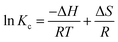DOI:
10.1039/C6RA20422H
(Paper)
RSC Adv., 2016,
6, 100248-100261
Rapid and selective separation of molybdenum ions using a novel magnetic Mo(VI) ion imprinted polymer: a study of the adsorption properties
Received
12th August 2016
, Accepted 10th October 2016
First published on 10th October 2016
Abstract
In this work, we have developed a convenient method for the rapid and selective separation of Mo(VI) from aqueous solution by using a novel magnetic Mo(VI) ion imprinted polymer (Mo(VI)-MIIP) as the sorbent. This sorbent was synthesized using methacrylic acid (MAA) as the functional monomer, the Mo6+ anion as a template, ethylene glycol dimethacrylate (EGDMA) as a cross-linker, and Fe3O4@SiO2 as the magnetic component. The template Mo6+ ions were removed from the polymer using a solution containing 10% (v/v) methanol in HCl (0.5 mol L−1). The thermal stability, chemical structure, and magnetic properties of the synthesized sorbent were characterized using different techniques. Various parameters which affect the adsorption efficiency were evaluated in a batch system to determine the optimal adsorption conditions and reduce non-specific interactions. The results showed that the maximum adsorption capacity was 28 mg g−1, which was observed at 25 °C. The equilibrium time for the adsorption of the Mo(VI) analyte was determined over 30 min, and the amount of adsorbent which gave the maximum adsorption capacity was 1 g L−1. The kinetic data, obtained under optimum conditions, could be fitted with a pseudo-second order kinetic model with a high correlation coefficient (R2 = 0.9971). The adsorption isotherm data could be well described using Langmuir adsorption isotherms, and the maximum adsorption capacity calculated from the Langmuir isotherm was 31.08 mg g−1. This was very close to the maximum adsorption capacity obtained under optimal conditions. The selectivity studies indicated that the synthesized sorbent had a high single selectivity sorption for the Mo6+ anion in the presence of competing ions. The values of the thermodynamic parameters proved that the adsorption process of molybdenum onto the synthesized sorbent was exothermic (ΔH < 0) and spontaneous (ΔG < 0). In addition, the spent MIIP can be reused several times without a significant decrease in adsorption capacity.
1. Introduction
The necessity for scarce metals has been increasing in recent years, so the separation and recovery of scarce metals are still active issues. Among several recovery technologies for scarce metals, selective adsorption is one of the most effective methods. Molybdenum (Mo) is one of the scarce metals, which is used in the steel industry as an alloying agent, and in the petrochemical industry as a catalyst.1 Molybdenum is a refractory metallic element, which is widely used in electron tubes, vacuum tubes, heat-resistant materials, and in thermo-couples for high temperature measurements.2,3 Molybdenum can exist in various oxidation states ranging from −2 to +6, and the form Mo(VI) is generally assumed to be the dominant oxidation state in toxic natural water.4,5 Molybdenum is considered an essential trace element for both plants and animals, and the recommended dietary intake is 75–250 μg per day for adults and older children.6 Molybdenum may be toxic at high concentrations for both plants and humans, as it leads to some diseases such as anemia, bone and joint deformities, liver and kidney abnormalities, and disturbances in the metabolism of fats and proteins in humans.7,8 The World Health Organization (WHO) recommends a maximum level of 0.07 mg L−1 of Mo in drinking water.9 On the other hand, molybdenum is a strategic metal in the industrial sector and in medicine. It is used in radios, thermocouples, the anticathodes of X-ray tubes, and the production of alloys such as special steels. Mo(VI) is also included in the high level liquid waste (HLLW) solution of spent nuclear fuel. The separation and recovery of Mo(VI) from HLLW is required for the nuclear fuel cycle process, since the solubility of Mo(VI) for vitrification is quite low. Therefore, the development of a new sorbent that can either selectively separate or adsorb molybdenum (Mo(VI)) is a greatly significant goal. In the past decades, several methods, such as chemical precipitation,10 solvent extraction,11 micellar ultrafiltration,12 organic and inorganic ion exchange,13 electrolysis methods,14 liquid membrane separation,15 biological treatment,16 and adsorption,17 have been adopted for the removal of heavy metal ions from aqueous solution. Most of these methods encounter various difficulties, such as technical, economic, environmental, and health problems, which are related to low efficiency, long processing time, high energy consumption, and large quantities of hazardous materials. Among these methods, the adsorption technique appears to be an efficient and economically feasible method, especially for effluents with moderate and low concentrations of heavy metal ions. Adsorption is most often utilized, owing to its high efficiency and simple operation, and the availability of different adsorbents. Several adsorbents have been studied as adsorbents for the removal of Mo(VI), including mineral substances, natural materials (like pyrite, goethite, kaolinite, siliceous materials, and zeolites), resins, and bioadsorbents (chitin, chitosan, and biomass).18–22 However, these adsorbents have some disadvantages such as poor selectivity, low reuse capacity and slow adsorption–desorption kinetics. The fast and selective separation of heavy metal ions will facilitate environment protection and the reuse of precious heavy metals. Therefore, there is a need to develop a novel adsorbent which has high selectivity, fast adsorption–desorption kinetics, and high regeneration ability.
Molecular imprinting technology (MIT) is an emerging technology that has gained much attention in generating recognition sites through the reversible immobilization of template molecules on cross-linked macromolecular polymer matrixes. Molecularly imprinted polymers (MIP) represent a new class of materials with high selectivity and good affinity for target molecules. If some ions are applied as templates, the polymers obtained are called ion-imprinted polymers (IIPs). For comparison, IIPs maintain all the virtues of MIPs in an essentially similar fashion, but IIPs can recognize ionic templates other than the molecular templates in MIPs as their targets. The ionic imprinting technique is the best used method for the adsorption or separation of trace metal ions from aqueous solution. In an ion-imprinting process, the high selectivity of a polymeric adsorbent can be explained on the basis of the polymer memory effect towards a metal ion interaction between a specific ligand, and the coordination geometry and coordination number of the metal ion, as well as its size and charge. However, it is still time-consuming and complicated to use the imprinted polymer process due to indispensable filtration, centrifugation, and other handling processes. Also, these materials suffer from a lack of surface area.23,24
To increase the active surface area of the imprinted polymers and their mechanical stability, these polymers have been coated on various supports, such as carbon nanotubes (CNTs),25 mesoporous silica,26 colloidal SiO2 particles,27 and Fe3O4 nanoparticles (NPs).28 Among these supports, MNPs have the desirable property that they can be easily separated with an external magnetic field without either additional centrifugation or filtration procedures. When IIP particles are incorporated with Fe3O4, they can be easily separated through the application of an external magnetic field. Therefore, the use of magnetic ion-imprinted polymers for the selective adsorption of heavy metal ions is rather common.29–31 Magnetic ion imprinted polymers have consequently received much attention because of the ease of size and morphology modulation, their simple and convenient operation, the economization of materials, time and energy, as well as the homogeneous binding sites, and stable physical and chemical properties.32–37
Currently, several applications of IIPs to molybdenum adsorption and extraction have been reported. Ardestani et al.38 synthesized nanopore Mo(VI)-imprinted polymers through bulk polymerization for the preconcentration and separation of Mo(VI) trace amounts from water samples. Gao et al.39 prepared a molybdate anion surface-imprinted material with the new surface-imprinting technique of “pre-graft polymerizing and post-crosslinking/imprinting” for the selective removal of molybdate anions from a water medium. Also Mo(VI) oxy ion imprinted particles were prepared first through the surface metal ion imprinting technique with a sol–gel process on the surface of amino-silica by Ren et al.40 They used Mo(VI) oxy ion-imprinted particles as an adsorbent for the preconcentration and separation of Mo(VI) trace amounts from water samples. However, none of these studies have reported the use of magnetic ion imprinted polymers for the selective adsorption of Mo(VI). Our new idea was the synthesis of a new adsorbent with two highlighted features: high selectivity and magnetic properties.
