Simultaneous encapsulation and stabilization of Aloe vera extract on cotton fabric for wound dressing application
S. Ghayempour a,
M. Montazer*a and
M. Mahmoudi Radb
a,
M. Montazer*a and
M. Mahmoudi Radb
aTextile Engineering Department, Functional Fibrous Structures & Environmental Enhancement (FFSEE), Amirkabir University of Technology, Tehran, Iran. E-mail: tex5mm@aut.ac.ir
bSkin Research Centre, Shahid Beheshti University of Medical Sciences, Tehran, Iran
First published on 21st November 2016
Abstract
Utilization of some herbal products in wound dressing for rapid healing with no side effects is a highly interesting task. The present paper offers the use of Aloe vera extract in nanocapsules on cotton fabric for wound healing with antimicrobial properties. A simultaneous encapsulation of Aloe vera extract and stabilization method was carried out on the cotton fabric. FESEM images indicated successful formation of nanocapsules on the cotton fabric with spherical shape and average size of 55–70 nm. The cotton fabric loaded with Tragacanth nanocapsules containing Aloe vera extract showed relatively good antibacterial and antifungal activities with microbial reduction of 75 ± 0.1, 80 ± 0.1 and 81 ± 0.1% against E. coli, S. aureus and C. albicans, respectively. Also, the treated fabric is suitable for wound dressing application due to good wound healing effects with migration rate of 88% after 24 h.
Introduction
Natural plant extracts have been used in nanotechnology due to their properties including biodegradability, biocompatibility and non-toxicity.1–3 Aloe vera is a plant growing in dry and warm weather applied to accelerate wound healing. This contains polysaccharides, vitamin C, vitamin E, anthraquinone, lectin, glycoprotein, amino acids and several wound healing compounds. Medicinal properties of Aloe vera can be related to the anti-oxidant and anti-inflammatory characteristics.4–8Recently, application of Aloe vera on the textiles has been performed for several purposes such as antimicrobial, wound healing, non-cytotoxicity and others.9–14 The commercial microcapsules containing Aloe vera was used by Cheng et al. for the development of cosmetic textiles. They showed no cytotoxic effect to the fibroblast cells on the finished fabric. Also the remaining of microcapsules on the fibers was satisfactory after 15 washes; however, some broken microcapsule observed on the fibers after 20 and 25 washes.10 Ravi et al. in 2014 encapsulated Aloe vera and other extracts into Acacia gum and applied on the cotton fabric. They analyzed the dyeing of plant extracts through direct application and microencapsulation method indicated microencapsulation technique increased the durability and smoothness of finished fabric.11 Further, an antimicrobial and anti-crease silk fabric was prepared using Aloe vera in presence of butanetetracarboxylic acid as cross-linking agent and sodium hypophosphite as catalyst. Nadiger and Shukla indicated excellent antimicrobial activities of the treated fabric with 15% Aloe vera.12
Application of Aloe vera on the textiles to obtain a helpful wound dressing is a favorite approach. Wound healing properties of Aloe vera is related to its component such as vitamins, enzymes, polysaccharides and phenolic compounds.5,15–17 A wound dressing based on Aloe vera mixed with poly(vinyl alcohol), polyethylene oxide and carboxymethyl cellulose was prepared by Gupta et al. in 2014. They applied above compound on the nonwoven polyester fabric as the support layer via freeze-drying method. They confirmed that treated fabric could possibly be used as wound dressing with having an enough swelling, good antimicrobial activity and drug release during 2 days.18 In 2016, Tummalapalli et al. loaded Aloe vera and curcumin into oxidized pectin–gelatin matrices and applied on nonwoven cotton fabric to obtain composite wound dressing. The drug release characteristics of the prepared wound dressings indicated the use of Aloe vera in wound dressing led to accelerated wound healing.13 Also, preparation of a wound dressing containing nanosilver nanohydrogels, Aloe vera and curcumin was reported by Anjum et al. in 2016. They used polyvinyl alcohol/polyethylene oxide/carboxymethyl cellulose as gelling system for coating obtained antimicrobial wound dressings. Best wound healing with minimum scarring was observed on fabric treated with Aloe vera.14
Recently, we introduce a one single step processing for simultaneous nano-encapsulation and stabilization of Chamomile extract on the cotton fabric using a sonochemical method.19 According to our results such as controlled release behavior and hydrogel properties of the treated cotton fabric in presence of natural, non-toxic, biodegradable and biocompatible polymer namely Tragacanth gum (TG), we convinced to encapsulate and stabilize Aloe vera extract on the cotton fabric for wound dressing applications. Properties of the prepared hydrogel wound dressing were investigated using field emission scanning electron microscope (FESEM), Fourier transform infrared (FT-IR) spectroscopy, antimicrobial activities and wound healing assay.
