growth mechanism (Fig. 3, left). However, PBNT2 with a large Dh (in toluene) do not cover all the facets of the nanoparticles. Some facets are exposed more and others are exposed less, resulting in an anisotropic growth. Also, the spread polymer provides more room for the small nanoparticles to agglomerate together, forming branch-like particles. Using TEM images obtained in the early stages of the reaction, we confirmed that even in toluene, wherein branch-like structures are generated, very small nanoparticle seeds formed first, which aggregated as the reaction proceeded (see ESI, Fig. S3†). In both pathways, since the polymer itself acts as the reductant, the high molecular weight of polymers provides more “reducing agents” in close proximity and effectively increases the concentration of Au atoms, resulting in larger AuNPs.
The CP/AuNP hybrids synthesized by the in situ method showed enhanced electrochemical properties. Fig. 4 compares the cyclic voltammograms (CVs) of PBNT1 and PBNT1/AuNP hybrids, drop casting on a working electrode. Because of the solubility issue particularly, the films were prepared by mixing PBNT1/AuNP (chloroform or toluene) or PBNT1 (1 mg) and Nafion® 117 solution (100 μL) in isopropyl alcohol (1 mL), drop casting on a glassy carbon electrode (GCE), and dried in air. Cyclic voltammetry was performed in N2-purged acetonitrile solution using 0.1 M TBAPF6 as the supporting electrolyte at room temperature. The potential was referenced to the redox potential of Fc/Fc+ (external standard). We observed the two-electron oxidation of PBNT in all materials, which is typical to polythiophenes (polaronic/bipolaronic states).22 Interestingly, the electroactivity of PBNT1/AuNPs with a sphere-like shape (chloroform), regardless of sphere-like or branch-like structure, the electroactivity of PBNT1 appeared slightly enhanced when compared to that of PBNT1 only. We attributed this enhancement to the inclusion of AuNPs, which may provide a pathway of electric conduction. However, the CV of PBNT1/AuNPs with a branched structure (toluene) was somewhat we did not observe significant differences; the oxidation onset potential of the polymer appeared shifted to higher values. However, it should be noted here that the electroactivity of PBNT1/AuNP was still enhanced and the difference of peak potentials (ΔEp = Epa − Epc) seems decreased (∼0.2 V for branched vs. ∼0.4 V for spheres and polymer only), which suggests the diffusion of counter-ions are slightly faster in PBNT1/AuNP with a branched structure. The reason why the oxidation onset potential of PBNT1/AuNP with a branched structure was unclear presently, but it is important that the electric conductivity of the material was enhanced by inclusion of branched AuNPs.24 These hybrid materials with enhanced electrochemical properties can be applied in solar cells, light-emitting diodes, and electronic memories.
Conclusions
In this study, we successfully synthesized in situ conjugated polymer/gold nanoparticle (CP/AuNP) hybrid nanocomposites, and the size and shape of the AuNPs were easily controlled by varying the CPs based on their molecular weights and the type of the reaction solvent. We utilized binaphthyl-containing conjugated polymers (PBNTs) with different molecular weights, which simultaneously reduced Au3+ to Au(0) and stabilized the formed AuNPs. TEM analysis of the PBNT/AuNP hybrids showed that in chloroform AuNPs grew to form sphere-like structures, while in toluene they grew to form branch-like structures. In addition, PBNT2 with higher molecular weight produced larger AuNPs. Since in toluene PBNT has a relatively larger hydrodynamic radius (measured by DLS) and a faster rate of Au3+ reduction, we proposed the agglomeration and fusion growth mechanism for the fast-generated AuNP seeds in toluene, which resulted in branched structures. In contrast, AuNPs grew stepwise in chloroform due to relatively better stabilization of PBNTs and slow reduction rates and formed sphere-like structures. The synthesized PBNT/AuNP hybrid nanocomposites showed enhanced electrochemical properties, which enable their applications in solar cells, light-emitting diodes, and electronic memories.Acknowledgements
This work was supported by the Small Grant Exploratory Research (SGER) Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (MEST), Republic of Korea (NRF-2015R1D1A1A02062095). In addition, this work was supported by the Nano Material Development Program through NRF.Notes and references
- R. Pacios, R. Marcilla, C. Pozo-Gonzalo, J. A. Pomposo, H. Grande, J. Aizpurua and D. Mecerreyes, Combined Electrochromic and Plasmonic Optical Responses in Conducting Polymer/Metal Nanoparticle Films, J. Nanosci. Nanotechnol., 2007, 7(8), 2938–2941 CrossRef CAS PubMed.
- M. A. G. Namboothiry, T. Zimmerman, F. M. Coldren, J. Liu, K. Kim and D. L. Carroll, Electrochromic properties of conducting polymer metal nanoparticles composites, Synth. Met., 2007, 157(13–15), 580–584 CrossRef CAS.
- H. Yoon, Current Trends in Sensors Based on Conducting Polymer Nanomaterials, Nanomaterials, 2013, 3(3), 524 CrossRef CAS.
- G. Kaur, R. Adhikari, P. Cass, M. Bown and P. Gunatillake, Electrically conductive polymers and composites for biomedical applications, RSC Adv., 2015, 5(47), 37553–37567 RSC.
- V. V. Kondratiev, V. V. Malev and S. N. Eliseeva, Composite electrode materials based on conducting polymers loaded with metal nanostructures, Russ. Chem. Rev., 2016, 85(1), 14 CrossRef CAS.
- G. W. W. Zhan and H. C. Zeng, Charge-Switchable Integrated Nanocatalysts for Substrate-Selective Degradation in Advanced Oxidation Processes, Chem. Mater., 2016, 28(13), 4572–4582 CrossRef CAS.