The aim of this work was to synthesize a novel magnetic-ion imprinted polymer (MIIP) which was prepared through the combination of an imprinting polymer and MNPs for the selective adsorption of Mo(VI) from aqueous solution. Fe3O4 nanoparticles were employed as magnetic materials and synthesized using an improved co-precipitation method. Iron oxide is easy to reunite due to van der Waals forces and mutual magnetic attraction. Also silica particles with stable chemical properties, a firm physical structure, and high mechanical strength were selected as protective molecules to effectively stabilize the iron oxide. The resultant magnetic ion imprinted polymers were systematically characterized, and the adsorption capacity and selectivity of the adsorbent were investigated. Furthermore, the thermodynamic, kinetic, and isothermal properties of the adsorption process were studied to explore the mechanism of adsorption.
2. Experimental
2.1 Chemicals and reagents
Ferric chloride (FeCl3·6H2O), ferrous chloride (FeCl2·4H2O), sodium hydroxide (NaOH), ammonium hydroxide (NH4OH), and tetraethyl orthosilicate (TEOS) were obtained from Merck (Darmstadt, Germany) for the synthesis of Fe3O4@SiO2 nanoparticles. For the preparation of the imprinted polymer, methacrylic acid (MAA), tetra-n-butylammonium hydroxide (20%, m/m), sodium thiocyanate (NaSCN), ethylene glycol dimethacrylate (EGDMA), and α,α′-azobisisobutyronitrile (AIBN) were obtained from Merck (Darmstadt, Germany), and Na2MoO4·2H2O (Merck, 99%) was used as the source of Mo(VI). Ethanol and acetonitrile (HPLC grade) were purchased from Merck (Darmstadt, Germany). A stock solution (2000 mg L−1) of Mo(VI) was prepared through dissolving appropriate amounts of sodium molybdate (Na2MoO4·2H2O) in double distilled water in a 100 mL volumetric flask. Working solutions were prepared daily from the stock solution through serial dilutions. The stock solution was stored at 4 °C when it was not being used.
2.2 Instrumentation and characterization
The concentration of Mo(VI) ions was determined using inductively coupled plasma-optical emission spectrometry (ICP-OES). Argon was used as a purge gas, and this was purchased from Roham Gas Co. The operating conditions for the ICP-OES are shown in Table 1. Furthermore, the measurements were made in triplicate for statistical purposes. The FT-IR spectra of imprinted and non-imprinted polymer particles were recorded over the frequency range of 400–4000 cm−1 with an FT-IR spectrophotometer (Bruker, VEATOR22 model, USA). A scanning electron microscope (SEM) equipped with an energy dispersive spectrometer (EDS) (Sigma model, Zeiss, Germany) was used to study the surface morphology and element content of the materials. The accelerating voltage was 15 kV. The particle size was determined at 200 nm and 1 μm resolutions with a magnification of 50k×. The crystal structures of the samples were characterized using X-ray diffraction (XRD) on an XPERT-PRO (Panalytical, USA) diffractometer system with Cu Kα radiation (λ = 0.154 nm) at 40 kV and 40 mA. The Brunauer–Emmett–Teller (BET) surface area and pore-size distribution (PSD) of the synthesized samples were obtained from the N2 adsorption–desorption isotherms at liquid N2 temperature on a BELSORP model (MicrotracBel Corp, Japan) analyser. Thermal gravimetric analysis was carried out on a Bahr STA-503 (BAHR, Germany) instrument with a heating rate of 10 °C min−1 under nitrogen flow. Magnetization measurements were performed at room temperature using a vibrating sample magnetometer (MDKFD-Meghnatis Daghigh Kavir Co., Iran).
Table 1 Operating conditions for ICP-OESa
| Parameter |
Setting |
| RFb: radiofrequency. |
| RFb generator power |
1.3 kW |
| Frequency of RFb generator |
40 MHz |
| Mo wavelength |
202.030 nm |
| Auxiliary gas flow rate |
1 L min−1 |
| Sample flow rate |
1.5 mL min−1 |
| Viewing height |
15 mm |
| Nebulizer pressure flow rate |
0.8 L min−1 |
| Plasma gas flow rate |
15 L min−1 |
2.3 Preparation of the adsorbent
2.3.1 Synthesis of Fe3O4 magnetic nanoparticles (MNPs). Fe3O4 magnetic nanoparticles were synthesized via the co-precipitation of ferrous and ferric salts in an alkaline medium.41 50 mL of an aqueous solution containing 10.4 g of FeCl3·6H2O, 4.0 g of FeCl2·4H2O, and 1.7 mL of HCl (12 mol L−1) was dissolved under magnetic stirring in order to prepare a stock solution of ferrous and ferric chloride in a beaker. Then the stock solution was degassed with nitrogen gas for 20 min in order to remove the oxygen. Simultaneously, 500 mL of NaOH solution (1.5 M) was purged with nitrogen gas for 15 min, while the temperature increased to 80 °C in a glass reactor. Then the stock solution was slowly added dropwise to the base of the NaOH solution under nitrogen gas and stirred (1000 rpm) using a glassware stirrer, and then the reaction was maintained for 30 min. During the whole process, the solution temperature was maintained at 80 °C, and the solution was purged with nitrogen gas to remove the dissolved oxygen. After the completion of the reaction, the black precipitate was collected using an external magnetic field, and the supernatant was decanted from the reaction medium. Finally, the obtained Fe3O4 MNPs were washed with doubly distilled water several times and re-suspended in 500 mL of degassed double distilled water, and capped with parafilm.
2.3.2 Synthesis of Fe3O4@SiO2. Silica coating was the next step after synthesizing the iron oxide nanoparticles. TEOS was used for this purpose. 200 mL of synthesized Fe3O4 MNPs dispersed in 600 mL of ethanol was placed into an ultrasonic bath for 10 min to prepare a steady suspension. Then the mixture solution temperature was increased to 80 °C, and in this time 20 mL of ammonium hydroxide (25%) was added to the mixture under vigorous stirring from a glassware stirrer. Ultimately, 10.8 mL of TEOS dissolved in 100 mL of ethanol was added drop by drop to this mixture, and the reaction was continued for 12 h at 80 °C under both reflux and stirring (1200 rpm) from a glassware stirrer. Finally the silica-coated Fe3O4 MNPs were separated using a permanent magnet, washed with ethanol and doubly distilled water several times, and dried in oven at 90 °C for 6 h.
