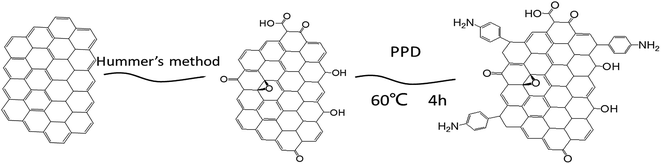
A sulfonated poly(ether ether ketone)/amine-functionalized graphene oxide hybrid membrane for vanadium redox flow batteries
Lingqian Kong,
Lanyue Zheng,
Ruiting Niu,
Haixia Wang* and
Haifeng Shi*
State Key Lab of Separation Membranes and Membrane Processes, Tianjin Key Laboratory of Advanced Fibers and Energy Storage, Institute of Functional Fiber, School of Materials Science and Engineering, Tianjin Polytechnic University, Tianjin 300387, China. E-mail: hxwang@tjpu.edu.cn; haifeng.shi@gmail.com
First published on 17th October 2016
Abstract
A series of hybrid membranes (SPEEK/PPD-GO) based on sulfonated poly(ether ether ketone) (SPEEK) and p-phenylene diamine-functionalized graphene oxide (PPD-GO) are prepared by a solution-casting method for vanadium redox flow batteries (VRB). The physicochemical properties such as water uptake, swelling ratio, ion exchange capacity, proton conductivity and vanadium ion permeability of SPEEK hybrid membranes are well controlled by the incorporated PPD-GO nanofillers. SPEEK/PPD-GO hybrid membranes show higher coulombic efficiency (CE, 96.9%) and energy efficiency (EE, 82.8%) at 30 mA cm−2 than Nafion 117 membrane (CE, 89% and EE, 72.5%). The self-discharge time of the SPEEK/PPD-GO-1 hybrid membrane (56 h) is longer than that of the Nafion 117 membrane (26 h), demonstrating its excellent vanadium ion selectivity. Moreover, the SPEEK/PPD-GO membrane exhibits a stable operation performance for up to 100 cycles, accompanied with no significant decay in CE and EE. The present experimental results demonstrate that it is a feasible method by controlling the interfacial interaction to realize a high performance hybrid membrane for VRB systems.
1. Introduction
The vanadium redox flow battery (VRB) has attracted lots of interest due to its high energy efficiency, deep discharge capability and flexible operation.1–4 As the key component, the structure–property of the proton exchange membrane (PEM) influences the commercialization perspective of VRB.5,6 The reported ion exchange membrane for VRB, the Nafion membrane, has a high proton conductivity and structure stability; however, its low ion selectivity and high cost limits further application.7–13 So, developing PEM with a high structure–property and low cost is necessary for pushing the commercialization process of VRB systems.14–18Recently, sulfonated aromatic polymer membranes and their hybrid composites, such as sulfonated poly(ether ether ketone) (SPEEK),19–24 sulfonated poly(fluorenyl ether ketone),25,26 and sulfonated poly(arylene ether sulfone),27 exhibit a potential VRB application because of their low cost, good ionic conductivity and excellent mechanical properties. For aromatic polymer membranes, i.e. SPEEK membranes, the VRB performance is highly dependent upon their sulfonated degrees (DS).19 However, SPEEK membranes with high DS lead to high water uptake and vanadium ion permeability, and thus decrease the mechanical property and thermal stability. Typically, Vehicle mechanism and Grotthuss mechanism are highly recognized for the proton transfer behavior inside the bulk membrane.28,29 To improve the proton conductivity of membrane, the chemical modifications and the post-functionalization technique are usually applied. Although introducing a certain amount of acidic groups, i.e. SO3H groups along the polymeric backbone may enhance the Vehicle transport,30,31 the hydrated water will decrease the dimensional stability of membrane. Thus, incorporation of the functional nanofillers offering the acid–base action or the hydrogen bonding formation is another choice, and thus it jointly provides the Grotthuss transport and the Vehicle transport.32–34 Dai et al.14 reported the single VRB performance of composite membrane based on SPEEK/graphene, indicating that SPEEK/graphene membrane shows a lower self-discharge rate, and a higher CE, EE and VE, compared with Nafion 117. Heo et al.23 analyzed the effect of the sulfonated graphene oxide (SGO) on the property of SPEEK membrane, and they found the incorporated sulfonic groups (SO3H) significantly increases the proton conductivity of SGO/SPEEK membrane. Similarly, our studies also demonstrated the functionalized GO nanofillers are beneficial to enhance the balance of hybrid membrane. For sulfonated polyimides (SPI) hybrid membranes containing amine-functionalized rGO, they present a good electrochemical energy storage efficiency (CE: 93% and EE: 78% at 40 mA cm−2), indicating the synergistic effect of the sulfonated and amine groups plays an active contribution to conduct the stable proton conductive pathways.35 The followed SPI/zwitterionic polymer-decorated GO hybrid membranes further show a much lower vanadium ion permeability value (7.4–26.3 × 10−7 cm2 min−1), compared with Nafion 117 (67.2 × 10 −7 cm2 min−1), due to the interaction between SPI and amine-functionalized GO.36 Therefore, incorporation of the functionalized nanofillers enhancing the interfacial interaction, and then promoting the formation of proton transfer channel is proved an effective way to improve the membrane structure–property-performance for VRB application.