Experimental
Materials
Aloe vera extract as the core material was extracted from leaf of Aloe vera plant. Iranian Tragacanth gum as the wall material was received from Fars province, Iran and milled to a powder with size ranged from 200 to 500 μm. Ethanol as the solvent, Triton X-100 as the surfactant and aluminum chloride as the cross-linking agent were purchased from Merck Co., Germany. Almond oil was received from Barij Essence Pharmaceutical Co., Iran. A 140 gm−2 bleached cotton fabric was used as the textile substrate. Escherichia coli (E. coli, ATCC 25922), Staphylococcus aureus (S. aureus, ATCC 25923) and Candida albicans (C. albicans, ATCC 3153) are the used microorganisms for antibacterial and antifungal assay. The primary human fibroblast for wound healing assay was received from Pastor Co, Iran.Preparation of Aloe vera extract
In order to extract Aloe vera, 0.25 g leaf of Aloe vera plant was added to 40 mL solution of ethanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) in a beaker and sonicated with a titanium sonotrode of 3 mm diameter connected to a 400 W and 24 kHz ultrasonic homogenizer (Hielscher, UP400S, Germany) at 50% amplitude and 0.5 cycle for 5 min. The obtained mixture was centrifuged using a RST 24&16 centrifuge, Iran for 10 min and supernatant was used as Aloe vera extract.
3) in a beaker and sonicated with a titanium sonotrode of 3 mm diameter connected to a 400 W and 24 kHz ultrasonic homogenizer (Hielscher, UP400S, Germany) at 50% amplitude and 0.5 cycle for 5 min. The obtained mixture was centrifuged using a RST 24&16 centrifuge, Iran for 10 min and supernatant was used as Aloe vera extract.
Encapsulation and stabilization of Aloe vera extract on the cotton fabric
Encapsulation process was started with sonication of a mixture containing 3 mL Aloe vera extract, 0.2 mL Triton X-100 0.1% and almond oil using an UP400S Hielscher ultrasonic homogenizer at 100% amplitude and 0.5 cycle for 5 min. The prepared microemulsion was added to 100 mL Tragacanth gum 1% and Triton X-100 0.1% and the solution was sonicated to form a white microemulsion. The cotton fabric was immersed into the microemulsion for 5 min and aluminum chloride 2% was added to the solution. Ultrasonic waves led to encapsulation and loading of Aloe vera extract on the cotton fabric. Finally, the cotton fabric loaded with nanocapsules was washed with distilled water.Characterization of the cotton fabric loaded with nanocapsules
Morphology of the treated cotton fabric was studied using a FESEM device (VEGA2-TESCAN scanning electron microscopy, Czech). A FT-IR spectrophotometer (Thermo Nicolet, model: Nexus 670, USA) was used to study FT-IR spectra of the samples. UV-Vis spectra of the samples were evaluated by a UV-Vis spectrophotometer (UV-Vis Array Spectrophotometer, Iran) to study the existence of Aloe vera extract in nanocapsules and investigate the release behaviour of the treated samples. To evaluate the release behaviour of Aloe vera extract from nanocapsules, the sample was cut to 5 cm × 5 cm pieces and immersed into phosphate buffer with 2, 4, 8, 12, 18, 24, 30 and 36 h shaking to release Aloe vera extract from the nanocapsules on the fabric.Antibacterial and antifungal assay
The antibacterial and antifungal activity of the treated cotton fabric was measured using colony count method according to AATCC Test 100 Standard Method.20 The inoculated microbes on a nutrient agar plate was cultivated at 37 °C for 24 h, inoculated in 20 mL broth and cultivated at 37 °C for 24 h. The live microbes was measured through optical density at 580 nm and adjusted to 1–2 × 106 CFU mL−1. 1 mL broth was added to 20 mL nutrient culture medium and cultivated at 37 °C for 2 h with shaking. The microbe's number was adjusted to 1 ± 0.3 × 105 CFU mL−1 and 0.2 mL of the prepared inoculator was poured on the samples. The mixture was cultivated at 37 °C for 18 h and the percentage of microbial reduction (% R) was calculated using eqn (1).