2.3.3 Synthesis of the magnetic Mo(VI) ion imprinted polymer (Mo(VI)-MIIP) and Mo(VI) non-imprinted polymer (Mo(VI)-MNIP). In the first step, a molybdenum complex was prepared with Na2MoO4·2H2O as a source of Mo(VI), sodium thiocyanate (NaSCN) as the ligand, and tetra-n-butylammonium hydroxide as the precipitator in an aqueous solution of 1.25 mol L−1 HCl. The molybdenum concentration in aqueous solution was 0.005 mol L−1. Sodium thiocyanate (NaSCN) was used to form a Mo–SCN complex in the aqueous phase, and tetra-n-butylammonium hydroxide (20%, m/m) was used to form an ion pair between the Mo–SCN inorganic complex as the bulk anion and tetrabutylammonium (NR4+) as the bulk cation. Then the ion pair was precipitated completely. The molar ratio of Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCN
SCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NR4+ was selected as 0.1
NR4+ was selected as 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, according to the literature.38
0.25, according to the literature.38Red crystals of molybdenum were formed after 48 h, and they were dissolved in acetonitrile as solvent on the basis of a stoichiometric ratio to form a metal–monomer complex. For the preparation of the magnetic molybdenum ion imprinted polymer through a typical polymerization reaction, a three-necked glass reactor was equipped with a condenser, a mechanical stirrer, and a gas inlet to maintain an argon atmosphere. 1.0 mmol of the synthesized molybdenum complex and 500 mg of the Fe3O4@SiO2 MNPs were dispersed in 100 mL of acetonitrile as solvent, and the mixture was stirred for 30 min. After this, MAA, EGDMA, and 100 mg of AIBN were added into the resulting mixture, and the solution was purged with argon gas for 10 min. The polymerization was performed at 80 °C under argon gas for 48 h. The molar ratio of MO to MAA to EGDMA was 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3
0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Finally, the synthesized Mo(VI)-MIIPs were separated using an external magnetic field and eluted using a mixture of methanol and double distilled water several times to remove all unreacted monomers and other ingredients. Then the Mo ions were removed successively with 10% (v/v) methanol in HCl (0.5 mol L−1), and this cycle was repeated several times until the Mo ion content was almost non-detectable. Finally, the particles were washed using distilled water and dried in an oven at 70 °C for 3 h. For comparison, magnetic non imprinted polymers (MNIPs) were synthesized simultaneously under the same procedure in the absence of Mo ions. Fig. 1 exhibits a schematic diagram of the preparation procedure of Mo(VI)-MIIPs.
2. Finally, the synthesized Mo(VI)-MIIPs were separated using an external magnetic field and eluted using a mixture of methanol and double distilled water several times to remove all unreacted monomers and other ingredients. Then the Mo ions were removed successively with 10% (v/v) methanol in HCl (0.5 mol L−1), and this cycle was repeated several times until the Mo ion content was almost non-detectable. Finally, the particles were washed using distilled water and dried in an oven at 70 °C for 3 h. For comparison, magnetic non imprinted polymers (MNIPs) were synthesized simultaneously under the same procedure in the absence of Mo ions. Fig. 1 exhibits a schematic diagram of the preparation procedure of Mo(VI)-MIIPs.
 |
| | Fig. 1 Schematic model for the synthesis of magnetic molybdenum ion imprinted polymers. | |
2.4 Static adsorption experiments
The adsorption of Mo(VI) from aqueous solution was investigated in order to determine the optimal values for pH, adsorbent dosage, and contact time. The adsorption experiments were performed at ambient temperature (25 °C) in a batch system. The optimization of these parameters was achieved by varying one parameter while the other parameters were kept constant. After optimization of the parameters, adsorption isotherm, adsorption kinetic, and thermodynamic studies were done. In all experiments, 50 mL of molybdenum solution was poured into Erlenmeyer glass flasks, and the flasks were placed inside a mechanical shaker, where the agitation speed was set to 300 rpm.
Each experiment was carried out two times and the average results are reported. The relative standard deviation values for the results are relatively low, in the order of ±2.5%, which shows the good reproducibility of the experiments. The adsorption capacity and removal efficiency of the MIIPs were calculated from the change in solution concentration using the following equations:42
| |
 | (1) |
| |
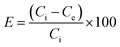 | (2) |
where
q (mg g
−1) and
E (%) represent the adsorption capacity and removal efficiency, respectively,
Ci (mg L
−1) and
Ce (mg L
−1) are the initial and equilibrium metal ion concentrations, respectively,
V (L) is the volume of added solution, and
m (g) is the mass of adsorbent (dry).
2.5 Selectivity experiments
The selectivity of the prepared magnetic ion imprinted polymers towards Mo(VI) was estimated using competitive adsorption studies in the presence of Ni2+, Cd2+, Mn2+, Zn2+ and Cu2+ metallic ions. A binary mixed solution containing 100 mg L−1 of Mo(VI) and a different amount of each metallic ion was prepared. The experiments were performed in batch mode under the optimum conditions. After adsorption equilibrium was reached, the adsorbent was separated via an external magnetic field, and the metals remaining in solution were quantified with ICP-OES. Also the respective magnetic NIP was used for the control experiment.
The distribution coefficient (Kd) and selectivity coefficient (K) of the competitive ions with respect to Mo(VI) were calculated using the following equations:
| |
 | (3) |
| |
 | (4) |
where B represents any other analyte that might have been used as a competitor to the template ion during the experiment. Furthermore, the imprinting effect (
K′) of the MIIP against the MNIP is calculated from the following equation:
| |
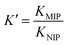 | (5) |
where
KMIP and
KNIP are the selectivity coefficients of the MIP and NIP, respectively.
2.6 Desorption and regeneration studies
For economic reasons, the regeneration of the spent adsorbent is likely to be a key evaluation factor. In order to test the reusability of the synthesized sorbent, several adsorption–desorption cycles were performed using the same magnetic imprinted adsorbent. For this purpose, several solutions were tried for the regeneration of the adsorbent. Among them, an acidified solution (10% (v/v) methanol in 0.5 M HCl) was found effective in desorbing Mo(VI) from the loaded adsorbent. After desorption, the Mo(VI)-MIIPs were washed thoroughly with double distilled water to neutrality and dried in an oven at 50 °C for adsorption in the next cycle.
3. Results and discussion
3.1 Characterization of adsorbent
3.1.1 FT-IR analysis. The FT-IR spectra of Fe3O4@SiO2, Mo(VI)-MNIP, and Mo(VI)-MIIP samples are shown in Fig. 2. In Fig. 2a, the peak at 586 cm−1 can be assigned to the Fe–O bands from the magnetite phase. The strong peak at 1097 cm−1 can be attributed to Si–O–Si bonds, which indicates the formation of a silica layer on the magnetite. Also the peaks at 468 and 802 cm−1 were attributed to Si–O and Si–O–H bonds respectively. In Fig. 2c the typical characteristic peak of Si–O–Si at 1097 cm−1 from Fe3O4@SiO2 (Fig. 2a) was shifted to a higher wave number of 1150 cm−1. The new strong peak at 1728 cm−1 indicated carbonyl groups, and corresponded to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of the polymer. It was shown that the oxygen of the C
O stretching vibrations of the polymer. It was shown that the oxygen of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group is involved in Mo interactions. The peak at 2952 cm−1 indicated the methylene groups of the polymer, and corresponded to the C–H aromatic stretching vibrations, which can be identified as the characteristic bands of EGDMA. The appearances of these bands confirmed that polymerization was successful. Also, the absence of bands in the region of 1638–1648 cm−1 indicates the absence of vinyl groups in the polymer particles. This observation could be possibly attributed to the polymerization of both MAA and EGDMA. Moreover, the peaks at 1108, 2042, and 2354 cm−1 were assigned to the C–N and C
O group is involved in Mo interactions. The peak at 2952 cm−1 indicated the methylene groups of the polymer, and corresponded to the C–H aromatic stretching vibrations, which can be identified as the characteristic bands of EGDMA. The appearances of these bands confirmed that polymerization was successful. Also, the absence of bands in the region of 1638–1648 cm−1 indicates the absence of vinyl groups in the polymer particles. This observation could be possibly attributed to the polymerization of both MAA and EGDMA. Moreover, the peaks at 1108, 2042, and 2354 cm−1 were assigned to the C–N and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups in TBAOH and SCN, respectively. Ultimately, in all spectra, the broad peak in the 3300–3500 cm−1 range indicated OH stretching vibrations in the structure of the adsorbent.
N groups in TBAOH and SCN, respectively. Ultimately, in all spectra, the broad peak in the 3300–3500 cm−1 range indicated OH stretching vibrations in the structure of the adsorbent.