Therein, in this work, we report a novel SPEEK/PPD-GO hybrid membrane, in which p-phenylene diamine-functionalized graphene oxide (PPD-GO) are involved into SPEEK matrix to improve the physicochemical properties and decrease vanadium ion permeability. The effect of PPD-GO nanofillers on the proton conductivity, vanadium ion permeability, swelling ratio and water uptake of SPEEK membrane are characterized and analyzed. VRB single cell performance is further determined and discussed considering the assembled proton channel from the interaction between NH2 and SO3H groups.
2. Experimental
2.1 Materials
Natural graphite powders (NGP) (325 mesh) were purchased from Qingdao Laixi Graphite Co., Ltd. Poly(ether ether ketone) was provided by Panjin ZhongRun Special plastic co., Ltd. Nafion 117 membrane was purchased from DuPont company. N,N-Dimethyl formamide (DMF), NaOH, dichloromethane were purchased from Tianjin Fengchuan Chemical Reagent Technologies co., Ltd., and used as received.2.2 Preparation of PPD-GO nanofillers
The graphite oxide (GO), as reported in our previous work,37 is used to prepare the functionalized products. Fig. 1 presents the preparation process of PPD-GO nanofillers. 0.1 g GO was redispersed in 100 mL deionized water, and then 6 mmol p-phenylenediamine and H2SO4 was dissolved into GO solution. 6 mmol NaNO2 was dissolved in 5 mL deionized water, and slowly dropped into the above solution. In the following, the solution was heated to 60 °C for 6 h, and then the reaction completed. The obtained PPD-GO was further centrifuged and washed with lot of deionized water for three times, and then it was freeze-dried until the constant weight.2.3 Preparation of SPEEK
4 g of PEEK was gradually dissolved into 100 mL of H2SO4 (98 wt%) with vigorous mechanical stirring at room temperature for 24 h, and then heated up to 50 °C for a certain time. Afterwards, the solution was slowly poured into lots of ice-cold water under continuous agitation. The precipitate was filtered and washed several times with deionized water until neutral pH, and then dried in a vacuum oven at 100 °C for 24 h. The sulfonated degree of SPEEK was 62%, and used as the VRB membrane.2.4 Preparation of SPEEK/PPD-GO hybrid membrane
SPEEK hybrid membranes with different content of PPD-GO were prepared by a solution-casting method. Firstly, PPD-GO was dispersed in DMF under ultrasonication for 45 min, and then dropped into SPEEK/DMF solution. The solution was cast into a clean framework and dried at 60 °C for 12 h, and then vacuum-dried at 100 °C for 12 h to remove the residual solvent. SPEEK/PPD-GO membranes were acidified by 1 mol L−1 H2SO4 solution for 48 h. After that, they were washed by deionized water for three times, and then stored in deionized water before use. The SPPEEK/PPD-GO membranes with various contents of PPD-GO were denoted as SPEEK/PPD-GO-X, where X is the mass fraction of PPD-GO.2.5 Characterization of hybrid membrane
The chemical compositions are characterized by Fourier transform infrared spectrometer (FT-IR, Nicolet IS5, US) in the range of 4000–500 cm−1. The surface and cross-section of membranes are observed by field-emission scanning electron microscopy (FE-SEM, S4800 Hitachi, Japan) with an accelerating voltage of 10 kV. X-ray photoelectron spectroscopy (XPS) (GENESIS EDAX, US) with Al Kα radiation (hν = 1486.4 eV) is used.where, Wwet and Wdry are the membrane weight before and after water adsorption; Lwet and Ldry are the length of wet and dry membrane, respectively.
where CNaOH and VNaOH are the concentration and the volume of NaOH solution, respectively, and Wdry is the weight of dry membrane.