 | (1) |
Wound healing assay
Wound healing assay of the treated cotton fabrics was investigated using scratch assay. A suitable extra cellular matrix (ECM) of fibroblast was coated on 60 mm dishes by incubating at 4 °C for one night. The coated dishes were blocked with 3 mL bovine serum albumin (2 mg mL−1) for 1 h at 37 °C. They were washed with phosphate buffered saline (PBS) and refill with 3–5 mL media. The versene containing trypsin was added to the cells washed twice with PBS. The cells were mixed with medium containing serum to re-suspend growing cells. The solution was pipetted, while the dish was rocked. Cells were cultured into a 60 mm dish to create a confluent monolayer and a “scratch” was scraped using a pipet tip. The scratch edge was smoothed by washing the cells with 1 mL of the growth medium and replaced with 5 mL of specific medium for the in vitro scratch assay. The first image of the scratch was recorded by a phase-contrast microscope. The dish was incubated at 37 °C for 8–18 h and the second image was taken through matching dish with the reference point in the first image. For each image, distances between one side of scratch and the other can be measured at certain intervals (μm). The cell migration rate in the certain time into scratch (% Mt) was determined using eqn (2).
 | (2) |
Results and discussion
The aim of this work was to apply Aloe vera as the medical plant extract on the cotton fabric in nano-encapsulated form for wound dressing. Fig. 1 shows a schematic of simultaneous encapsulation and stabilization of aqueous Aloe vera extract on the cotton fabric. Ultrasonic irradiation of mixture of Aloe vera, almond oil and Triton X-100 leads to W/O microemulsion and adding Tragacanth gum results formation of W/O/W microemulsion. Finally, the nanocapsules were formed and stabilized on the cotton fabric by ultrasonic irradiation of the prepared microemulsion in the presence of aluminum chloride. Aluminum chloride acts as a cross-linking agent to form nanocapsules through interaction between aluminum ions and carboxylic groups of Tragacanth gum. In fact, W/O/W microemulsion was provided a suitable situation for sonochemical interaction between aluminum ions and carboxylic groups of Tragacanth gum resulted spherical-shape nanocapsules. | ||
| Fig. 1 A schematic of simultaneous encapsulation and stabilization of aqueous Aloe vera extract on the cotton fabric. | ||
Morphology
The morphology of the cotton fabric loaded with nanocapsules was studied by FESEM images. Fig. 2 indicates the FESEM images of raw and treated cotton fabrics. As observed, Aloe vera extract successfully encapsulated and stabilized on the cotton fabric. The prepared nanocapsules formed with spherical shape and average size of 55–70 nm. Stabilization of formed nanocapsules on the cotton fabric performs through the intermolecular hydrogen bindings between Tragacanth gum and cellulose and the interaction between aluminum ions and hydroxyl groups of cellulose. | ||
| Fig. 2 FESEM images of (a) raw cotton fabric and (b) cotton fabric loaded with nanocapsules containing Aloe vera extract. | ||
FT-IR