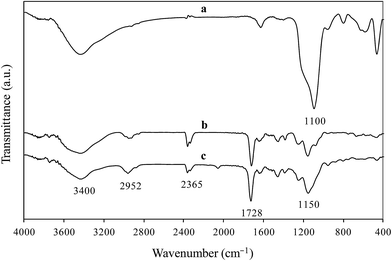 |
| | Fig. 2 IR spectra of Fe3O4@SiO2 (a), Mo(VI)-MNIP (b), and Mo(VI)-MIIP (c). | |
3.1.2 SEM and EDAX analysis. In order to investigate the morphology and size of the synthesized sorbent, SEM micrographs of the material were recorded, and the results are shown in Fig. 3. Fig. 3a shows that the Fe3O4@SiO2 nanoparticles were spherical in shape, with an average diameter of 70 nm. As shown in this figure, these nanoparticles were fully coated in a silica layer. From the SEM micrograph of Mo(VI)-MIIP (Fig. 3b), it seems that the beads are not isolated but connected to one another. The SEM micrograph also shows some agglomerates, suggesting the formation of an imprinting layer. In comparison with Fe3O4@SiO2, the particles of Mo(VI)-MIIP were porous and gathered together, which was attributed to successful polymerization. The micrograph shown in Fig. 3c illustrates that the porosity changed after the removal of the molybdenum ions. In addition, it is clear that the pore size in the leached polymers was in the nano-range, corresponding to the ion size.
 |
| | Fig. 3 SEM images of Fe3O4@SiO2 (a), Mo(VI)-MIIP (unleached) (b), and Mo(VI)-MIIP (leached) (c). | |
EDS analysis of Fe3O4@SiO2, Mo(VI)-MIIP (unleached) and Mo(VI)-MIIP (leached) was carried out, and the related results of the elemental analysis are shown in Fig. 4. The signals for carbon, oxygen, iron, and silica appeared for the Fe3O4@SiO2 nanoparticles (Fig. 4a). In Fig. 4b, the main elements were Fe, Si, O, C, N, and Mo. The presence of an Fe band in the EDS spectra of Mo(VI)-MIIP confirms the incorporation of the magnetic core within the polymer matrix. Higher percentages of Si and C, and a lower percentage of Fe in the pattern of Fig. 4b confirmed the successfully synthesis of magnetic IIPs. Also, a comparison of the EDS analysis for the unleached and leached magnetic polymer particles confirmed the absence of molybdenum in the EDS spectrum of the leached polymer particles, illustrating the complete removal of molybdenum from the polymer matrix in the leaching step.
 |
| | Fig. 4 EDS spectra of Fe3O4@SiO2 (a), Mo(VI)-MIIP (unleached) (b), and Mo(VI)-MIIP (leached) (c). | |
3.1.3 XRD analysis. The X-ray powder diffraction spectra of Fe3O4@SiO2 and Mo(VI)-MIIP are shown in Fig. 5. As can be seen in Fig. 5a, five characteristic peaks at 2θ = 29, 35, 42, 53, 57, and 65°, which are attributed to d220, d311, d400, d422, d511, and d440, were observed for Fe3O4@SiO2, confirming the presence of Fe3O4 nanoparticles in this composite. In Fig. 5b, a characteristic diffraction peak was observed at 2θ = 35°, the other peaks indexed to 29, 42, 53, 57, and 65° were not clear, and the intensity of these peaks was decreased. This result demonstrated that the Fe3O4 nanoparticles were indeed incorporated into the IIPs, and the crystalline structure of the Fe3O4 nanoparticles essentially remained stable during the polymerization process.
 |
| | Fig. 5 X-ray diffraction patterns of Fe3O4@SiO2 (a) and Mo(VI)-MIIP (b). | |
3.1.4 BET surface area and average pore diameter analysis. The specific surface area, total pore volume, and average pore diameter for the unleached magnetic Mo(VI) ion imprinted polymer and leached magnetic Mo(VI) ion imprinted polymer are listed in Table 2. According to IUPAC recommendations, total porosity can be classified into three groups, according to diameter (d). The three groups are macroporous (d > 50 nm), mesoporous (2 < d < 50 nm), and microporous (d < 2 nm). Based on Table 2, it can be concluded that the synthesized sorbents are mesoporous, which is suitable for metal ion binding. It is clearly seen that the specific surface area and average pore diameter of the magnetic polymers increased after the removal of the molybdenum ions from the adsorbent. This may be due to the formation of larger pores during the Mo(VI)-imprinting process.
Table 2 Physical properties of the magnetic polymers
| Magnetic polymer |
Specific surface area (m2 g−1) |
Total pore volume (cm3 g−1) |
Average pore diameter (nm) |
| Unleached |
23.22 |
0.1735 |
29.88 |
| Leached |
37.08 |
0.2305 |
24.81 |
3.1.5 Thermo-gravimetric analysis (TGA). The thermal stabilities of Fe3O4@SiO2 and Mo(VI)-MIIP were investigated using TGA analysis, and the results are shown in Fig. 6. From Fig. 6a, we can see that the Fe3O4@SiO2 has good thermal stability. From room temperature to 800 °C, there was only a little weight loss for Fe3O4@SiO2, which was related to the dehydration of the organic solvent or water in the SiO2 layer. The TGA curves show that the decreased weight, as a result of solvent or water, was about 8%. In Fig. 6b, we can see that the TGA curve was stable up to 200 °C. The rapid weight loss occurred over the temperature ranging from 300 °C to 500 °C, which was due to the loss of the polymer layer. The weight loss in Mo(VI)-MIIP was approximately 82%. Hence, the results completely demonstrated the formation of an imprinted polymer.
 |
| | Fig. 6 TGA curves of Fe3O4@SiO2 (a) and Mo(VI)-MIIP (b). | |
3.1.6 Magnetism analysis (VSM). The magnetic hysteresis loops for Fe3O4@SiO2 and Mo(VI)-MIIP are shown in Fig. 7. The saturation magnetization values obtained at room temperature were about 10 and 1 emu g−1. The decrease in magnetization value can be attributed to the existence of the polymer on the surface of the Fe3O4@SiO2 nanoparticles. Also, it was apparent that the magnetic hysteresis loops, which consist of coercive forces and remnant magnetization, were relatively low. It is indicated that the two samples were superparamagnetic and could be gathered thoroughly in a very short time when an external magnetic field was applied.
 |
| | Fig. 7 Magnetization curves obtained using VSM at room temperature for Fe3O4@SiO2 (a) and Mo(VI)-MIIP (b). | |
3.2 Optimization for maximum adsorption
3.2.1 Effect of HCl concentration. The effect of HCl concentration was investigated on the adsorption of molybdenum ions using Mo(VI)-MIIP, and the results are shown in Fig. 8. For this purpose, we investigated the effect of HCl concentration over the range from 0.1–4.0 mol L−1. The obtained results indicate that the adsorption capacity increased with increasing HCl concentration from 0.1 to 3.0 mol L−1, and then slightly decreased. This is attributed to the influence of solution pH on the solution chemistry of metals and the activity of the functional groups on the adsorbent. Therefore, maintaining the solution acidity with 3.0 mol L−1 HCl can help attain good adsorption results, and all further experiments were carried out under these conditions.
