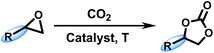Construction of solvent-dependent self-assembled porous Ni(II)-coordinated frameworks as effective catalysts for chemical transformation of CO2†
Zhen Zhoua,
Lu Yanga,
Yefei Wanga,
Cheng He*a,
Tao Liua and
Chunying Duanab
aState Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, P. R. China 116024. E-mail: hecheng@dlut.edu.cn
bCollaborative Innovation Center of Chemical Science and Engineering, Tianjin, P. R. China 300071
First published on 26th October 2016
Abstract
Coordinated frameworks having large pores can accommodate organic molecules and adsorb gas, such as waste carbon dioxide, to transform into valuable chemical products. By incorporating a tetraphenylethylene moiety as the four-point connected node, herein, two types of Ni(II)-based coordinated frameworks have been solvothermally synthesized via solvent driven self-assembly and structurally characterized. Notably, these two complexes both contain a trinuclear cluster but with different styles and construction modes. The IR and XRD spectra suggested that the two compounds are highly stable in various solvents, including water, DMF, C2H5OH, C2H2Cl2, DMSO and 1,4-dioxane. Catalysis experiments indicated that both of them could act as effective heterogeneous catalysts for cycloaddition of CO2 with epoxides, forming cyclic carbonates under ambient conditions. Notably, the two catalysts contained the same amount of catalytic sites, but the activity of Ni-1 was significantly lower than that of Ni-2, demonstrating a size-selective catalytic performance.
Introduction
From the viewpoint of crystal engineering, the design and self-assembly of versatile coordination frameworks has attracted a great deal of attention over the past two decades.1–3 Considered to be promising analogues of porous materials, the construction of metal–organic frameworks (MOFs) by various organic linkers and metal ions or clusters has been of special interest because of their intriguing structural diversity and outstanding physical and chemical properties that can be used in numerous fields.4–6 Carboxylate ligands represent one of the most popular ligand classes in the assembly of MOFs, mainly due to their excellent stability and diverse bridging modes, which can lead to abundant topological architectures with different size and shape of pores.7–9 However, the self-assembly procedure remains inscrutable, and it is difficult to accurately tailor their structures. As a consequence, the fabrication of carboxylate-based MOFs requires relatively extreme synthesis conditions for the strict control of the resulting structures and there still remains large room to explore.10–12As we know, porous MOF materials exhibit remarkable properties for gas storage and adsorption, which make them useful for solving the environmental problem of increasing CO2 emissions.13–15 In terms of catalysis and green chemistry, gaseous waste CO2 is an ideal and abundant C1 source due to its characteristics of safety, non-toxicity, renewability and ready availability;16–18 thus, the utilization and transformation of CO2 into valuable and usable chemical products show attractive prospects for the satisfaction of atom economy.19–21 In this respect, the cycloaddition of carbon dioxide with epoxides to produce cyclic carbonates is quite promising, as the latter compounds are widely used in industry and the incorporation of CO2 to fabricate these chemicals does not result in any side products.22–24 More efficient conversion under mild conditions from CO2 to cyclic carbonates is an ongoing challenge, although this conversion has been widely studied.25–28
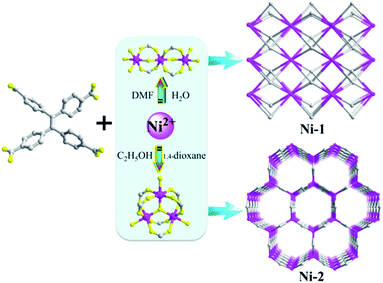
MOF materials, incorporating active metal sites and designable ligands, display the combined advantages of homogeneous and heterogeneous catalyst; for example, high activity and selectivity, as well as recovery and high controllability.29–31 From a catalytic perspective, MOF catalysts based on permanently porous or channel frameworks are able to utilize minimum amount of metal ions and organic ligands to build maximum surface areas with predictable, controllable, tailorable and post modifiable pores and cavities.32–34 We herein report the synthesis and catalytic properties of two Ni(II)-based porous frameworks for the cycloaddition of CO2 by the incorporation of a tetrakis(4-carboxyphenyl)ethylene (H4TCPE) moiety as the four-point connected node (Scheme 1). These two compounds have been solvothermally synthesized and exhibit diverse structures via solvent driven self-assembly. We envisioned that the partially twisted ethyl core and multiple rotational phenyl rings of the H4TCPE moiety, together with its diverse coordination modes, would cause different MOF configurations. Both the two structures contain a trinuclear cluster to provide an identical amount of open metal sites during catalysis.