FT-IR spectra of TG, Aloe vera extract, raw cotton fabric and treated cotton fabric are showed in Fig. 3. The stretching vibrations of hydroxyl in 3420 cm−1, carboxylate in 1629 cm−1 and carbonyl in 1743 cm−1 are the important peaks in FT-IR spectrum of TG.1,24 Aloe vera extract is composed of various chemical compounds such as carvone, limonene and hexadecanoic acid that are determinant in the appeared peaks of FT-IR spectrum.25 Aromatic double bonds in carvone and limonene lead to the peaks in 1543 and 1604 cm−1 and the carboxylic groups in hexadecanoic acid create peak in 1696 cm−1. The important peaks of almond oil are appeared at 1746 and 3006 cm−1 related to the ester carbonyl groups of the triacylglycerides and the cis-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H groups in almond oil.26,27 In the spectrum of cotton fabric loaded with nanocapsules, the peaks of double bonds in aromatic compounds of Aloe vera extract are observed in 1542 and 1614 cm−1 and the peak of the carboxylic group is appeared in 1696 cm−1. The appeared peak in 1743 cm−1 is attributed to the stretching vibration of the carbonyl groups in TG and the ester carbonyl groups of almond oil. Successful encapsulation process confirms with observe the peak related to Al–O–C bonds in 1111 cm−1. Aluminium ion acts as the cross-linking agent and encapsulates the trapped extract in TG.28,29
C–H groups in almond oil.26,27 In the spectrum of cotton fabric loaded with nanocapsules, the peaks of double bonds in aromatic compounds of Aloe vera extract are observed in 1542 and 1614 cm−1 and the peak of the carboxylic group is appeared in 1696 cm−1. The appeared peak in 1743 cm−1 is attributed to the stretching vibration of the carbonyl groups in TG and the ester carbonyl groups of almond oil. Successful encapsulation process confirms with observe the peak related to Al–O–C bonds in 1111 cm−1. Aluminium ion acts as the cross-linking agent and encapsulates the trapped extract in TG.28,29
 | ||
| Fig. 3 FT-IR spectra of TG, Aloe vera extract, almond oil, raw cotton fabric and treated cotton fabric. | ||
Presence of Aloe vera extract into formed nanocapsules on the cotton fabric
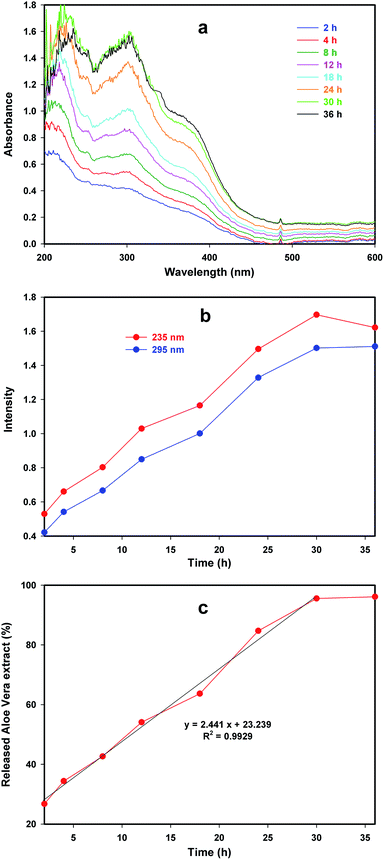
Aloe vera extract has a specific UV-Vis spectrum used for qualitative and quantitative identification in the solutions. Therefore, the presence of Aloe vera extract into nanocapsules formed on the cotton fabric was proved by UV-Vis spectra of pure Aloe vera extract and the remaining solution from the treated cotton fabric. Fig. 4 shows the UV-Vis spectrum of pure Aloe vera extract involves two intense absorbance peaks in 235 and 295 nm. These peaks in the spectrum of cotton fabric loaded with nanocapsules confirm the existence of Aloe vera extract into nanocapsules. | ||
| Fig. 4 UV-Vis spectra of pure Aloe vera extract and the remaining solution from treated cotton fabric. | ||
Release of Aloe vera extract from the treated cotton fabric
The main purpose of encapsulate materials is the controlled release. Release behavior of Aloe vera extract from cotton fabric loaded with nanocapsules was investigated using UV-Vis spectra during diverse stirring time. Fig. 5a indicates UV-Vis spectra of the remaining solution of the treated fabric in different stirring time. The more intense spectra can be seen with more stirring. Aloe vera extract encapsulated into nanocapsules and stirring led to breakage of nanocapsules wall and leakage of Aloe vera extract into solution. For more clarity, the intensity in maximum wavelength of 235 and 295 nm was plotted against time (Fig. 5b). The absorbance intensity in 235 nm was 0.53 that increased to 1.62 after 36 h.Also, the absorbance intensity of 0.42 in 2 h gained to 1.51 after 36 h in 295 nm. These results indicate good release behavior of the treated fabric as the nanocapsules wall slowly destroyed with stirring and Aloe vera extract released from the nanocapsules into the solution.