The determination procedure of proton conductivity has been reported in our previous studies. The membrane is soaked in a solution of 1.0 mol L−1 VOSO4/2.0 mol L−1 H2SO4 for 24 h before test. The conductivity cell is separated into two parts filled with 1.0 mol L−1 VOSO4/2.0 mol L−1 H2SO4. The electric resistances of the conductivity cell with membrane (r1) and without membrane (r2) are measured by electrochemical impedance spectroscopy (EIS) over a frequency range of 1 MHz to 1 Hz using an electrochemical workstation (CHI604E, China) at room temperature. The effective membrane area S of the cell is 7.065 cm2. The area resistance of membrane R and the proton conductivity (σ) are calculated by the following equation.
| R = (r1 − r2) × S |
where L is the thickness of membrane (cm), R is the area resistance of membrane (Ω), and A is the effective area of membrane (cm2).
where VB is the volume of VO2+ concentration, CB is the VO2+ concentration in MgSO4 solution, t is time. A is the effective area of membrane, L is the thickness of membrane, P is the VO2+ permeability, CA is the initial concentration of the VO2+ in the left cell. It is supposed that the change of CA and CB is so small and can always be negligible. So, the (CA − CB(t)) is almost constant, equaled with the initial concentration of the VO2+ in the left cell.
where Ic and Id are the charge current and discharge current, respectively, and Vc and Vd are the charge voltage and discharge voltage, respectively.
3. Results and discussion
3.1 Characterization of PPD-GO
Fig. 2 shows the FT-IR spectra of GO (a) and PPD-GO (b). The GO characteristic bands present at 1726 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1623 cm−1 (C
O stretching), 1623 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching), 1100 cm−1 (C–O stretching of epoxy groups), respectively. For PPD-GO, new bands at 1581 cm−1 (NH2 stretching), 1265 cm−1 (C–N stretching) and 830 cm−1 (N–H wagging)38 appear. Moreover, the characteristic band of benzene ring is also detected at 1500 cm−1, indicating that PPD has been successfully grafted onto GO surface.
C stretching), 1100 cm−1 (C–O stretching of epoxy groups), respectively. For PPD-GO, new bands at 1581 cm−1 (NH2 stretching), 1265 cm−1 (C–N stretching) and 830 cm−1 (N–H wagging)38 appear. Moreover, the characteristic band of benzene ring is also detected at 1500 cm−1, indicating that PPD has been successfully grafted onto GO surface.
XPS spectra of GO and PPD-GO are shown in Fig. 3. GO spectrum shown in Fig. 3a gives the C 1s and O 1s peaks at 287 and 535 eV, respectively; while for PPD-GO, an additional peak appears at 402 eV, which is attributed to the N 1s peak (Fig. 3b). The atomic compositions of GO and PPD-GO shown in Table 1. The increased N composition proves the successful grafting process of PPD. The C 1s XPS spectra of GO and PPD-GO are shown in Fig. 3c and d. The peak at 287 eV in Fig. 3d is assigned to C–NH, arising from the nucleophilic reaction between amine (PPD) and epoxy groups in GO.39 This further demonstrates that PPD has been successfully grafted onto GO surface via the covalent bonding.
| Samples | C (atomic%) | O (atomic%) | N (atomic%) |
|---|---|---|---|
| GO | 59.3 | 40.7 | 0 |
| PPD-GO | 71.39 | 25.75 | 2.86 |
3.2 Structure and properties of SPEEK/PPD-GO hybrid membranes
SEM images of SPEEK and SPEEK/PPD-GO hybrid membranes are shown in Fig. 4. The cross-section picture of SPEEK exhibits a smooth surface (Fig. 4a), while SPEEK/PPD-GO gives a relatively rough morphology. The increased roughness obviously shows agreement with the incorporated content of PPD-GO, as shown in Fig. 4b–d. In addition, the PPD-GO nanofillers are well dispersed into the SPEEK matrix, and no agglomeration is found. The well-dispersed behavior of PPD-GO nanofillers is attributed to the good interfacial miscible and possible interaction between PPD-GO and SPEEK matrix.40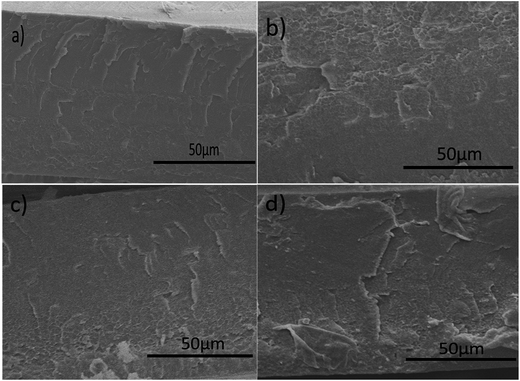 | ||
| Fig. 4 SEM images of the cross-section of SPEEK (a), SPEEK/PPD-GO-0.5 (b), SPEEK/PPD-GO-1 (c) and SPEEK/PPD-GO-2 (d). | ||
The physicochemical properties such as water uptake (WU), swelling ratio (SR) and ion exchange capacity (IEC) of hybrid membranes are detailed analyzed, and they are shown in Table 2. WU and SR of SPEEK/PPD-GO hybrid membrane both decrease with the increased PPD-GO contents, as compared with the pristine SPEEK and Nafion 117 membrane. This is possible originated from the dense membrane morphology in light of the interfacial interaction of NH2 and SO3H groups. The formed interfacial interaction in the interfacial zone between PPD-GO and SPEEK matrix decreases the water-absorbing ability of SO3H groups, and further restricts the mobility of polymer chains. Both decreased WU and SR for hybrid membranes exhibit an obvious advantage over typical SPEEK and Nafion membrane because these two parameters will influence the stability of proton transfer channel. However, it is should be noted that IEC value shows a decrement tendency against the incorporated PPD-GO nanofillers, as compared with SPEEK membrane. This is possible ascribed to the influence of the doped PPD-GO nanofillers, and the incorporated PPD-GO nanofillers consume SO3H groups of SPEEK due to the acid–base action.