- R. Gangopadhyay and A. De, Conducting Polymer Nanocomposites: A Brief Overview, Chem. Mater., 2000, 12(3), 608–622 CrossRef CAS.
- Z. Wang and L. J. Rothberg, Structure and Dynamics of Single Conjugated Polymer Chromophores by Surface-Enhanced Raman Spectroscopy, ACS Nano, 2007, 1(4), 299–306 CrossRef CAS PubMed.
- H.-C. Liao, C.-P. Hsu, M.-C. Wu, C.-F. Lu and W.-F. Su, Conjugated Polymer/Nanoparticles Nanocomposites for High Efficient and Real-Time Volatile Organic Compounds Sensors, Anal. Chem., 2013, 85(19), 9305–9311 CrossRef CAS PubMed.
- R. U. Islam, M. J. Witcomb, M. S. Scurrell, E. van der Lingen, W. Van Otterlo and K. Mallick, Conjugated polymer stabilized palladium nanoparticles as a versatile catalyst for Suzuki cross-coupling reactions for both aryl and heteroaryl bromide systems, Catal. Sci. Technol., 2011, 1(2), 308–315 CAS.
- W. J. E. Beek, M. M. Wienk and R. A. J. Janssen, Efficient Hybrid Solar Cells from Zinc Oxide Nanoparticles and a Conjugated Polymer, Adv. Mater., 2004, 16(12), 1009–1013 CrossRef CAS.
- A. Prakash, J. Ouyang, J.-L. Lin and Y. Yang, Polymer memory device based on conjugated polymer and gold nanoparticles, J. Appl. Phys., 2006, 100(5), 054309 CrossRef.
- N. T. K. Thanh, N. Maclean and S. Mahiddine, Mechanisms of Nucleation and Growth of Nanoparticles in Solution, Chem. Rev., 2014, 114(15), 7610–7630 CrossRef CAS PubMed.
- L. Yuan, C. Wan, X. Ye and F. Wu, Facial Synthesis of Silver-Incorporated Conductive Polypyrrole Submicron Spheres for Supercapacitors, Electrochim. Acta, 2016, 213, 115–123 CrossRef CAS.
- L. Zhai and R. D. McCullough, Regioregular polythiophene/gold nanoparticle hybrid materials, J. Mater. Chem., 2004, 14(2), 141–143 RSC.
- P. E. Williams, S. T. Jones, Z. Walsh, E. A. Appel, E. K. Abo-Hamed and O. A. Scherman, Synthesis of Conducting Polymer–Metal Nanoparticle Hybrids Exploiting RAFT Polymerization, ACS Macro Lett., 2015, 4(2), 255–259 CrossRef CAS.
- Y.-H. Lee, Y.-P. Lee, C.-J. Chiang, F.-K. Wei, C.-H. Wu, W.-C. Chen, C. Shen, H.-A. Jeng, L. Wang, M.-W. Liu, Y.-F. Chen, T. Yokozawa and C.-A. Dai, A new strategy for co-assembling [small pi]-conjugated polymer/cadmium sulfide hybrids into an efficient charge-transporting nanochannel array by using an all-conjugated diblock copolymer motif, J. Mater. Chem. A, 2014, 2(35), 14600–14612 CAS.
- S. H. Min, H.-I. Kim, K.-s. Kim, I. Cha, S. Ha, W. S. Yun, S. K. Kwak, J.-H. Kim, B.-S. Kim and C. Song, Selective dispersion of single-walled carbon nanotubes by binaphthyl-based conjugated polymers: Integrated experimental and simulation approach, Polymer, 2016, 96, 63–69 CrossRef CAS.
- D.-C. Jeong, H. Lee, K. S. Yang and C. Song, Effects of Substituent on Binaphthyl Hinge-Containing Conductive Polymers, Macromolecules, 2012, 45(24), 9571–9578 CrossRef CAS.
- S. Kang, I. Cha, J. G. Han and C. Song, Electroactive polymer sensors for chiral amines based on optically active 1,1-binaphthyls, Mater. Express, 2013, 3(2), 119–126 CrossRef CAS.
- K. Naka and Y. Chujo, Nanohybridized Synthesis of Metal Nanoparticles and Their Organization, Nanohybridization of Organic–Inorganic Materials, ed. A. Muramatsu and T. Miyashita, Springer, Berlin, Heidelberg, 2009; pp. 3–40 Search PubMed.
- S. Cook, A. Furube and R. Katoh, Analysis of the excited states of regioregular polythiophene P3HT, Energy Environ. Sci., 2008, 1(2), 294–299 CAS.
- (a) P. Dey, I. Blakey, K. J. Thurecht and P. M. Fredericks, Self-Assembled Hyperbranched Polymer–Gold Nanoparticle Hybrids: Understanding the Effect of Polymer Coverage on Assembly Size and SERS Performance, Langmuir, 2013, 29(2), 525–533 CrossRef CAS PubMed; (b) T. Sakai and P. Alexandridis, Mechanism of Gold Metal Ion Reduction, Nanoparticle Growth and Size Control in Aqueous Amphiphilic Block Copolymer Solutions at Ambient Conditions, J. Phys. Chem. B, 2005, 109(16), 7766–7777 CrossRef CAS PubMed.
- G. Zotti, B. Vercelli and A. Berlin, Gold Nanoparticles Linked by Pyrrole- and Thiophene-Based Thiols. Electrochemical, Optical, and Conductive Properties, Chem. Mater., 2008, 20(2), 397–412 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images, time dependent TEM images of PBNT/AuNP hybrid materials and DLS spectra of PBNTs. See DOI: 10.1039/c6ra22234j |
| This journal is © The Royal Society of Chemistry 2016 |