The kinetic release of Aloe vera from nanocapsules was plotted as a fraction of the initial amount of encapsulated Aloe vera on the treated fabric. The amount of encapsulated Aloe vera extract on the treated cotton fabric considered as the reference and the amount of released Aloe vera extract obtained from the absorbance intensity according to Beer–Lambert law.30 As seen in Fig. 5c, Aloe vera was released from nanocapsules with constant rate of 2.44% h−1 with R2 = 0.9929 during 30 h stirring. More stirring leads to a low increase in percentage of released Aloe vera extract from nanocapsules, thus the percentage of released Aloe vera changed from 95.54 after 30 h to 96.10 after 36 h. This means that Aloe vera was released from nanocapsules with constant rate of 0.093% h−1 with prolonged 6 h stirring. Also, the percentage of released Aloe vera extract from nanocapsules indicated no changes when more stirring applied.
Antibacterial and antifungal assay
Antibacterial and antifungal activities of cotton fabric loaded with nanocapsules were studied using colony count method. Fig. 6 indicates the images of antibacterial/antifungal assay of the treated cotton fabric and blank sample (raw cotton fabric). The results indicated that the treated cotton fabric had relative good antibacterial and antifungal activities with microbial reduction of 75 ± 0.1, 80 ± 0.1 and 81 ± 0.1% against E. coli, S. aureus and C. albicans, respectively. The existence of Aloe vera extract into the formed nanocapsules on the treated cotton fabric leads to antibacterial and antifungal activity. This is related to the presence of important compounds in Aloe vera extract such as limonene and α-bisabolol. Also, acemannon, anthroquinone, and salicylic acid component in Aloe vera extract help to promote antibacterial and antifungal activities.9,31 The Aloe vera releases from the nanocapsules and prevents growth of microbes. The components of Aloe vera act as prooxidants affecting inner cell membranes and organelles such as mitochondria killing microbe.32–37 Various researches are reported on the antimicrobial activity of Aloe vera. Habeeb et al. used the standard methods based on a microtiter assay using a metabolic colour indicator Alamar blue™ to investigate antimicrobial activity of Aloe vera. According to their results, all standard methods indicated that Aloe vera has an antimicrobial effect; however, the microtiter assay has high screening with lower wasteful of material.38 Wazed Ali et al. studied the antimicrobial activity of cotton fabric treated with Aloe vera gel using the pad-dry-cure method. They indicated the treated cotton fabric inhibited the growth of E. coli and S. aureus bacteria.39 Furqan Khurshid et al. prepared an antimicrobial textile using Aloe vera and Neem extracts. They claimed combination of Aloe vera and Neem extracts is more effective against E. coli and Aspergillus niger, but less so against S. aureus as compared to alone Aloe vera and Neem extracts.40 Sogvar et al. studied the effects of an edible coating based on Aloe vera gel in combination with ascorbic acid. The results of antimicrobial activity in different times of storage indicated Aloe vera has potential to maintain postharvest fruit quality of strawberry fruit through reduce microbial populations.41Wound healing assay
The “scratch” assay is commonly used to in vitro study of wound healing. This method is an inexpensive method that covers the second phase of wound healing characterized by a proliferation and migration of either keratinocytes or fibroblasts.23 This is based on the scratching on a cell monolayer with a pipette tip or syringe needle. Cell responds to disruption of cell–cell contact established through scratch on the cell monolayer by increasing the concentration of growth factors and cytokines at the wound edge. Also, keratinocytes and fibroblasts proliferate and migrate to the wound place. Therefore, the cells on the scratch edge move to the centre of scratch and re-establish new cell–cell contact.23,42–44 In this assay, the migration of fibroblast cells was investigated after 0 and 24 h. The results of wound healing for raw and treated cotton fabrics are indicated in Fig. 7. The created scratch on the treated cotton fabric is approximately closed with migration rate of 88% after 24 h, while very low migration was observed