| Samples | WU (%) | SR (%) | IEC (mmol g−1) |
|---|---|---|---|
| Nafion 117 | 21.2 | 29.0 | 0.88 |
| SPEEK | 43.0 | 20.0 | 2.03 |
| SPEEK/PPD-GO-0.5 | 24 | 8 | 1.1 |
| SPEEK/PPD-GO-1 | 22 | 6.8 | 1.08 |
| SPEEK/PPD-GO-1.5 | 21 | 6.2 | 1.06 |
| SPEEK/PPD-GO-2 | 20 | 6.1 | 1.02 |
For VRB application, proton conductivity, vanadium ion permeability and selectivity are important parameters to verify the membrane performance. Fig. 5a compares the proton conductivity of hybrid membranes and Nafion 117. From Fig. 5a, SPEEK/PPD-GO hybrid membranes at low content of PPD-GO, from 0.5 to 1.0 wt%, the proton conductivity is much higher than that of pristine SPEEK and Nafion 117 membrane. However, further increasing to 2.0 wt%, SPEEK/PPD-GO hybrid membranes show a little lower value, compared with the control ones. Additionally, for the proton transfers of PEM, the Vehicle mechanism and the Grotthuss mechanism are generally accepted, and the proton conductivity is mainly dependent on the concentration of proton and the mobility of proton in membrane. For a low PPD-GO content ≤1.0 wt%, the proton conductivity increased with increasing amounts of PPD-GO, which created more interconnected transfer channels throughout the SPEEK matrix. The introduction of PPD-GO provides more proton transfer channels, thus lowering the energy-barrier for proton transport into SPEEK membrane. However, with still further PPD-GO loading beyond 1.0 wt%, the “blocking effect” started to predominate, thus reducing the conductivity of the hybrid membrane.41 Moreover, SPEEK/PPD-GO-1.0 exhibits the highest proton conductivity (16.4 mS cm−1) among these hybrid membranes, and is almost 3 times higher than that of pristine SPEEK due to its good WU property. So, suitable amounts of PPD-GO (i.e. less than or equal to 1.0 wt%) were seen to enhance the proton transport properties for SPEEK membrane.
 | ||
| Fig. 5 Proton conductivity (a), vanadium ion permeability (b) and ion selectivity (c) of hybrid membranes and Nafion 117 membrane. | ||
The vanadium ion permeability (P) of membranes are given in Fig. 5b. All hybrid membranes exhibit much lower P value (13.5–22.5 × 10−7 cm2 min−1) than that of Nafion 117 (67.2 × 10−7 cm2 min−1) and pristine SPEEK membrane (43 × 10−7 cm2 min−1), which is attributed to the different interfacial microstructures.15 For SPEEK hybrid membranes, the P value firstly decreases with the content of PPD-GO changing from 0.5 to 1.0 wt%, and then increases again with the incorporated PPD-GO contents from 1.5 to 2.0 wt%. The lower P value, 13.5 × 10−7 cm2 min−1, shows at 1.0 wt% PPD-GO, indicating that SPEEK/PPD-GO-1.0 has an optimized vanadium ion permeability. The incorporated PPD-GO nanofillers change the membrane morphology and structure, and the formed transfer pathway enhances the dense state of SPEEK matrix,42 and thus prohibits the transportation phenomenon of vanadium ions through the membrane.
Ion selectivity is another important parameter to evaluate the membrane performance, and membrane with a higher ion selectivity presents a better performance in VRB system. Ion selectivity of hybrid membrane and Nafion 117 is shown in Fig. 5c. All SPEEK hybrid membranes show a higher ion selectivity value than that of Nafion 117 and pristine SPEEK membrane. With increasing the content of PPD-GO from 0.5 to 2.0 wt%, ion selectivity value firstly increases, and then decreases. The maximum value, 12.2 × 103 S min cm−3, is shown at 1.0 wt% PPD-GO among these SPEEK hybrid membranes. This is contributed from the low vanadium ion permeability and high proton conductivity, and the good balance between vanadium ion permeability and proton conductivity leads to a higher ion selectivity, as compared with Nafion 117 membrane. So, in the following VRB single cell performance, SPEEK/PPD-GO-1 hybrid membrane is used and discussed.