 |
| | Fig. 8 The effect of HCl concentration on the adsorption of molybdenum(VI) on Mo(VI)-MIIP (initial concentration = 20 mg L−1, mass of adsorbent = 1.5 g L−1, time = 120 min, and T = 25 °C). | |
3.2.2 Effect of the amount of adsorbent. The adsorbent dose is a parameter which greatly affects the adsorption capacity and determines the equilibrium state of the adsorption system. The dependence of Mo(VI) adsorption on the amount of adsorbent was studied through varying the amount of adsorbent while keeping the other parameters constant. The amount of adsorbent was varied between 0.5 and 2.5 g L−1, and the results are shown in Fig. 9. The obtained results indicate that by increasing the amount of adsorbent, the adsorption percentage of molybdenum was increased. This was attributed to an increase in surface area and the availability of active sites for Mo(VI) ions. Besides, at a constant concentration, increasing the adsorbent led to more unsaturated sites, which can decrease the adsorption capacity per unit weight of adsorbent. Hence, the optimum amount of magnetic polymer was chosen as 1.0 g L−1.
 |
| | Fig. 9 The effect of adsorbent dose on the adsorption of molybdenum(VI) on Mo(VI)-MIIP (initial concentration = 20 mg L−1 (prepared in 3 mol L−1 HCl), time = 120 min, and T = 25 °C). | |
3.3 Adsorption kinetics
The kinetics of adsorption are vital because they control the process efficiency. So adsorption kinetics experiments for Mo(VI) on Mo(VI)-MIIP were investigated under optimum conditions at an initial concentration of 20 mg L−1 of molybdenum, and the results are shown in Fig. 10. As can be seen in Fig. 10, the initial adsorption rate was very fast and reached equilibrium after 10 min. There was no obvious change from 10 to 30 min. The rapid adsorption in the beginning can be attributed to the availability of a greater number of active sites and small mass transfer resistance on the surface of the adsorbent. The small mass transfer resistance causes the templates to enter the cavities easily and bind to the recognition sites. In other words, the faster adsorption rate compared with other adsorbents reported elsewhere could be attributed to the absence of internal diffusion resistance.
 |
| | Fig. 10 The effect of contact time on the adsorption of molybdenum(VI) on Mo(VI)-MIIP (initial concentration = 20 mg L−1 (prepared in 3 mol L−1 HCl), mass of adsorbent = 1 g L−1, and T = 25 °C). | |
In order to investigate the controlling mechanisms of the adsorption process, such as mass transfer and chemical reactions, the kinetic data obtained from batch experiments were analyzed using two common semi-empirical kinetic models: the pseudo-first-order equation (eqn (6)) proposed by Lagergren,43 and the pseudo-second-order equation (eqn (7)) proposed by Ho and Mckay:44
| |
 | (7) |
In the equations above, qe and qt (mg g−1) represent the adsorbed amount of molybdenum at equilibrium and at time (t), respectively. Also, k1 (min−1) and k2 (g mg−1 min−1) are the pseudo-first-order and pseudo-second-order adsorption rate constants, respectively.
Supposing that the adsorption capacity of an adsorbent is proportional to the number of active sites on its surface, the pseudo-second-order kinetics model is better than the pseudo-first-order kinetics model. The kinetics modeling for the adsorption of molybdenum onto Mo(VI)-MIIP is shown in Fig. 11. Furthermore, the adsorption kinetics constants, determination coefficient values (R2), and root mean square errors (RMSE) from the two rate equations are summarized in Table 3. It can be seen that the kinetics data was better fitted to a pseudo-second-order equation than to a pseudo-first-order equation, according to the correlation coefficient (R2). A relatively high R2 value demonstrated the fact that the model can successfully describe the kinetics of Mo(VI) adsorption. Also the results indicate that the adsorption process of molybdenum on Mo(VI)-MIIP was controlled through a chemisorption mechanism, through either sharing or exchange of electrons between the adsorbent surface and the adsorbate.
 |
| | Fig. 11 Kinetic models for the adsorption of molybdenum(VI) on Mo(VI)-MIIP: pseudo-first-order model (a), and pseudo-second-order model (b). | |
Table 3 Kinetic parameters for the pseudo-first-order and pseudo-second-order rate equations for Mo(VI) adsorption on the Mo(VI)-MIIP adsorbent
| Pseudo-first-order |
Pseudo-second-order |
| Ci (mg L−1) |
k1 (min−1) |
qe (mg g−1) |
R2 |
RMSE |
k2 (g mg−1 min−1) |
qe (mg g−1) |
R2 |
RMSE |
| 20 |
0.1551 |
6.662 |
0.9950 |
0.0569 |
0.0237 |
7.7011 |
0.9971 |
0.0437 |
3.4 Adsorption isotherms
In order to optimize the design of an adsorption system, it is important to study the interactive behavior between the solution and adsorbent. For this purpose, the equilibrium adsorption isotherm was investigated. Adsorption isotherms can explain how the adsorbate is distributed on the adsorbent, and this was tested at a given temperature with different initial concentrations of molybdenum. The adsorption experiments were performed by varying the initial concentration of molybdenum at three temperatures, 25 °C, 35 °C, and 45 °C. The results of the tests are shown in Fig. 12. It is clearly demonstrated that the adsorption capacity of the MIIP increased when the initial concentration of Mo(VI) increased. As the initial concentration increased to 150 mg L−1, the maximum adsorption capacity was obtained. It was observed that the maximum adsorption capacity was 28 mg g−1 for Mo(VI)-MIIP.
 |
| | Fig. 12 Adsorption isotherms for molybdenum(VI) on Mo(VI)-MIIP (solutions prepared in 3 mol L−1 HCl, T = 25, 35 and 45 °C, and mass of adsorbent = 1 g L−1). | |
In order to design and establish the adsorption system, two isotherm models were used to analyze the equilibrium values, the Langmuir (eqn (8)) and Freundlich (eqn (9)) models. The Langmuir model assumes that adsorption takes place on surfaces that are energetically homogeneous and distant to each other, and there is no interaction between neighboring adsorbed ions on the surface of the adsorbent. This isotherm shows a limiting sorption capacity.45 Unlike the Langmuir model, the Freundlich model assumes that adsorption occurs on a heterogeneous surface.46
The Langmuir and Freundlich isotherms are described using the following equations:
| |
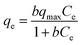 | (8) |
where
Ce (mg L
−1) and
qe (mg g
−1) are the concentration and adsorbed amount of Mo(
VI) at adsorption equilibrium, respectively,
b (L mg
−1) is the Langmuir constant,
Kf ((mg g
−1) (L mg
−1)
1/n) is the Freundlich constant,
n is the Freundlich exponent, and
qmax (mg g
−1) is the maximum adsorption capacity. The isotherm parameters calculated from
eqn (8) and
(9) are listed in
Table 4. Also, the equilibrium isotherms for the adsorption of Mo(
VI) onto Mo(
VI)-MIIP at various temperatures (25, 35, and 45 °C) are depicted in
Fig. 13.
Table 4 Langmuir and Freundlich isotherm constants for molybdenum(VI) adsorption onto the Mo(VI)-MIIP adsorbent
| Temperature (°C) |
Langmuir isotherm model |
Freundlich isotherm model |
| qmax (mg g−1) |
b (L mg−1) |
R2 |
RMSE |
n |
Kf (mg g−1) (L mg−1)1/n |
R2 |
RMSE |
| 25 |
31.08 |
0.0547 |
0.9914 |
0.2836 |
3.387 |
6.198 |
0.9045 |
0.9439 |
| 35 |
24.49 |
0.0452 |
0.993 |
0.1975 |
3.202 |
4.354 |
0.9145 |
0.6896 |
| 45 |
21.28 |
0.0415 |
0.9951 |
0.1409 |
3.145 |
3.623 |
0.9222 |
0.5635 |
 |
| | Fig. 13 Isotherm models for the adsorption of molybdenum(VI) on Mo(VI)-MIIP at different temperatures: Langmuir (a) and Freundlich (b). | |
The results show that at all tested temperatures, the Langmuir isotherm model is in better agreement with the experimental data than the Freundlich isotherm model, in terms of a higher correlation coefficient (R2). This was due to the existence of homogeneous cavities on the surface of the adsorbent. Also, the value of qmax, which was obtained from the Langmuir curve, was very close to the experimental value. In other words, the difference in maximum adsorption capacity between experiment (28 mg g−1) and calculation (31.08 mg g−1) was quite small.
The essential characteristic parameters of the Langmuir isotherm model are defined using the following equation:
| |
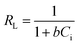 | (10) |
where
Ci is the initial molybdenum concentration (mg L
−1) and
b is the Langmuir constant (L mg
−1). The value of the separation factor constant (
RL) indicates the favorability and possibility of the adsorption process occurring. Favorable adsorption will occur when the
RL value is in the range of 0–1.
47 The values of
RL are shown in
Table 5. In our study, at all temperatures
RL was found to be in the range of 0–1, which indicated the favorable adsorption of molybdenum on the Mo(
VI)-MIIP adsorbent.
Table 5 Values of RL at three different temperatures
| Temperature (°C) |
Ci (mg L−1) |
RL |
| 25 |
10–150 |
0.76–0.06 |
| 35 |
10–150 |
0.79–0.07 |
| 45 |
10–150 |
0.81–0.08 |
3.5 Evaluation of selective adsorption
In order to investigate the selectivity of the synthesized sorbent toward the Mo(VI) ion, the effects of interfering ions on the separation of Mo(VI) ions were investigated. The data for distribution coefficients and selectivity coefficients are summarized in Table 6. As can be seen in Table 6, Mo(VI)-MIIP had a much higher selectivity for Mo(VI) in the presence of competitors. This is attributed to the specific recognition cavities for Mo(VI) ions created in Mo(VI)-MIIP, which were developed using ion imprinting. These imprinting cavities are complementary to the Mo(VI) ions in size, shape, and coordination geometry, and have a strong ability to selectively adsorb MoO22+ very well, even in the presence of Ni2+, Cd2+, Mn2+, Zn2+ and Cu2+ interference to a quite high extent.
Table 6 Distribution coefficient and selectivity coefficient data for Mo(VI)-MIIP and Mo(VI)-MNIP
| Metal ions |
Imprinted adsorbents |
Non-imprinted adsorbents |
K′ |
| q (mg g−1) |
Kd |
K |
q (mg g−1) |
Kd |
K |
| Mo(VI) |
20.56 |
0.335 |
19.25 |
10.35 |
0.145 |
1.91 |
10.08 |
| Ni(II) |
1.72 |
0.017 |
7.06 |
0.076 |
| Mo(VI) |
19.8 |
0.32 |
10.67 |
9.36 |
0.129 |
1.5 |
7.11 |
| Cd(II) |
2.87 |
0.03 |
7.92 |
0.086 |
| Mo(VI) |
16.39 |
0.25 |
12.5 |
7.45 |
0.1 |
2.44 |
5.12 |
| Mn(II) |
1.93 |
0.02 |
3.88 |
0.041 |
| Mo(VI) |
12.9 |
0.187 |
22.61 |
5.4 |
0.07 |
2 |
11.31 |
| Zn(II) |
0.8 |
0.008 |
3.3 |
0.035 |
| Mo(VI) |
14.7 |
0.218 |
23.9 |
6.9 |
0.092 |
3.03 |
7.88 |
| Cu(II) |
0.9 |
0.009 |
2.9 |
0.03 |
3.6 Adsorption thermodynamics
In environmental engineering, both Gibbs free energy and entropy parameters should be considered in order to determine what processes will occur spontaneously. The thermodynamic parameters, including Gibbs free energy (ΔG), enthalpy (ΔH), and entropy (ΔS) were calculated using the following equations:| |
 | (11) |
| |
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc Kc
| (12) |
| |
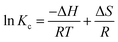 | (13) |
where Ce is the equilibrium concentration in solution (mg L−1), Kc (mL g−1) is the distribution coefficient at each temperature, R is the universal gas constant (8.314 J mol K−1), and T is the adsorption absolute temperature (K).
The adsorption experiments were carried out at an initial concentration of 100 mg L−1 molybdenum and at three temperatures (25 °C, 35 °C and 45 °C), and the results are shown in Fig. 14. The values of ΔH and ΔS can be calculated from the slope and intercept of the straight line obtained from the thermodynamic plot, respectively. The obtained thermodynamic parameters for molybdenum are listed in Table 7. The negative values of ΔG verify that Mo(VI) adsorption was spontaneous and thermodynamically favorable. The negative value of enthalpy proves that adsorption was exothermic. Also, the negative value of entropy can be explained through the decreased degree of randomness during the adsorption process, which might be ascribed to the stable structure formed by the combination of molybdenum ions and binding sites from the magnetic polymer.
 |
| | Fig. 14 Linear plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc versus 1/T for the adsorption of molybdenum(VI) on Mo(VI)-MIIP. Kc versus 1/T for the adsorption of molybdenum(VI) on Mo(VI)-MIIP. | |
Table 7 Thermodynamic parameters for molybdenum(VI) adsorption on Mo(VI)-MIIP at Ci = 100 mg L−1
| Sorbent |
T (°C) |
Kc (mL g−1) |
ΔG (kJ mol−1) |
ΔH (kJ mol−1) |
ΔS (J mol−1 K−1) |
| Mo(VI)-MIIP |
25 |
433.99 |
−15.05 |
−22.95 |
−26.57 |
| 35 |
304.15 |
−14.65 |
| 45 |
244.68 |
−14.55 |
3.7 Desorption and repeated use
The regeneration of a sorbent is very important for improving adsorption process economics. Adsorption–desorption cycles with Mo(VI) ions and the adsorbent were repeated five times, using the same adsorbent in a batch experimental set-up, and the desorption time was found to be 30 min. The adsorption results for the reutilization experiments are shown in Fig. 15. The results show that Mo(VI)-MIIP was stable and reusable for adsorption, and can be used repeatedly at least 5 times without any significant loss of adsorption capacity.
 |
| | Fig. 15 Influence of regeneration cycles on molybdenum(VI) adsorption on Mo(VI)-MIIP (initial concentration = 150 mg L−1 (prepared in 3 mol L−1 HCl), mass of adsorbent = 1 g L−1, T = 25 °C). | |
4. Conclusions
In this study, a novel magnetic Mo(VI) ion imprinted polymer was prepared using precipitation polymerization and applied to the selective adsorption of molybdenum from aqueous solution. The combination of imprinting technology with magnetic separating techniques can produce a novel adsorbent with unique characteristics, such as high selectivity and magnetic properties. Thus, the synthesized adsorbent exhibited excellent specific recognition and saturation magnetization, and allowed magnetic separation to replace the centrifugation and filtration steps in a convenient and economical way. Also the synthesized adsorbent exhibited excellent characteristics such as high thermal stability, fast adsorption kinetics, suitable reusability, and strong acid resistance. All adsorption experiments were carried out in batch sorption mode. For molybdenum adsorbed on the MIIP, the pseudo-second-order kinetics model provided the best description of the adsorption process. Equilibrium experiments fitted well with the Langmuir isotherm model, which means that the adsorption process involved monolayer adsorption on the surface of the imprinted polymer. The maximum sorption calculated from the Langmuir isotherm was 31.08 mg g−1 at 25 °C. The results indicated that Mo(VI)-MIIP had much higher selectivity for Mo(VI) in the presence of competitors. Thermodynamic calculations indicated that the adsorption process was spontaneous and exothermic. They also revealed that Mo(VI)-MIIP could be regenerated for repeated use without decreasing its adsorption capacity significantly. From the results obtained in this study, it can be concluded that this new magnetic ion-imprinted polymer is a promising candidate for the selective adsorption of molybdenum ion from aqueous solution.
Acknowledgements
This work was financially supported by the Iranian Nanotechnology Initiative Council.
References
- L. Zeng and C. Y. Cheng, A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurization catalysts, Part 1: metallurgical processes, Hydrometallurgy, 2009, 98, 1–9 CrossRef CAS.
- Y. Xiong, H. T. Wang, Z. N. Lou, W. J. Shan, Z. Q. Xing, G. C. Deng, D. D. Wu, D. W. Fang and B. K. Biswas, Selective adsorption of molybdenum(VI) from Mo–Re bearing effluent by chemically modified astringent persimmon, J. Hazard. Mater., 2011, 186, 1855–1861 CrossRef CAS PubMed.
- J. P. Gustafsson, Modelling molybdate and tungstate adsorption to ferrihydrite, Chem. Geol., 2003, 200, 105–115 CrossRef CAS.
- J. X. Du, J. J Li, L. J. Yang and J. R. Lu, Sensitive and selective determination of molybdenum by flow injection chemiluminescence method combined with controlled potential electrolysis technique, Anal. Chim. Acta, 2003, 481, 239–244 CrossRef CAS.
- D. Wang, R. C. Aller and S. A. Sanudo-Wilhelmyc, A new method for the quantification of different redox-species of molybdenum(V and VI) in seawater, Mar. Chem., 2009, 113, 250–256 CrossRef CAS.
- C. Namasivayam and D. Sangeetha, Removal of molybdate from water by adsorption onto ZnCl2 activated coir pith carbon, Bioresour. Technol., 2006, 97, 1194–1200 CrossRef CAS PubMed.
- A. A. Atia, Adsorption of chromate and molybdate by cetylpyridinium bentonite, Appl. Clay Sci., 2008, 41, 73–84 CrossRef CAS.
- A. A. Atia, A. M. Donia and H. A. Awed, Synthesis of magnetic chelating resins functionalized with tetraethylenepentamine for adsorption of molybdate anions from aqueous solutions, J. Hazard. Mater., 2008, 155, 100–108 CrossRef CAS PubMed.
- C. Ardau, F. Frau, E. Dore and P. Lattanzi, Molybdate sorption by Zn–Al sulphate layered double hydroxides, Appl. Clay Sci., 2012, 65–66, 128–133 CrossRef CAS.
- Z. Djedidi, M. Bouda, M. A. Souissi, R. Ben Cheikh, G. Mercier, R. D. Tyagi and J. F. Blais, Metals removal from soil, fly ash and sewage sludge leachates by precipitation and dewatering properties of the generated sludge, J. Hazard. Mater., 2009, 172, 1372–1382 CrossRef CAS PubMed.
- U. Domanska and A. Rekawek, Extraction of metal ions from aqueous solutions using imidazolium based ionic liquids, J. Solution Chem., 2009, 38, 739–751 CrossRef CAS.
- C. Cojocaru, G. Zakrzewska-Trznadel and A. Jaworska, Removal of cobalt ions from aqueous solutions by polymer assisted ultrafiltration using experimental design approach. Part 1: optimization of complexation conditions, J. Hazard. Mater., 2009, 169, 599–609 CrossRef CAS PubMed.
- L. Rafati, A. H. Mahvi, A. R. Asgari and S. S. Hosseini, Removal of chromium(VI) from aqueous solutions using Lewatit FO36 nano ion exchange resin, Int. J. Environ. Sci. Technol., 2010, 7, 147–156 CrossRef CAS.
- J. Q. Pan, C. Zhang, Y. Z. Sun, Z. H. Wang and Y. S. Yang, A new process of lead recovery from waste lead-acid batteries by electrolysis of alkaline lead oxide solution, Electrochem. Commun., 2012, 19, 70–72 CrossRef CAS.
- M. Soylak and R. S. Cay, Separation/preconcentration of silver(I) and lead(II) in environmental samples on cellulose nitrate membrane filter prior to their flame atomic absorption spectrometric determinations, J. Hazard. Mater., 2007, 146, 142–147 CrossRef CAS PubMed.
- J. E. Morgan and A. J. Morgan, Earthworms as biological monitors of cadmium, copper, lead and zinc in metalliferous soils, Environ. Pollut., 1988, 54, 123–138 CrossRef CAS PubMed.
- W. L. Yan and R. Bai, Adsorption of lead and humic acid on chitosan hydrogel beads, Water Res., 2005, 39, 688–698 CrossRef CAS PubMed.
- B. C. Bostick, S. Fendorf and G. R. Helz, Differential adsorption of molybdate and tetrathiomolybdate on pyrite (FeS2), Environ. Sci. Technol., 2003, 37, 285–291 CrossRef CAS PubMed.
- N. Xu, C. Christodoulatos and W. Braida, Adsorption of molybdate and tetrathiomolybdate onto pyrite and goethite: effect of pH and competitive anions, Chemosphere, 2006, 62, 1726–1735 CrossRef CAS PubMed.
- A. A. Atia, A. M. Donia and K. Z. Elwakeel, Adsorption behavior of non-transition metal ions on a synthetic chelating resin bearing iminoacetate functions, Sep. Purif. Technol., 2005, 43, 43–48 CrossRef CAS.
- A. Moret and J. Rubio, Sulphate and molybdate ions uptake by chitin-based shrimp shells, Miner. Eng., 2003, 16, 715–722 CrossRef CAS.
- C. Namasivayam and D. Sangeetha, Removal of molybdate from water by adsorption onto ZnCl2 activated coir pith carbon, Bioresour. Technol., 2006, 97, 1194–1200 CrossRef CAS PubMed.
- E. Birlik, S. Buyuktiryaki, A. Ersoz, R. Say and A. Denizli, Selective separation of thorium using ion imprinted chitosan-phthalate particles via solid phase extraction, Sep. Sci. Technol., 2006, 41, 3109–3121 CrossRef CAS.
- B. J. Gao, J. Wang, F. Q. An and Q. Liu, Molecular imprinted material prepared by novel surface imprinting technique for selective adsorption of pirimicarb, Polymer, 2008, 49, 1230–1238 CrossRef CAS.
- H. Ebrahimzadeh, E. Moazzen, M. M. Amini and O. Sadeghi, Novel ion imprinted polymer coated multiwalled carbon nanotubes as a high selective sorbent for determination of gold ions in environmental samples, Chem. Eng. J., 2013, 315, 215–216 Search PubMed.
- Y. Liu, Z. Liu, J. Gao, J. Dai, J. Han, Y. Wang, J. Xie and Y. Yan, Selective adsorption behavior of Pb(II) by mesoporous silica SBA-15-supported Pb(II)-imprinted polymer based on surface molecularly imprinting technique, J. Hazard. Mater., 2011, 186, 197–205 CrossRef CAS PubMed.
- F. Q. An, B. J. Gao and X. Q. Feng, Adsorption and recognizing ability of molecular imprinted polymer MIP-PEI/SiO2 towards phenol, J. Hazard. Mater., 2008, 157, 286–292 CrossRef CAS PubMed.
- Y. M. Ren, X. Z. Wei and M. L. Zhang, Adsorption character for removal Cu(II) by magnetic Cu(II) ion imprinted composite adsorbent, J. Hazard. Mater., 2008, 158, 14–22 CrossRef CAS PubMed.
- M. L. Zhang, Z. H. Zhang, Y. N. Liu, X. Yang, L. J. Luo, J. T. Chen and S. Z. Yao, Preparation of core–shell magnetic ion-imprinted polymer for selective extraction of Pb(II) from environmental samples, Chem. Eng. J., 2011, 178, 443–450 CrossRef CAS.
- S. Sadeghi and E. Aboobakri, Magnetic nanoparticles with an imprinted polymer coating for the selective extraction of uranyl ions, Microchim. Acta, 2012, 178, 89–97 CrossRef CAS.
- Y. Cui, J. Q. Liu, Z. J. Hu, X. W. Xua and H. W. Gaoa, Well-defined surface ion-imprinted magnetic microspheres for facile onsite monitoring of lead ions at trace level in water, Anal. Methods, 2012, 4, 3095–3097 RSC.
- F. Aboufazeli, H. R. L. Z. Zhad, O. Sadeghi, M. Karimi and E. Najafi, Novel ion imprinted polymer magnetic mesoporous silica nano-particles for selective separation and determination of lead ions in food samples, Food Chem., 2013, 141, 3459–3465 CrossRef CAS PubMed.
- Y. Hiratsuka, N. Funaya, H. Matsunaga and J. Haginaka, Preparation of magnetic molecularly imprinted polymers for bisphenol a and its analogues and their application to the assay of bisphenol a in river water, J. Pharm. Biomed. Anal., 2013, 75, 180–185 CrossRef CAS PubMed.
- A. Mehdinia, T. B. Kayyal, A. Jabbari, M. O. Aziz-Zanjani and E. Ziaei, Magnetic molecularly imprinted nanoparticles based on grafting polymerization for selective detection of 4-nitrophenol in aqueous samples, J. Chromatogr. A, 2013, 1283, 82–88 CrossRef CAS PubMed.
- A. Bagheri, M. Behbahani, M. M. Amini, O. Sadeghi, A. Tootoonchi and Z. Dahaghin, Preconcentration and separation of ultra-trace palladium ion using pyridine-functionalized magnetic nanoparticles, Microchim. Acta, 2012, 178, 261–268 CrossRef CAS.
- M. Liu, C. Chen, T. Wen and X. Wang, Synthesis of magnetic ion-imprinted composites and selective separation and preconcentration of U(VI), Dalton Trans., 2014, 43, 7050–7056 RSC.
- C. L. Chen, X. K. Wang and M. Nagatsu, Europium adsorption on multiwall carbon nanotube/iron oxide
magnetic composite in the presence of polyacrylic acid, Environ. Sci. Technol., 2009, 43, 2362–2367 CrossRef CAS PubMed.
- F. Ardestani, M. H. Hosseini, M. Taghizadeh, M. R. Pourjavid and M. Rezaee, Synthesis and characterization of nanopore MoVI-imprinted polymer and its application as solid phase for extraction, separation and preconcentration of molybdenum ions from water samples, J. Braz. Chem. Soc., 2016, 27, 1279–1289 Search PubMed.
- B. Gao, X. Li, T. Chen and L. Fang, Preparation of molybdate anion surface-imprinted material for selective removal of molybdate anion from water medium, Ind. Eng. Chem. Res., 2014, 53, 4469–4479 CrossRef CAS.
- Y. Ren, P. Liu, J. Feng, J. Ma, Q. Wen and M. Zhang, Selective recognition of molybdenum(VI) from water by Mo(VI) oxy ion-imprinted particle as an adsorbent, Chem. Eng. J., 2013, 219, 286–294 CrossRef CAS.
- M. Faraji, Y. Yamini, E. Tahmasbi, A. Saleh and F. Nourmohammadian, Cetyltrimethylammonium bromide-coated magnetite nanoparticles as highly efficient adsorbent for rapid removal of reactive dyes from the textile companies wastewaters, J. Iran. Chem. Soc., 2010, 7, 130–144 CrossRef.
- Y. Prasanna Kumar, P. King and V. S. R. K. Prasad, Adsorption of zinc from aqueous solution using marine green algae—Ulva fasciata sp., Chem. Eng. J., 2007, 129, 161–166 CrossRef.
- S. Azizian, Kinetic models of sorption: a theoretical analysis, J. Colloid Interface Sci., 2004, 276, 47–52 CrossRef CAS PubMed.
- Y. S. Ho and G. McKay, Sorption of dye from aqueous solution by peat, Chem. Eng. J., 1998, 70, 115–124 CrossRef CAS.
- I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- H. M. F. Freundlich, Uber die adsorption in losungen, Z. Phys. Chem., 1906, 57, 385–470 CAS.
- T. W. Webi and R. K. Chakravort, Pore and solid diffusion models for fixed-bed adsorbers, AIChE J., 1974, 20, 228–238 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCN
SCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NR4+ was selected as 0.1
NR4+ was selected as 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, according to the literature.38
0.25, according to the literature.38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3
0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Finally, the synthesized Mo(VI)-MIIPs were separated using an external magnetic field and eluted using a mixture of methanol and double distilled water several times to remove all unreacted monomers and other ingredients. Then the Mo ions were removed successively with 10% (v/v) methanol in HCl (0.5 mol L−1), and this cycle was repeated several times until the Mo ion content was almost non-detectable. Finally, the particles were washed using distilled water and dried in an oven at 70 °C for 3 h. For comparison, magnetic non imprinted polymers (MNIPs) were synthesized simultaneously under the same procedure in the absence of Mo ions. Fig. 1 exhibits a schematic diagram of the preparation procedure of Mo(VI)-MIIPs.
2. Finally, the synthesized Mo(VI)-MIIPs were separated using an external magnetic field and eluted using a mixture of methanol and double distilled water several times to remove all unreacted monomers and other ingredients. Then the Mo ions were removed successively with 10% (v/v) methanol in HCl (0.5 mol L−1), and this cycle was repeated several times until the Mo ion content was almost non-detectable. Finally, the particles were washed using distilled water and dried in an oven at 70 °C for 3 h. For comparison, magnetic non imprinted polymers (MNIPs) were synthesized simultaneously under the same procedure in the absence of Mo ions. Fig. 1 exhibits a schematic diagram of the preparation procedure of Mo(VI)-MIIPs.
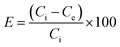


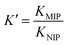
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of the polymer. It was shown that the oxygen of the C
O stretching vibrations of the polymer. It was shown that the oxygen of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group is involved in Mo interactions. The peak at 2952 cm−1 indicated the methylene groups of the polymer, and corresponded to the C–H aromatic stretching vibrations, which can be identified as the characteristic bands of EGDMA. The appearances of these bands confirmed that polymerization was successful. Also, the absence of bands in the region of 1638–1648 cm−1 indicates the absence of vinyl groups in the polymer particles. This observation could be possibly attributed to the polymerization of both MAA and EGDMA. Moreover, the peaks at 1108, 2042, and 2354 cm−1 were assigned to the C–N and C
O group is involved in Mo interactions. The peak at 2952 cm−1 indicated the methylene groups of the polymer, and corresponded to the C–H aromatic stretching vibrations, which can be identified as the characteristic bands of EGDMA. The appearances of these bands confirmed that polymerization was successful. Also, the absence of bands in the region of 1638–1648 cm−1 indicates the absence of vinyl groups in the polymer particles. This observation could be possibly attributed to the polymerization of both MAA and EGDMA. Moreover, the peaks at 1108, 2042, and 2354 cm−1 were assigned to the C–N and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups in TBAOH and SCN, respectively. Ultimately, in all spectra, the broad peak in the 3300–3500 cm−1 range indicated OH stretching vibrations in the structure of the adsorbent.
N groups in TBAOH and SCN, respectively. Ultimately, in all spectra, the broad peak in the 3300–3500 cm−1 range indicated OH stretching vibrations in the structure of the adsorbent.




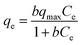

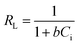

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc
Kc