in control sample. In the treated cotton fabric, the fibroblast cells are well proliferated and migrated from the scratch edge to scratch centre. Aloe vera extract slowly releases from nanocapsules into scratch and helps to proliferation and migration of fibroblast cells. Aloe vera extract has water-soluble vitamins, enzymes, polysaccharides and phenolic compounds such as α-bisabolol that help to accelerate the wound healing process. α-Bisabolol acts as the lowering fever and reducing wound healing time. It has a positive effect on migration of around cells to the wound. Other effective factors in wound healing properties of Aloe vera relate to the powerful antioxidant component called α-tocopherol a form of vitamin E presented in Aloe vera.5,15–17 | ||
| Fig. 7 Cell migration in (a) 0 and (b) 24 h for raw cotton fabric and (c) 0 and (d) 24 h for treated cotton fabric. | ||
Aloe vera heals the wound through several suggested mechanisms. An assay for cell migration was used by Topman et al. to study the effect of Aloe vera on wound healing. They found no evidence that Aloe vera directly effect on the kinematics of fibroblast migration in damaged cultures and suggested it may still influence the wound healing through other pathways;45 however, Atiba et al. indicated the potential application of Aloe vera to improve the acute radiation-delayed wound healing by increasing transforming growth factor β-1 and basic fibroblast growth factor production.46 Increasing collagen, activating macrophages, reducing vasoconstriction, oxygenating, reducing platelet aggregation and scavenging free radicals are the important suggested mechanisms. Also, Aloe vera increases growth factor production and angiogenesis.5,15–17 Recently, we have been reported the study on a wound healing product prepared through encapsulation of Aloe vera extract into Tragacanth gum. Relative high antimicrobial activity, good cell viability and well wound healing activity of prepared wound healing product caused to increase our motivation for application of Aloe vera to enhance performance of wound dressing.47 Uslu and Aytimur prepared a biocompatible fibers containing (hydroxypropyl)methyl cellulose as the water retention agent and Aloe vera as the wound healing agent by electrospinning method. They indicated the prepared fibers can be used in produce dressing wounds.48 Galehdari et al. studied a combination of several plant material including Aloe vera, botanicals, Henna, Commiphora molmol and Adiantum capillus-vernis to improve the wound healing in streptozotocin-induced diabetic and non-diabetic rats. They claimed that the above combination accelerates wound healing process through improving conditions of wound and promoting better closure.49
Conclusions
Aloe vera has been traditionally used to accelerate the wound healing process. Application of plant extracts and essential oils on the textiles can be modified through encapsulate in a polymeric wall to control the release. In this work, Aloe vera extract was simultaneously encapsulated and stabilized on the cotton fabric using Tragacanth gum in the presence of ultrasound irradiation. FESEM images showed spherical nanocapsules containing Aloe vera extract stabilized on the cotton fabric with average size of 55–70 nm. Displaying the peaks related to Aloe vera extract and TG and the peak related to Al–O–C bonds in FT-IR spectrum of the treated cotton fabric confirmed encapsulation of Aloe vera into Tragacanth gum. The absorbance peaks in 235 and 295 nm in UV-Vis spectrum of the treated cotton fabric confirmed the presence of Aloe vera extract into the formed nanocapsules. The slow increase of UV-Vis spectra intensity during stirring showed a good release behavior of Aloe vera extract from the nanocapsules on the cotton fabric. The finished fabric is a suitable substrate for wound dressing application due to good antibacterial and antifungal activities against E. coli, S. aureus and C. albicans and also good wound healing properties with migration rate of 88% after 24 h in scratch assay.Note added after first publication
This article replaces the version published on 25th November 2016, in which the role of corresponding author was misattributed through editorial error.References
- S. Ghayempour, M. Montazer and M. Mahmoudi Rad, Carbohydr. Polym., 2016, 136, 232–241 CrossRef PubMed.