3.3 VRB performance
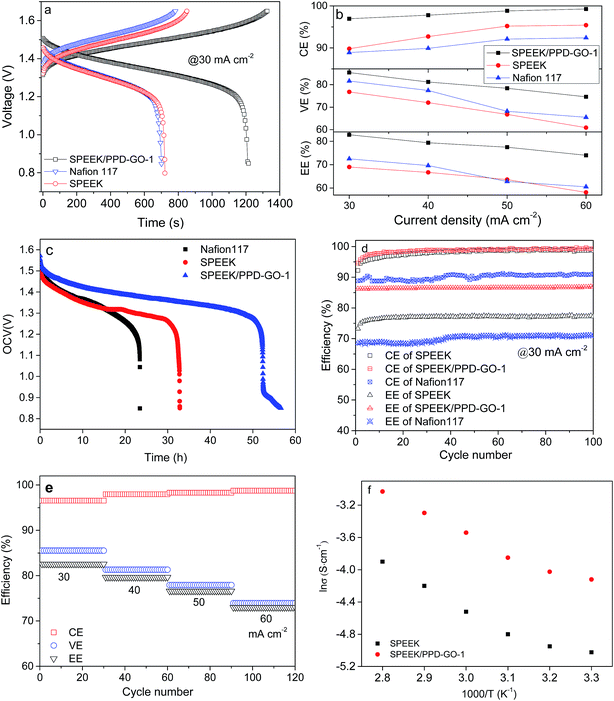
Single cell VRB assembled with SPEEK/PPD-GO-1.0 hybrid membrane is cycled and detected at a current density from 30 to 60 mA cm−2. Similarly, VRB cell with Nafion 117 and SPEEK membrane also is compared under the same condition. Fig. 6a presents the compared charge–discharge curves of VRB assembled with Nafion 117, SPEEK and SPEEK/PPD-GO-1.0 membrane. It can be find that SPEEK/PPD-GO-1 hybrid membrane possess the longest charge–discharge time, a relative lower charge voltage and a higher discharge voltage during the charge–discharge process, as compared with SPEEK and Nafion 117 membranes. This should be attributed to the lower vanadium ion permeability and higher proton conductivity for SPEEK/PPD-GO-1.0 membrane.The coulombic efficiency (CE), voltage efficiency (VE) and energy efficiency (EE) of VRB at current density of 30–60 mA cm−2 presents in Fig. 6b. CE shows a linear increasing behavior, while VE and EE give a decreased tendency with the increased current density from 30 to 60 mA cm−2. SPEEK/PPD-GO-1.0 hybrid membrane illustrates a better CE, VE and EE performance than Nafion 117 and SPEEK membrane based on its good vanadium ion selectivity and proton conductivity. For our present work, SPEEK/PPD-GO-1.0 hybrid membrane gives the maximum EE value against Nafion 117 and SPEEK membrane, indicating that SPEEK/PPD-GO-1.0 hybrid membrane has a good balance between vanadium ion permeability and proton conductivity. The decreased VE against the current density is mainly influenced by a high overpotential and ohmic polarization at high current density.35 For these proton exchange membrane, VRB cell assembled with SPEEK/PPD-GO-1.0 hybrid membrane exhibits a much higher performance (CE: 96.9%, EE: 82.8%) at 30 mA cm−2 current density, as compared with Nafion 117 membrane (CE: 89%, EE: 72.5%). This demonstrates high ion selectivity and low vanadium ion permeability contribute a good single cell performance when PPD-GO nanofillers are used as the interfacial structure modifying composition for hybrid membrane.
The open circuit voltage (OCV) is an important parameter to evaluate the self-discharge process of VRB system. Fig. 6c presents the compared OCV results of VRB cell with SPEEK/PPD-GO-1, SPEEK, and Nafion 117 membrane. With time increasing, the OCV value of SPEEK and Nafion 117 membranes decrease, and then drop quickly at about 1.27 V. For SPEEK/PPD-GO-1 hybrid membrane, it decreases with a lower speed than that of Nafion 117 and SPEEK membranes before 1.3 V, and then continues to decrease after 0.92 V. The OCV value of SPEEK/PPD-GO-1 hybrid membrane keeps ca. 0.85 V for 56 h, which is much longer than that of SPEEK (ca. 34 h) membrane and is 2 times longer than that of Nafion 117 membrane (ca. 26 h). This result proves that PPD-GO nanofillers play an effective barrier to prohibit the cross-mixing of vanadium ion, and the acid–base interaction between NH2 and SO3H groups also prevents the vanadium ion crossing the membranes depending upon the Donnan exclusion effect. Actually, Donnan exclusion effect means one charged substance near membrane that sometimes fails to distribute evenly across the two sides of membrane. The reason of unable passing through membrane is ascribed to the influence of another different charged substance, creating an uneven electrical charge. The incorporated –NH2 groups from PPD-GO nanofillers interact with proton, and then the positive charged –NH3+ is formed, which hinder vanadium ions across the hybrid membrane due to the electrostatic exclusion effect. Therefore, the as-prepared SPEEK/PPD-GO hybrid membranes have a lower self-discharge rate than Nafion 117 membrane.
