Improved performances of a LiNi0.6Co0.15Mn0.25O2 cathode material with full concentration-gradient for lithium ion batteries†
Abstract
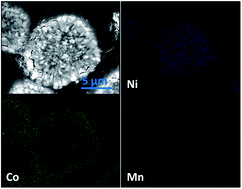
A novel high capacity cathode material with a full concentration-gradient (FCG) structure has been successfully synthesized by a modified hydroxide co-precipitation method. A continuous concentration change of Ni, Co, and Mn from the core LiNi0.8Co0.1Mn0.1O2 to the shell LiNi0.4Co0.2Mn0.4O2 in each particle with an average composition of LiNi0.6Co0.15Mn0.25O2 is confirmed by EDX and ICP, respectively. Charge–discharge tests demonstrate that the FCG cathode delivers a high specific capacity of 190 mA h g−1 at a current density of 0.05 mA cm−2, exceptional rate capacity (a high capacity of 125 mA h g−1 at an ultrafast rate of 10C-rate), and an ultralong cycle life up to 1000 cycles (capacity retention of 80% at 5C-rate), which is obviously superior to the conventional cathode LiNi0.6Co0.15Mn0.25O2. Meanwhile, the Mn-rich in the outer layer of the FCG cathode contributes to more excellent thermal stability than the conventional cathode LiNi0.6Co0.15Mn0.25O2.

 Please wait while we load your content...
Please wait while we load your content...