- F.-P. Chang, L.-Y. Kuang, C.-A. Huang, W.-N. Jane, Y. Hung, Y.-i. C. Hsing and C.-Y. Mou, J. Mater. Chem. B, 2013, 1, 5279–5287 RSC.
- R. Aladpoosh and M. Montazer, Carbohydr. Polym., 2016, 141, 116–125 CrossRef PubMed.
- A. Balaji, M. V. Vellayappan, A. A. John, A. P. Subramanian, S. K. Jaganathan, M. SelvaKumar, A. A. b. Mohd Faudzi, E. Supriyanto and M. Yusof, RSC Adv., 2015, 5, 86199–86213 RSC.
- A. Ahmadi, Chin. J. Integr. Med., 2012, 18, 635–640 CrossRef PubMed.
- R. F. Pereira, A. Carvalho, M. H. Gil, A. Mendes and P. J. Bártolo, Carbohydr. Polym., 2013, 98, 311–320 CrossRef PubMed.
- X. Guo, S. Zhang, S. L. Dial, M. D. Boudreau, Q. Xia, P. P. Fu, D. D. Levy, M. M. Moore and N. Mei, Toxicol. Res., 2014, 3, 487–496 RSC.
- A. A. Moosavi-Movahedi, F. Ghamari, S. M. Ghaffari, M. Salami, F. Farivar, F. Moosavi-Movahedi, A. Johari and A. L. N. Aminin, RSC Adv., 2015, 5, 248–254 RSC.
- L. Ammayappan and J. Jeyakodi Moses, Fibers Polym., 2009, 10, 161–166 CrossRef.
- S. Y. Cheng, C. W. M. Yuen, C. W. Kan, K. K. L. Cheuk and J. C. O. Tang, Text. Res. J., 2010, 80, 524–536 CrossRef.
- D. Ravi, R. B. D. Lekshmi, V. Vijayabharathi and R. Parthasarathy, J. Pharm., Chem. Biol. Sci., 2014, 2, 150–157 Search PubMed.
- V. G. Nadiger and S. R. Shukla, Fibers Polym., 2015, 16, 1012–1019 CrossRef.
- M. Tummalapalli, M. Berthet, B. Verrier, B. L. Deopura, M. S. Alam and B. Gupta, Int. J. Biol. Macromol., 2016, 82, 104–113 CrossRef PubMed.
- S. Anjum, A. Gupta, D. Sharma, D. Gautam, S. Bhan, A. Sharma, A. Kapil and B. Gupta, Mater. Sci. Eng., C, 2016, 64, 157–166 CrossRef PubMed.
- E. Barrantes and M. a. Guinea, Life Sci., 2003, 72, 843–850 CrossRef PubMed.
- S. Heggie, G. P. Bryant, L. Tripcony, J. Keller, P. Rose, M. Glendenning and J. Heath, Canc. Nurs., 2002, 25, 442–451 CrossRef.
- A. Atiba, H. Ueno and Y. Uzuka, J. Vet. Med. Sci., 2011, 73, 583–589 CrossRef PubMed.
- B. Gupta, R. Agarwal and M. Sarwar Alam, J. Mater. Sci.: Mater. Med., 2014, 25, 1613–1622 CrossRef PubMed.
- S. Ghayempour and M. Montazer, Cellulose, 2016, 23, 2561–2572 CrossRef.
- AATCC test method 100–2004 (2005) Antibacterial finishes on textile materials: assessment of AATCC technical manual, American Association of textile chemists and colorists, Research Triangle Park.
- A. J. Kora and J. Arunachalam, J. Nanomater., 2012, 2012, 8 Search PubMed.
- C.-C. Liang, A. Y. Park and J.-L. Guan, Nat. Protoc., 2007, 2, 329–333 CrossRef PubMed.
- N. Balekar, N. G. Katkam, T. Nakpheng, K. Jehtae and T. Srichana, J. Ethnopharmacol., 2012, 141, 817–824 CrossRef PubMed.
- S. Ghayempour and M. Montazer, Ultrason. Sonochem., 2017, 34, 458–465 CrossRef PubMed.
- F. Nejatzadeh-Barandozi, Org. Med. Chem. Lett., 2013, 3, 1–8 CrossRef PubMed.
- L. Brambilla, C. Riedo, C. Baraldi, A. Nevin, M. C. Gamberini, C. D’andrea, O. Chiantore, S. Goidanich and L. Toniolo, Anal. Bioanal. Chem., 2011, 401, 1827–1837 CrossRef PubMed.
- A. Beltrán, M. Ramos, N. Grané, M. L. Martín and M. C. Garrigós, Food Chem., 2011, 126, 603–609 CrossRef.
- F. Wypych, in Interface Science and Technology, ed. W. Fernando and S. Kestur Gundappa, Elsevier, 2004, vol. 1, pp. 1–56 Search PubMed.
- S. Ghayempour, M. Montazer and M. Mahmoudi Rad, Int. J. Biol. Macromol., 2015, 81, 514–520 CrossRef PubMed.
- D. Calloway, J. Chem. Educ., 1997, 74, 744 CrossRef.
- A. Femenia, E. S. Sánchez, S. Simal and C. Rosselló, Carbohydr. Polym., 1999, 39, 109–117 CrossRef.
- F. Bakkali, S. Averbeck, D. Averbeck and M. Idaomar, Food Chem. Toxicol., 2008, 46, 446–475 CrossRef PubMed.
- S. Ghayempour and S. M. Mortazavi, Cellulose, 2015, 22, 4065–4075 CrossRef.
- M. Dilamian, M. Montazer and J. Masoumi, Carbohydr. Polym., 2013, 94, 364–371 CrossRef PubMed.
- S. Ghayempour and S. M. Mortazavi, J. Essent. Oil Res., 2014, 26, 492–498 CrossRef CAS.
- A. Sadeghian Maryan, M. Montazer and T. Harifi, Carbohydr. Polym., 2015, 115, 568–574 CrossRef PubMed.
- S. Ghayempour and M. Montazer, J. Microencapsulation, 2016, 33, 497–510 CrossRef CAS PubMed.
- F. Habeeb, E. Shakir, F. Bradbury, P. Cameron, M. R. Taravati, A. J. Drummond, A. I. Gray and V. A. Ferro, Methods, 2007, 42, 315–320 CrossRef CAS PubMed.
- S. Wazed Ali, R. Purwar, M. Joshi and S. Rajendran, Cellulose, 2014, 21, 2063–2072 CrossRef.
- M. F. Khurshid, M. Ayyoob, M. Asad and S. N. H. Shah, Fibres Text. East. Eur., 2015, 23, 120–123 CrossRef CAS.
- O. B. Sogvar, M. Koushesh Saba and A. Emamifar, Postharvest Biol. Technol., 2016, 114, 29–35 CrossRef CAS.
- M. Schäfer and S. Werner, Annu. Rev. Cell Dev. Biol., 2007, 23, 69–92 CrossRef PubMed.
- G. C. Gurtner, S. Werner, Y. Barrandon and M. T. Longaker, Nature, 2008, 453, 314–321 CrossRef CAS PubMed.
- A. Adetutu, W. A. Morgan and O. Corcoran, J. Ethnopharmacol., 2011, 137, 50–56 CrossRef PubMed.
- G. Topman, F.-H. Lin and A. Gefen, J. Biomech., 2013, 46, 170–174 CrossRef PubMed.
- A. Atiba, M. Nishimura, S. Kakinuma, T. Hiraoka, M. Goryo, Y. Shimada, H. Ueno and Y. Uzuka, Am. J. Surg., 2011, 201, 809–818 CrossRef CAS PubMed.
- S. Ghayempour, M. Montazer and M. Mahmoudi Rad, Int. J. Biol. Macromol., 2016, 93, 344–349 CrossRef CAS PubMed.
- İ. Uslu and A. Aytimur, J. Appl. Polym. Sci., 2012, 124, 3520–3524 CrossRef.
- H. Galehdari, S. Negahdari, M. Kesmati, A. Rezaie and G. Shariati, BMC Complementary Altern. Med., 2016, 16, 386 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |