A novel pyrazole based single molecular probe for multi-analyte (Zn2+ and Mg2+) detection in human gastric adenocarcinoma cells†
Abstract
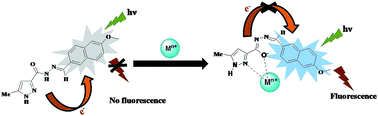
A new fluorescent sensor, 5-methyl-1H-pyrazole-3-carboxylic acid (6-methoxy-naphthalen-2-ylmethylene)-hydrazide (PYN), composed of a naphthalene group as the fluorogenic unit and a pyrazole carbohydrazide as the binding unit for metal ions has been designed and synthesized. The sensor shows excellent selectivity and sensitivity with a fluorescence enhancement towards Zn2+ and Mg2+ over other cations in aqueous acetonitrile solution. Turn-on fluorescent enhancements (FE) as high as ∼49 fold and ∼41 fold in mixed media for Zn2+ and, Mg2+ respectively were noticed. The signal enhancement of the sensor is based on chelation-enhanced fluorescence (CHEF) effect of PYN-Zn2+/Mg2+ with the inhibition of the photoinduced electron transfer (PET) effect. Moreover, the Job's plot established 1 : 1 stoichiometry of the complex formation between PYN and Zn2+ or Mg2+ ions. The limit of detection for Zn2+ and Mg2+ is as low as 2.2 × 10−7 M and 3.9 × 10−7 M respectively. PYN exhibited a second mode of selectivity for Zn2+ as it displaces Mg2+ from the PYN-Mg2+ complex. Density Functional Theory (DFT) calculations have been performed in order to show the structure and electronic properties of PYN and its complexes [PYN-Zn2+/Mg2+]. Cell imaging experiments confirmed that PYN can be used for monitoring intracellular Zn2+ and Mg2+ levels in living cells in vitro.

 Please wait while we load your content...
Please wait while we load your content...