 | ||
| Scheme 1 Presentation of the H4TCPE ligand and the assembly of Ni(II) ions in different solvents, resulting in isolation of two types of trinuclear clusters and construction frameworks. | ||
Experimental section
Materials and methods
All chemicals were of reagent grade, were obtained from commercial sources, and were used without further purification. All epoxides were purchased from Acros and distilled under a nitrogen atmosphere from CaH2 prior to use. Carbon dioxide (99.995%) was purchased from Dalian Institute of Special Gases and used as received. The elemental analyses of C, H and N were performed on a Vario EL III elemental analyzer. Inductively coupled plasma (ICP) analysis was performed on a Jarrel-AshJ-A1100 spectrometer. The powder XRD diffractograms were obtained on a Rigaku D/MAX-2400 X-ray diffractometer with Cu sealed tube (λ = 1.54178 Å). IR spectra were obtained as KBr pellets on a NEXUS instrument. Thermogravimetric analyses (TGA) were carried out at a ramp rate of 10 °C min−1 in a nitrogen flow with a SDTQ600 instrument. Gas sorption isotherms were measured using an Autosorb-IQ-C analyzer of Quantachrome. The N2 and CO2 adsorption isotherms for desolvated compounds were collected in a relative pressure range from 10 to 1.0 × 105 Pa. 1H NMR spectra were obtained on a Varian INOVA-400 MHz type spectrometer. Their peak frequencies were referenced versus an internal standard (TMS) at 0 ppm for 1H NMR.Preparation
All reagents were used as purchased without further purification. Tetrakis(4-carboxyphenyl)ethylene (H4TCPE) was prepared according to the literature methods35 and was characterized by 1H NMR.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) in a screw-capped vial. The resulting mixture was kept in an oven at 100 °C for 3 days. After cooling the autoclave to room temperature, green block single crystals were separated, washed with water and air-dried. Yield: 60% (based on H4TCPE). Anal. calcd for C15H8Ni0.75O6.75: C 52.95, H 2.37, Ni 12.94%. Found: C 52.35, H 2.03, Ni 12.61%. IR (KBr): 3450 (br, s), 1638 (s), 1542 (m), 1403 (s), 1187 (m), 1108 (w), 1053 (m), 777 (m) cm−1.
1, v/v) in a screw-capped vial. The resulting mixture was kept in an oven at 100 °C for 3 days. After cooling the autoclave to room temperature, green block single crystals were separated, washed with water and air-dried. Yield: 60% (based on H4TCPE). Anal. calcd for C15H8Ni0.75O6.75: C 52.95, H 2.37, Ni 12.94%. Found: C 52.35, H 2.03, Ni 12.61%. IR (KBr): 3450 (br, s), 1638 (s), 1542 (m), 1403 (s), 1187 (m), 1108 (w), 1053 (m), 777 (m) cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Yield: 32% (based on H4TCPE). Anal. calcd for C3.75H2.25Ni0.25O1.34: C 53.99, H 2.72, Ni 17.59%. Found: C 54.06, H 2.49, Ni 17.32%. IR (KBr): 3434 (br, s), 1605 (s), 1547 (w), 1404 (vs), 1204 (s), 1106 (w), 1018 (w), 789 (m), 771 (m) cm−1.
1, v/v). Yield: 32% (based on H4TCPE). Anal. calcd for C3.75H2.25Ni0.25O1.34: C 53.99, H 2.72, Ni 17.59%. Found: C 54.06, H 2.49, Ni 17.32%. IR (KBr): 3434 (br, s), 1605 (s), 1547 (w), 1404 (vs), 1204 (s), 1106 (w), 1018 (w), 789 (m), 771 (m) cm−1.Crystallography
X-Ray intensity data were measured on a Bruker SMART APEX CCD-based diffractometer (Mo-Kα radiation, λ = 0.71073 Å) using the SMART and SAINT programs.36,37 The crystal data were solved by direct methods and further refined by full-matrix least-squares refinements on F2 using the SHELXL-97 software and an absorption correction was performed using the SADABS program.38 The remaining atoms were found from successive full-matrix least-squares refinements on F2 and Fourier syntheses. Non-H atoms were refined with anisotropic displacement parameters. The hydrogen atoms within the ligand backbones were fixed geometrically at calculated distances and allowed to ride on the parent non-hydrogen atoms. No satisfactory disorder model could be achieved, and therefore the SQUEEZE program implemented in PLATON was used to remove these electron densities.39 Crystallographic data for Ni-1 and Ni-2 are summarized in Table S1.†Catalysis details
Before the catalysis experiments, solvent exchange experiments were carried out to activate the catalysts by removing the coordinated water molecules and solvent. The crystal samples were immersed in ethanol for 2 days, and then fully dried out at 100 °C under high vacuum for 6 h to obtain the activated samples. TGA was used to demonstrate the activation process. The mol number of the catalysts (30 μmol) was calculated based on the Ni sites in each catalyst after being activated. For catalyst Ni-1 (18.2 mg, 60 μmol), the molecular weight of the asymmetric unit after removal of solvent and coordinated water (C15H8Ni0.75O4.5) was 304.24 g mol−1, with 0.5 catalytically active Ni ions, equivalent to 30 μmol. For catalyst Ni-2 (9.5 mg, 120 μmol), the molecular weight of the asymmetric unit after removal of solvent and coordinated water (C3.75H2Ni0.25O1.083) was 79.05 g mol−1, with 0.25 catalytically active Ni ions, equivalent to 30 μmol.In a typical catalytic reaction, a 10 mL Schlenk tube was purged with 1 atm CO2 using a balloon under solvent free environment. The vessel was set in an oil bath with frequent stirring at a temperature of 313 K for 40 h. At the end of the reaction, the reactor was placed in an ice bath for 20 min and then opened. The catalysts were separated by centrifugation. Then, 1,3,5-trimethylbenzene (120 mg, 1 mmol) was added as an internal standard for the analysis of the product yields by 1H NMR. The catalysts were collected after each cycle, washed with CH2Cl2 and dried by vacuum. The remaining mixture was degassed and fractionally distilled under reduced pressure or purified by column chromatography on silica gel to obtain cyclic carbonate.
Results and discussion
Synthetic effects
The solvothermal reaction of Ni(NO3)2·6H2O and H4TCPE at 100 °C for 3 days produced stick-shaped crystals Ni-1 in the mixture of DMF and H2O (v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and block-shaped crystals Ni-2 in the mixture of DMF and CH3CN (v/v = 2
1), and block-shaped crystals Ni-2 in the mixture of DMF and CH3CN (v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The only difference in the synthesis of the isolated crystals was the mixed solvents. As we know, solvent plays an essential role in the coordination capability because of its steric effect, polarity and solubility, leading to the synthesis of supramolecular polymers with diverse connectivity and architectures. Changing the reaction temperature or ratio of raw materials cannot obtain our target compounds, and the accurate modulation of solvent is the key factor to influence the formation of Ni-1 and Ni-2. Elemental and powder X-ray diffraction (XRD) analyses indicate that the bulk samples consist of a pure, single phase.
1). The only difference in the synthesis of the isolated crystals was the mixed solvents. As we know, solvent plays an essential role in the coordination capability because of its steric effect, polarity and solubility, leading to the synthesis of supramolecular polymers with diverse connectivity and architectures. Changing the reaction temperature or ratio of raw materials cannot obtain our target compounds, and the accurate modulation of solvent is the key factor to influence the formation of Ni-1 and Ni-2. Elemental and powder X-ray diffraction (XRD) analyses indicate that the bulk samples consist of a pure, single phase.
 | ||
| Fig. 2 (a) The asymmetric unit of Ni-2; (b) the trinuclear Ni3 cluster in Ni-2; (c) perspective view of the three-dimensional framework along the c axis; (d) the schematic of Ni-2 network as tiling. | ||
| Entry | Substrate | Product | Yieldb (%) | |
|---|---|---|---|---|
| Ni-1 | Ni-2 | |||
| a Reaction conditions: epoxide (10 mmol), catalyst (30 μmol, based on the active Ni sites), and TBABr (0.5 mmol) under carbon dioxide (1 atm), 313 K and 40 h.b The yields were determined by 1H NMR analysis using 1,3,5-trimethylbenzene as internal standard for integration.c 1f (1 mmol) with 2 mL CDCl3 as solvent. | ||||
| 1 | 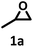 |
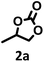 |
98.4 | 99.0 |
| 2 | 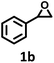 |
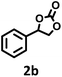 |
67.8 | 69.5 |
| 3 | 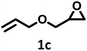 |
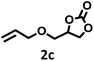 |
96.9 | 97.8 |
| 4 | 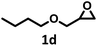 |
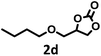 |
88.9 | 89.6 |
| 5 | 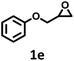 |
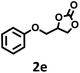 |
83.7 | 85.5 |
| 6c |  |
 |
— | 4.9 |
A tentative mechanism based on these observations and previous reports for the cycloaddition of epoxide and CO2 in the presence of Ni-MOFs co-catalyzed by TBABr is illustrated (Scheme S1†).47–49 After the coordinating water molecules are removed, the Ni active sites in the pores might be activated and can serve as Lewis acid catalytic sites to activate the epoxy ring through the oxygen atom. Then, Br− generated from TBABr attacks the less-hindered methylene carbon atom of the activated epoxide to open the epoxy ring. Concomitantly, the activated ring-opening intermediate reacts with CO2 to form an alkylcarbonate anion. Finally, ring-closure through an intramolecular nucleophilic attack by the oxyanion at the carbon center of CO2 occurs to generate a cyclic carbonate and regenerate the catalyst.
Conclusions
In conclusion, two types of Ni(II)-based coordination frameworks have been solvothermally synthesized via the elaborate regulation of solvent driven self-assembly, incorporating the tetraphenylethylene moiety as the backbone. These two complexes both contain a trinuclear metal cluster, but with different styles and construction modes, probably due to the different steric effects or polarity of the different solvent systems toward the rotation of the carboxylic ligand. IR and XRD spectra demonstrate the significant stabilities of the two frameworks toward various solvents. Importantly, both of them could act as effective heterogeneous catalysts for cycloaddition of CO2 with epoxides forming cyclic carbonates under ambient conditions and provide appropriately sized pores to encapsulate the reaction substrates. The size-selectivity of the substrates suggested that the catalytic processes occurred within the pores of the catalysts. This study suggests a new approach for the solvent driven construction of different porous frameworks for use as efficient heterogeneous catalysts.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (21531001 and 21421005).Notes and references
- A. G. Slater and A. I. Cooper, Science, 2015, 348, aaa8075 CrossRef PubMed.
- J. P. Zhang, Y. B. Zhang, J. B. Lin and X. M. Chen, Chem. Rev., 2012, 112, 1001–1033 CrossRef CAS PubMed.
- J. P. Sculley and H. C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 12660–12661 CrossRef CAS PubMed.
- H. Furukawa, U. Müllerv and O. M. Yaghi, Angew. Chem., Int. Ed., 2015, 54, 3417–3430 CrossRef CAS PubMed.
- Y. B. He, W. Zhou, G. D. Qian and B. L. Chen, Chem. Soc. Rev., 2014, 43, 5657–5678 RSC.
- J. W. Liu, L. F. Chen, H. Cui, J. Y. Zhang, L. Zhang and C. Y. Su, Chem. Soc. Rev., 2014, 43, 6011–6061 RSC.
- M. Du, C. P. Li, C. S. Liu and S. M. Fang, Coord. Chem. Rev., 2013, 257, 1282–1305 CrossRef CAS.
- Z. Chang, D. H. Yang, J. Xu, T. L. Hu and X. H. Bu, Adv. Mater., 2015, 27, 5432–5441 CrossRef CAS PubMed.
- J. S. Qin, D. Y. Du, M. Li, X. Z. Lian, L. Z. Dong, M. Bosch, Z. M. Su, Q. Zhang, S. L. Li, Y. Q. Lan, S. Yuan and H. C. Zhou, J. Am. Chem. Soc., 2016, 138, 5299–5307 CrossRef CAS PubMed.
- J. Lü, J. X. Lin, M. N. Cao and R. Cao, Coord. Chem. Rev., 2013, 257, 1334–1356 CrossRef.
- M. Li, D. Li, M. O. Keeffe and O. M. Yaghi, Chem. Rev., 2014, 114, 1343–1370 CrossRef CAS PubMed.
- K. Shen, M. D. Zhang and H. G. Zheng, CrystEngComm, 2015, 17, 981–991 RSC.
- G. Sneddon, A. Greenaway and H. H. P. Yiu, Adv. Energy Mater., 2014, 4, 1301873 CrossRef.
- Y. G. Zhang and D. S. W. Lim, ChemSusChem, 2015, 8, 2606–2608 CrossRef CAS PubMed.
- Q. Wang, J. F. Bai, Z. Y. Lu, Y. Pan and X. Z. You, Chem. Commun., 2016, 52, 443–452 RSC.
- K. Huang, C. L. Sun and Z. J. Shi, Chem. Soc. Rev., 2011, 40, 2435–2452 RSC.
- S. Pulla, C. M. Felton, P. Ramidi, Y. Gartia, N. Ali, U. B. Nasini and A. Ghosh, J. CO2 Util., 2013, 2, 49–57 CrossRef CAS.
- P. Dürre and B. J. Eikmanns, Curr. Opin. Biotechnol., 2015, 35, 63–72 CrossRef PubMed.
- J. Bayardon, J. Holz, B. Schäffner, V. Andrushko, S. Verevkin, A. Preetz and A. Börner, Angew. Chem., Int. Ed., 2007, 46, 5971–5974 CrossRef CAS PubMed.
- M. Aresta, A. Dibenedetto and A. Angelini, Chem. Rev., 2014, 114, 1709–1742 CrossRef CAS PubMed.
- P. Markewitz, W. Kuckshinrichs, W. Leitner, J. Linssen, P. Zapp, R. Bongartz, A. Schreiber and T. E. Muller, Energy Environ. Sci., 2012, 5, 7281–7305 CAS.
- M. North and R. Pasquale, Angew. Chem., Int. Ed., 2009, 48, 2946–2948 CrossRef CAS PubMed.
- C. Martín, G. Fiorani and A. W. Kleij, ACS Catal., 2015, 5, 1353–1370 CrossRef.
- X. B. Lu and D. J. Darensbourg, Chem. Soc. Rev., 2012, 41, 1462–1484 RSC.
- Y. Xie, T. T. Wang, X. H. Liu, K. Zou and W. Q. Deng, Nat. Commun., 2013, 4, 1960 Search PubMed.
- W. Y. Gao, Y. Chen, Y. H. Niu, K. Williams, L. Cash, P. J. Perez, L. Wojtas, J. F. Cai, Y. S. Chen and S. Q. Ma, Angew. Chem., Int. Ed., 2014, 53, 2615–2619 CrossRef CAS PubMed.
- J. Zheng, M. Y. Wu, F. L. Jiang, W. P. Su and M. C. Hong, Chem. Sci., 2015, 6, 3466–3470 RSC.
- R. Babu, A. C. Kathalikkattil, R. Roshan, J. Tharun, D. W. Kim and D. W. Park, Green Chem., 2016, 18, 232–242 RSC.
- M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196–1231 CrossRef CAS PubMed.
- Y. Liu, W. M. Xuan and Y. Cui, Adv. Mater., 2010, 22, 4112–4135 CrossRef CAS PubMed.
- J. Y. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- J. Dong, P. Cui, P. F. Shi, P. Cheng and B. Zhao, J. Am. Chem. Soc., 2015, 137, 15988–15991 CrossRef CAS PubMed.
- H. Q. Xu, J. H. Hu, D. K. Wang, Z. H. Li, Q. Zhang, Y. Luo, S. H. Yu and H. L. Jiang, J. Am. Chem. Soc., 2015, 137, 13440–13443 CrossRef CAS PubMed.
- T. Zhang and W. B. Lin, Chem. Soc. Rev., 2014, 43, 5982–5993 RSC.
- N. B. Shustova, B. D. McCarthy and M. Dincă, J. Am. Chem. Soc., 2011, 133, 20126–20129 CrossRef CAS PubMed.
- SMART Data collection software (version 5.629), Bruker AXS Inc., Madison, WI, 2003 Search PubMed.
- SAINT, Data reduction software (version 6.45), Bruker AXS Inc., Madison, WI, 2003 Search PubMed.
- G. M. Sheldrick, SHELXTL97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS.
- Z. Zhou, C. He, J. H. Xiu, L. Yang and C. Y. Duan, J. Am. Chem. Soc., 2015, 137, 15066–15069 CrossRef CAS PubMed.
- X. Q. Wang, L. L. Zhang, J. Yang, F. L. Liu, F. N. Dai, R. M. Wang and D. F. Sun, J. Mater. Chem. A, 2015, 3, 12777–12785 CAS.
- S. N. Wang, T. T. Cao, H. Yan, Y. W. Li, J. Lu, R. R. Ma, D. C. Li, J. M. Dou and J. F. Bai, Inorg. Chem., 2016, 55, 5139–5151 CrossRef CAS PubMed.
- A. C. Kathalikkattil, D. W. Kim, J. Tharun, H. G. Soek, R. Roshan and D. W. Park, Green Chem., 2014, 16, 1607–1616 RSC.
- P. Z. Li, X. J. Wang, J. Liu, J. S. Lim, R. Q. Zou and Y. L. Zhao, J. Am. Chem. Soc., 2016, 138, 2142–2145 CrossRef CAS PubMed.
- W. Y. Gao, C. Y. Tsai, L. Wojtas, T. Thiounn, C. C. Lin and S. Q Ma, Inorg. Chem., 2016, 55, 7291–7294 CrossRef CAS PubMed.
- Z. H. Xu, L. L. Han, G. L. Zhuang, J. Bai and D. Sun, Inorg. Chem., 2015, 54, 4737–4743 CrossRef CAS PubMed.
- L. Liu, S. M. Wang, Z. B. Han, M. L. Ding, D. Q. Yuan and H. L. Jiang, Inorg. Chem., 2016, 55, 3558–3565 CrossRef CAS PubMed.
- Y. H. Han, Z. Y. Zhou, C. B. Tian and S. W. Du, Green Chem., 2016, 18, 4086–4091 RSC.
- M. H. Beyzavi, R. C. Klet, S. Tussupbayev, J. Borycz, N. A. Ver-meulen, C. J. Cramer, J. F. Stoddart, J. T. Hupp and O. K. Farha, J. Am. Chem. Soc., 2014, 136, 15861–15864 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and catalysis details, characterization data for the new compounds. CCDC 1502000 and 1502001. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra22971a |
| This journal is © The Royal Society of Chemistry 2016 |