The following 100 times charge–discharge cycles are carried out to analyze the operation stability of SPEEK/PPD-GO hybrid membranes. A static VRB system with SPEEK/PPD-GO-1 hybrid membrane, SPEEK membrane and Nafion 117 membrane are determined at current density of 30 mA cm−2. Fig. 6d gives the compared results with the above membranes. Both CE and EE of SPEEK/PPD-GO-1.0 membrane are much higher than that of Nafion 117. And, both CE and EE of VRB are highly stable and no obvious decay appears over 100 cycles. Additionally, a stable EE for VRB is closely depended upon the good stability of hybrid membranes, especially under the strong acidic and oxidizing condition. Compared with SPEEK membrane, the EE of SPEEK/PPD-GO-1 membrane is much higher, illustrating that the PPD-GO nanofillers can effectively enhance its operating stability. The stable CE and higher EE of hybrid membrane demonstrates that the doped PPD-GO nanofillers into SPEEK membrane effectively enhance the membrane stability, originated from the interaction between NH2 and SO3H groups.
Additionally, the rate capability of VRB system assembled with SPEEK/PPD-GO-1 membrane is further analyzed, and 120 times charge–discharge tests are conducted at the current density from 30 to 60 mA cm−2. Fig. 6e presents this cycle performance of VRB with SPEEK/PPD-GO-1 membrane at different current densities. The cell efficiencies keep constant at each current density. CE increases with the current density from 96.5% at 30 mA cm−2 to 98.7% at 60 mA cm−2, which is due to the decreased vanadium ion crossover at high current density. VE drops from 85.5% at 30 mA cm−2 to 73.9% at 60 mA cm−2 due to the high ohmic polarization. EE decreases from 82.5% at 30 mA cm−2 to 73% at 60 mA cm−2. Thus, VRB with SPEEK/PPD-GO-1 hybrid membrane demonstrates a higher rate capability, and the incorporated PPD-GO nanofillers effectively enhance the proton transfers for SPEEK matrix.
Fig. 6f illustrates the Arrhenius plot of conductivity vs. temperature for SPEEK and SPEEK/PPD-GO-1 hybrid membrane. The proton conductivities of the membranes are determined at 100% RH as a function of temperature from 30 to 80 °C. The proton conductivities of SPEEK/PPD-GO-1 are higher than SPEEK membrane, contributed from the effect of acid–base pairs. The activation energy values (Ea) for proton conduction can be estimated from the Arrhenius equation  where σ0 is the pre-exponential factor, R is the gas constant and T is the Kelvin temperature. For Vehicle mechanism, the Ea should be around 0–14 kJ mol−1;32 while for the hybrid membrane, its value is 18.87 kJ mol−1. This indicates that both Grotthuss mechanism and Vehicle mechanism coexist in SPEEK/PPD-GO hybrid membrane, where the former is predominant.
where σ0 is the pre-exponential factor, R is the gas constant and T is the Kelvin temperature. For Vehicle mechanism, the Ea should be around 0–14 kJ mol−1;32 while for the hybrid membrane, its value is 18.87 kJ mol−1. This indicates that both Grotthuss mechanism and Vehicle mechanism coexist in SPEEK/PPD-GO hybrid membrane, where the former is predominant.
SEM images of SPEEK/PPD-GO-1 membrane before and after 100 times tests are detected to give a clear understanding of structure stability under harsh condition. Fig. 7 shows the compared results. After 100 times tests, the hybrid membrane surface (Fig. 7b) and cross-section (Fig. 7d) is similar to the membrane morphology before test (Fig. 7a and c), and no structure decomposition appears, indicating that SPEEK/PPD-GO hybrid membranes are highly stable at such strong oxidizing and acid condition.
 | ||
| Fig. 7 SEM images of SPEEK/PPD-GO-1 before (a) and after (b) 100 cycles charge–discharge test; cross-section images before (c) and after (d) cycles. | ||
The obtained high cell efficiency and good operating stability of SPEEK/PPD-GO hybrid membranes are mainly attributed to the dense membrane structure. The interaction between PPD-GO and SPEEK matrix prohibits vanadium ions crossover in VRB system, and the NH2 groups of PPD-GO and the SO3H groups of SPEEK matrix create the proton transport pathway followed by enhancing the proton conductivity. In addition, the formed interfacial interactions also control the swelling ratio of membrane, realizing a good balance between ion selectivity and proton conductivity. Therefore, the present SPEEK/PPD-GO hybrid membranes indicate a feasible way by controlling the interfacial interaction to realize a high performance hybrid membrane for VRB application.
4. Conclusion
A series of SPEEK/PPD-GO hybrid membranes for VRB system are fabricated successfully. The formed interfacial interaction can be controlled by the incorporated PPD-GO nanofillers, in which effectively regulate the ion selectivity, vanadium ion permeation and structure stability of hybrid membranes. The incorporated PPD-GO nanofillers into SPEEK matrix successfully enhance the proton transports process and block the vanadium ion permeation. The VRB with SPEEK/PPD-GO-1 hybrid membrane exhibits a high cell performance (CE: 96.5–98.7% and EE: 73–82.5%) at current density of 30–60 mA cm−2. Moreover, self-discharge time of VRB with SPEEK/PPD-GO-1 hybrid membrane is much longer than that of Nafion 117 and SPEEK membrane. After 100 times charge–discharge tests, SPEEK/PPD-GO-1 hybrid membrane exhibits a good structure stability against the strong acidic and oxidizing condition. Therefore, controlling the interfacial interaction to realize a high performance hybrid membrane is a feasible way for VRB application.Acknowledgements
This work was supported by Program for New Century Excellent Talents in University (NECT-13-0928), National Natural Science Foundation of China (Grant No. 21404080, 20904040 and 21174105), and the Key Project of Tianjin Municipal Natural Science Foundation (Grant No. 16JCZDJC37000 and 12JCZDJC26800).Notes and references
- G. Kear, A. A. Shah and F. C. Walsh, Int. J. Energy Res., 2012, 36, 1105–1120 CrossRef CAS.
- M. Skyllas-Kazacos, M. Rychcik, R. G. Robins, A. G. Fane and M. A. Green, J. Electrochem. Soc., 1986, 133, 1057–1058 CrossRef CAS.
- E. Sum, M. Rychcik and M. Skyllas-kazacos, J. Power Sources, 1985, 16, 85–95 CrossRef CAS.
- E. Sum and M. Skyllas-Kazacos, J. Power Sources, 1985, 15, 179–190 CrossRef CAS.
- Z. Fu, J. Liu and Q. Liu, J. Electron. Mater., 2016, 45, 666–671 CrossRef CAS.
- B. Schwenzer, J. L. Zhang, S. Kim, L. Y. Li, J. Liu and Z. G. Yang, ChemSusChem, 2011, 4, 1388–1406 CrossRef CAS PubMed.
- Z. Mai, H. Zhang, X. Li, S. Xiao and H. Zhang, J. Power Sources, 2011, 196, 5737–5741 CrossRef CAS.
- X. Teng, J. Dai, F. Bi and G. Yin, J. Power Sources, 2014, 272, 113–120 CrossRef CAS.
- X. Teng, J. Dai, J. Su, Y. Zhu, H. Liu and Z. Song, J. Power Sources, 2013, 240, 131–139 CrossRef CAS.
- X. Teng, J. Lei, X. Gu, J. Dai, Y. Zhu and F. Li, Ionics, 2012, 18, 513–521 CrossRef CAS.
- X. Teng, C. Sun, J. Dai, H. Liu, J. Su and F. Li, Electrochim. Acta, 2013, 88, 725–734 CrossRef CAS.
- X. Teng, Y. Zhao, J. Xi, Z. Wu, X. Qiu and L. Chen, J. Membr. Sci., 2009, 341, 149–154 CrossRef CAS.
- M. Vijayakumar, B. Schwenzer, S. Kim, Z. Yang, S. Thevuthasan, J. Liu, G. L. Graff and J. Hu, Solid State Nucl. Magn. Reson., 2012, 42, 71–80 CrossRef CAS PubMed.
- W. Dai, Y. Shen, Z. Li, L. Yu, J. Xi and X. Qiu, J. Mater. Chem. A, 2014, 2, 12423 CAS.
- N. Wang, J. Yu, Z. Zhou, D. Fang, S. Liu and Y. Liu, J. Membr. Sci., 2013, 437, 114–121 CrossRef CAS.
- W. Wei, H. Zhang, X. Li, H. Zhang, Y. Li and I. Vankelecom, Phys. Chem. Chem. Phys., 2013, 15, 1766–1771 RSC.
- X. Wei, Z. Nie, Q. Luo, B. Li, V. Sprenkle and W. Wang, J. Electrochem. Soc., 2013, 160, A1215–A1218 CrossRef CAS.
- H. Zhang, H. Zhang, F. Zhang, X. Li, Y. Li and I. Vankelecom, Energy Environ. Sci., 2013, 6, 776–781 CAS.
- L. Q. Li, J. W. Chen, H. Lu, C. P. Jiang, S. Gao, X. Yang, X. J. Lian, X. J. Liu and R. L. Wang, Acta Chim. Sin., 2009, 67, 2785–2790 CAS.
- Z. H. Li, W. J. Dai, L. H. Yu, J. Y. Xi, X. P. Qiu and L. Q. Chen, J. Power Sources, 2014, 257, 221–229 CrossRef CAS.
- Z. H. Li, J. Y. Xi, H. P. Zhou, L. Liu, Z. H. Wu, X. P. Qiu and L. Q. Chen, J. Power Sources, 2013, 237, 132–140 CrossRef CAS.
- X. Ling, C. K. Jia, J. G. Liu and C. W. Yan, J. Membr. Sci., 2012, 415, 306–312 CrossRef.
- Y. Heo, H. Im and J. Kim, J. Membr. Sci., 2013, 425–426, 11–22 CrossRef CAS.
- S. Winardi, S. C. Raghu, M. O. Oo, Q. Y. Yan, N. Wai, T. M. Lim and M. Skyllas-Kazacos, J. Membr. Sci., 2014, 450, 313–322 CrossRef CAS.
- D. Y. Chen, S. J. Wang, M. Xiao, D. M. Han and Y. Z. Meng, J. Power Sources, 2010, 195, 7701–7708 CrossRef CAS.
- D. Y. Chen, S. J. Wang, M. Xiao, D. M. Han and Y. Z. Meng, Polymer, 2011, 52, 5312–5319 CrossRef CAS.
- D. Y. Chen, S. J. Wang, M. Xiao and Y. Z. Meng, Energy Convers. Manage., 2010, 51, 2816–2824 CrossRef CAS.
- T.-A. Chen, A. K. Y. Jen and Y. Cai, Chem. Mater., 1996, 8, 607–609 CrossRef CAS.
- J. Wang, Z. Zhang, X. Yue, L. Nie, G. He, H. Wu and Z. Jiang, J. Mater. Chem. A, 2013, 1, 2267–2277 CAS.
- R. Marschall, J. Rathouský and M. Wark, Chem. Mater., 2007, 19, 6401–6407 CrossRef CAS.
- Y. Yin, T. Xu, G. He, Z. Jiang and H. Wu, J. Power Sources, 2015, 276, 271–278 CrossRef CAS.
- H. Wu, X. Shen, T. Xu, W. Hou and Z. Jiang, J. Power Sources, 2012, 213, 83–92 CrossRef CAS.
- J. Wang, H. Zhang, X. Yang, S. Jiang, W. Lv, Z. Jiang and S. Z. Qiao, Adv. Funct. Mater., 2011, 21, 971–978 CrossRef CAS.
- B. Smitha, S. Sridhar and A. A. Khan, Macromolecules, 2004, 37, 2233–2239 CrossRef CAS.
- L. Cao, Q. Sun, Y. Gao, L. Liu and H. Shi, Electrochim. Acta, 2015, 158, 24–34 CrossRef CAS.
- L. Cao, L. Kong, L. Kong, X. Zhang and H. Shi, J. Power Sources, 2015, 299, 255–264 CrossRef CAS.
- H. Liu, T. Kuila, N. H. Kim, B.-C. Ku and J. H. Lee, J. Mater. Chem. A, 2013, 1, 3739–3746 CAS.
- Y. Wen, Y. Zhu, A. Langrock, A. Manivannan, S. H. Ehrman and C. Wang, Small, 2013, 9, 2810–2816 CrossRef CAS PubMed.
- L. Q. Xu, W. J. Yang, K.-G. Neoh, E.-T. Kang and G. D. Fu, Macromolecules, 2010, 43, 8336–8339 CrossRef CAS.
- C. Xue, J. Zou, Z. Sun, F. Wang, K. Han and H. Zhu, Int. J. Hydrogen Energy, 2014, 39, 7931–7939 CrossRef CAS.
- C.-Y. Tseng, Y.-S. Ye, M.-Y. Cheng, K.-Y. Kao, W.-C. Shen, J. Rick, J.-C. Chen and B.-J. Hwang, Adv. Energy Mater., 2011, 1, 1220–1224 CrossRef CAS.
- L. Cao, Q. Sun, H. Wang, X. Zhang and H. Shi, Composites, Part A, 2015, 68, 140–148 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |