Effect of the temperature on the dissolution of corn straw in ethanol solution
Xinghua Zhang *,
Zhengqiu Yuan,
Tiejun Wang*,
Qi Zhang and
Longlong Ma
*,
Zhengqiu Yuan,
Tiejun Wang*,
Qi Zhang and
Longlong Ma
Key Laboratory of Renewable Energy, Guangzhou Institute of Energy Conversion, Chinese Academy of Science, Guangzhou 510640, PR China. E-mail: zhangxh@ms.giec.ac.cn; wangtj@ms.giec.ac.cn; Fax: +86 20 8705 7789; Tel: +86 20 8705 7751
First published on 21st October 2016
Abstract
Lignocellulosic biomass is undissolvable in water and conventional solvents for its complex natural recalcitrance, which significantly hinders its efficient utilization. Herein, we provided an efficient process for the dissolution of corn straw under mild conditions with the solution of ethanol, H2SO4, H2O2 and H2O. The research emphasis is the effect of temperature on the dissolution process. Results showed that the components of cellulose, hemicellulose and lignin can be separated from the corn straw by adjusting the dissolution temperature. Part of the dissolved cellulose and hemicellulose were further converted into sugars and chemicals (such as furfural, 5-hydroymethylfurfural, ethyl levulinate, levulinic acid) during the dissolution process. Released lignin was destroyed into fragments and was recovered as organosolv lignin with lower molecular weight. Furthermore, residues and the recovered lignin obtained at different dissolution temperature were characterized carefully by FTIR, NMR, GPC and elemental analysis and the dissolution mechanism was discussed.
Introduction
Lignocellulosic biomass is the largest renewable carbon resource on earth and its use does not compete directly with food supplies.1 This renewable carbon resource offers great potential for green production of fuels and chemicals.2,3 As we all know, lignocellulosic biomass is recalcitrant complex polymers comprised with cellulose, hemicellulose and lignin by noncovalent and covalent cross linkages. It is hard to obtain target products with high yield and selectivity through direct conversion under mild conditions. Therefore, depolymerization of these naturally recalcitrant complex polymers into simpler molecules is needed for further refining the lignocellulosic biomass into useful fuels and chemicals. However, lignocellulosic biomass is insoluble in water and traditional organic solvent under a common condition, which leads to its depolymerization under severe conditions. This has already become a notable barrier that has to be overcome.4,5Chemical dissolution process in the presence of solvent is a convenient and less condition-harsh method to convert solid lignocellulosic biomass into liquid products,6–8 which are potential intermediates to produce various value-added polymers or chemicals.9 It was reported that complete dissolution of willow and hydrolysis had been achieved in hot water, leading to the production of sugars, chemicals and bio-fuels.10 Generally, ethanol and water are common solvents used in the dissolution process of biomass because ethanol can be obtained from biomass via fermentation and water can be easily obtained at a low price.
In addition, sulfuric acid was frequently used for the hydrolysis of lignocellulose due to its high catalytic activity for depolymerization of polysaccharides.11,12 Hydrogen peroxide will react readily with lignin and hemicellulose and yield an array of water soluble liquidation products with low molecular weight.13,14 Based on these, we developed a novel complete dissolution process for the corn straw under mild conditions, where the mixed solution of ethanol and water was used as solvent while hydrogen peroxide and sulfuric acid were used as catalysts.15 In this process, hemicellulose was converted into small-molecule chemicals (such as ethyl levulinate, furfural and 5-hydroymethylfurfural). Lignin was also be recovered with lower molecular weight while the dissolved cellulose can be efficiently used in subsequent hydrolysis and fermentation.16,17 Obviously, the components of lignin, hemicellulose and cellulose can be more easily converted after a complete dissolution process than that of raw lignocellulosic biomass in followed process.
Temperature usually plays an important role during the dissolution process of lignocellulosic biomass. Therefore, the effect of temperature on the dissolution process of corn straw would be investigated in this work. More specifically, under an optimized solution (75.0% C2H5OH + 21.5% H2O + 2.0% H2SO4 + 1.5% H2O2),15 the relationships between dissolution temperature and dissolution degree of corn straw, compositions of low-boiling chemicals, elemental compositions and molecular weight distribution of lignin would be examined in detail. Characteristic groups of the dissolved corn straw obtained at different dissolution temperature would be characterized carefully by FTIR and NMR technology, and the changes of inner molecular structural units of corn straw during the dissolution process would be also discussed.
Results and discussion
Dissolution of corn straw
In the mixed solution (75.0% C2H5OH + 21.5% H2O + 2.0% H2SO4 + 1.5% H2O2), the effect of temperature on the dissolution of corn straw was investigated. As shown in Fig. 1, the degree of the dissolution for the raw corn straw gradually increased with the elevated temperature. In general, susceptible components of lignocellulose (lignin, hemicellulose and amorphous zones of cellulose) were released and depolymerized firstly when the dissolution process was carried out in the mixed solution. The increased temperature would increase the accessibility of the cellulose, promoting the degradation of the crystalline region of the cellulose.18–20 In addition, the delignification generally was endothermic,21 therefore, it is plausible that the recovered lignin increased gradually with the increase of temperature.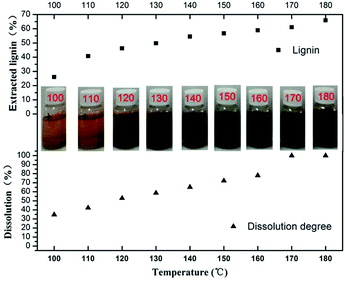 | ||
| Fig. 1 Yields of residue (related to raw material) and extracted lignin (related to lignin in raw material) at different temperature. | ||
From the photographs presented in Fig. 1, it can also be seen that the colour of dissolved liquid is gradually darkening with the increasing of temperature, suggesting that dissolved components of corn straw are also changing with temperature. As shown in Fig. 2a, xylose was released from the dissolved hemicellulose at 100 °C, suggesting that xylan was the most easily depolymerized due to its amorphous and hydrophilic properties. Within the temperature of 100–170 °C, the yield of xylose increased gradually because that the hydrolysis of hemicellulose was promoted by the evaluated temperature. However, the yield of xylose decreased obviously when the dissolution temperature further increased to 180 °C. Similar to xylose, the yield of glucose and arabinose also increased with temperature, and then decreased obviously when the dissolution temperature increased to 180 °C. These results suggest that higher temperature not only favors the dissolution of corn straw, but also promotes the depolymerization of hemicellulose and cellulose to saccharides.
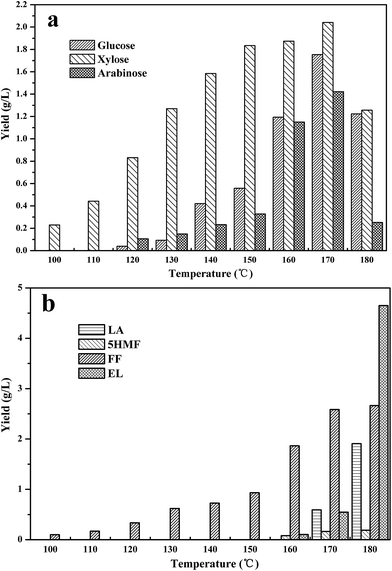 | ||
| Fig. 2 Sugars and low-boiling chemical products yielded at various temperatures. (a) Sugars; (b) low-boiling chemical products. | ||
The sugars could be further converted into low-boiling chemical products under acid environment. As shown in Fig. 2b, it can be clearly seen that the yields of chemical products were highly temperature dependent. For example, the yield of furfural (FF), which was originated from pentose, increased with the increased dissolution temperature. Chemicals 5-hydroymethylfurfural (5-HMF) and ethyl levulinate (EL), which were originated from glucose, appeared at 160 °C when levulinic acid (LA) appeared at 170 °C. Particularly, in the water-soluble fraction obtained at 170 °C, the yields of EL and LA were 0.55 and 0.59 g L−1, respectively. They dramatically increased to 4.65 and 1.91 g L−1 when the dissolution temperature increased to 180 °C. This is the reason that the yields of glucose, xylose and arabinose obviously decreased when the dissolution temperature increased from 170 °C to 180 °C as shown in Fig. 3a. Plainly, higher temperature favors the conversion of saccharides to chemical products. It should be noted that the obtained chemicals FF, 5-HMF, LA and EL can be used as platform chemicals for the production of intermediates with longer carbon chain by condensation, which can be converted into alkane fuel via hydrodeoxygenation.22,23
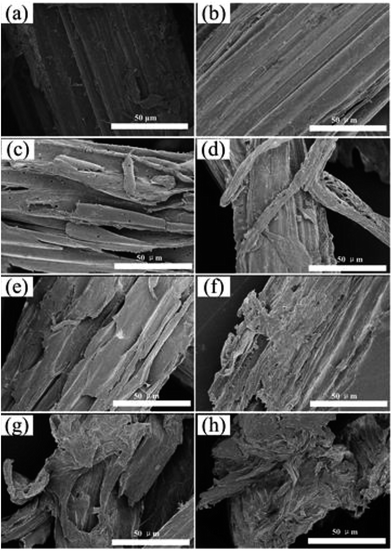 | ||
| Fig. 3 SEM images of raw corn straw and residues obtained at different dissolution temperatures. (a) Raw corn straw; (b–h) residues obtained at the temperature of 100–160 °C, respectively. | ||
Characterization of residues
The component of raw corn straw and residues were determined by NREL analytical method,24 and the results were listed in Table 1. The content of hemicellulose in residue decreased with the increasing temperature and was completely removed when the reaction temperature increased to 140 °C. Generally, lignin can be extracted by the mixed solution of ethanol and water with the acid catalyst.25 Furthermore, lignin content in the residue also decreased with the increased temperature from 100 °C to 160 °C. This indicates that the dissolubility of lignin in the mixture solution increases with the increased temperature. However, a small amount of 0.4% lignin remained in the residue when raw corn straw was dissolved at the 160 °C. This might be explained by the limited diffusion of the cell walls during the dissolution process. Deeply analyzing the data presented in Table 1, it can be said that the components of cellulose, hemicellulose and lignin can be separated roughly from corn straw by adjusting dissolution temperature.| Samples | Residues yield/% | Hemicellulose/% | Cellulose/% | Lignin/% | Hemicellulose removal/% | Cellulose removal/% | Lignin removal/% |
|---|---|---|---|---|---|---|---|
| a Notes: R100 refers to the residue obtained at 100 °C. | |||||||
| Raw | — | 16.59 | 37.62 | 23.78 | — | — | — |
| R100 | 65.37 | 15.61 | 57.17 | 11.20 | 38.49 | 0.66 | 68.00 |
| R110 | 57.81 | 12.05 | 61.50 | 10.66 | 58.01 | 5.50 | 73.70 |
| R120 | 47.13 | 10.94 | 65.62 | 7.28 | 68.92 | 17.79 | 85.00 |
| R130 | 41.33 | 7.12 | 71.29 | 6.71 | 82.26 | 21.68 | 87.88 |
| R140 | 34.84 | 0.00 | 78.12 | 4.76 | 100.00 | 27.65 | 92.75 |
| R150 | 27.78 | 0.00 | 88.84 | 2.42 | 100.00 | 34.41 | 97.08 |
| R160 | 21.99 | 0.00 | 97.36 | 0.40 | 100.00 | 43.10 | 99.62 |
The C, H, N, O contents of microcrystal cellulose, corn straw and residues obtained at different dissolution temperatures were showed in Table 2. It can be seen that the C content of the residue decreased while the O content increased with the gradually increased temperature. And the elemental compositions of residue closed to that of microcrystal cellulose when the temperature increased to 160 °C. Higher Heating Value (HHV) of the residue was lower than that of the raw corn straw due to the decrease of lignin content. The HHV of the residue also closed to the HHV of microcrystal cellulose when the dissolution temperature increased to 160 °C. These results also demonstrated that hemicellulose and lignin were successfully removed from the raw corn straw, forming the rich-cellulose residue when the dissolution process was carried out.
| C (%) | H (%) | O (%) | N (%) | H/C | O/C | N/C | HHV (MJ kg−1) | |
|---|---|---|---|---|---|---|---|---|
| a Notes: R100 refers to the residue obtained at 100 °C. | ||||||||
| Raw | 45.76 | 6.22 | 47.79 | 0.23 | 0.14 | 1.04 | 0.005 | 15.83 |
| Cellulose | 41.95 | 6.59 | 51.41 | 0.05 | 0.16 | 1.23 | 0.001 | 14.43 |
| R100 | 44.47 | 6.18 | 49.19 | 0.16 | 0.14 | 1.11 | 0.004 | 15.08 |
| R110 | 43.67 | 6.18 | 50.06 | 0.09 | 0.14 | 1.15 | 0.002 | 14.66 |
| R120 | 42.65 | 6.43 | 50.87 | 0.05 | 0.15 | 1.19 | 0.001 | 14.53 |
| R130 | 42.09 | 6.59 | 51.29 | 0.03 | 0.16 | 1.22 | 0.001 | 14.50 |
| R140 | 41.53 | 6.60 | 51.81 | 0.04 | 0.16 | 1.25 | 0.001 | 14.23 |
| R150 | 41.81 | 6.62 | 51.54 | 0.03 | 0.16 | 1.23 | 0.001 | 14.40 |
| R160 | 41.77 | 6.68 | 51.50 | 0.01 | 0.16 | 1.23 | 0.000 | 14.48 |
To determine morphological changes, the residues obtained at different temperatures were characterized by SEM. As shown is Fig. 3, an untreated vascular bundle with a tight and smooth surface was observed in the raw corn straw (Fig. 3a) and the residue obtained at 100 °C (Fig. 3b). The bundle became loose. The fibers were partially separated and the cell framework started to collapse when the dissolution increased to 110 °C (Fig. 3c). It can be clearly seen that a higher temperature resulted in considerable modifications for the cell wall structure (Fig. 3d–h). Particularly, corn straw cell walls were completely destroyed after dissolution at 150 °C and 160 °C (Fig. 3g and h).
To investigate the effect of dissolution temperature on the structural changes, the raw corn straw and the residues obtained at different temperature were characterized by FT-IR and the spectra were gathered in Fig. 4. According to the literatures,26,27 the peaks at about 1255 and 1731 cm−1 were assigned to the characteristic absorption of hemicellulose. The peaks at about 3390, 2900, 1375, and 1165 cm−1 were assigned to the characteristic absorption of cellulose. And the characteristic peaks for lignin were positioned at about 2900, 1600–1500, 1423, 1314, and 830–750 cm−1. As shown in Fig. 4, the characteristic peaks of cellulose strengthened while the peaks of hemicellulose and lignin were weakened gradually with the increased temperature. More specifically, the absorption peaks positioned at 1255 and 1731 cm−1, which were assigned to the C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of the acetyl ester unit of hemicellulose, weakened gradually with the increase of temperature and completely disappeared at 140 °C, implying the efficient removal of hemicellulose. The peaks of 1604, 1511 and 1461 cm−1, which were assigned to the C
O stretching vibrations of the acetyl ester unit of hemicellulose, weakened gradually with the increase of temperature and completely disappeared at 140 °C, implying the efficient removal of hemicellulose. The peaks of 1604, 1511 and 1461 cm−1, which were assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of the aromatic ring of lignin, decreased with the elevated temperature, suggesting the decrease of lignin content. These results are well in accordance with the aforementioned conclusions concluded in section of component analysis and ultimate analysis of residues.
C stretching of the aromatic ring of lignin, decreased with the elevated temperature, suggesting the decrease of lignin content. These results are well in accordance with the aforementioned conclusions concluded in section of component analysis and ultimate analysis of residues.
 | ||
| Fig. 4 FT-IR spectra of the residues obtained at various dissolution temperatures (R100 refers to the residue obtained at 100 °C). | ||
Characterization of lignin
Ultimate analysis of lignin samples recovered at different temperature was showed in Table 3. It can be clearly seen that the C content of lignin was higher than that of the MWL and the O content was lower than that of the MWL. It might be explained that a series of dehydration and condensation reactions occurred in the dissolution process of lignin, leading to the deoxygenation of the lignin sample. Furthermore, it should be noted that the O content and O/C ratio decrease with the increased temperature, implying that higher temperature favors the deoxygenation during the dissolution process with mixed solution.| C (%) | H (%) | O (%) | H/C | O/C | HHV (MJ kg−1) | |
|---|---|---|---|---|---|---|
| a Notes: R100 refers to the residue obtained at 100 °C. | ||||||
| MWL | 59.19 | 5.89 | 34.5 | 0.10 | 0.58 | 22.30 |
| L100 | 60.31 | 6.49 | 32.87 | 0.11 | 0.55 | 23.84 |
| L110 | 60.4 | 6.41 | 32.57 | 0.11 | 0.54 | 23.81 |
| L120 | 61.09 | 6.38 | 32.19 | 0.10 | 0.53 | 24.06 |
| L130 | 61.27 | 6.4 | 31.96 | 0.10 | 0.52 | 24.20 |
| L140 | 61.34 | 6.26 | 31.9 | 0.10 | 0.52 | 24.03 |
| L150 | 63.79 | 6.26 | 29.76 | 0.10 | 0.47 | 25.24 |
| L160 | 63.7 | 6.21 | 29.74 | 0.10 | 0.47 | 25.14 |
| L170 | 65.95 | 5.97 | 27.87 | 0.09 | 0.42 | 25.90 |
| L180 | 65.71 | 5.94 | 28.16 | 0.09 | 0.43 | 25.72 |
Fig. 5 gathered the FT-IR spectra of the lignin samples recovered from the dissolution process at various temperatures. The characteristic peaks were observed on the FT-IR spectra and the corresponding functional groups were listed in Table 4.28–30
 | ||
| Fig. 5 FT-IR spectra of the lignin samples extracted from corn straw at various temperatures (L100 refers to the lignin extracted at 100 °C). | ||
| Peak position (cm−1) | Functional groups |
|---|---|
| a All characteristic peaks were assigned based on the literature.28–30 | |
| 1710, 1656, 1266 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration O stretching vibration |
| 1035, 1127 and 1169 | Glycosidic linkage |
| 1601, 1512, 1423 | Aromatic skeleton vibrations |
| 1462 | C–H asymmetric vibrations and deformations (methyl and methylene) |
| 1330 | Syringyl ring plus guaiacyl ring condensed |
| 1226 | Ring breathing with C–C, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching O stretching |
| 837 | Aromatic C–H ring out-of-plane vibration breathing |
It was noted that the characteristic absorption of carbonyl stretching vibration at 1710 cm−1, conjugated carbonyl stretching vibration at 1656 cm−1 and aromatic skeleton vibrations at 1601, 1512 and 1423 cm−1 were observed in the FT-IR spectra of the lignin samples and MWL with similar shape and intensity, suggesting that the primary lignin structure did not be changed significantly during the dissolution process. However, some subtle differences caused by different dissolution temperature could be found in the FT-IR spectra of lignin samples. For example, the relative intensity of the characteristic absorptions at 1169 cm−1 (assigned to asymmetric C–O stretching of ester), 1330 cm−1 (assigned to syringyl ring plus guaiacyl ring condensed) and 837 cm−1 (assigned to C–H out-of-plane deformation in positions 2 and 6 of S units and all positions of p-hydroxyphenyl (H) units) gradually weaken with the increasing of dissolution temperature. These changes illustrated that evaluated temperature can promote the decomposition of lignin in the presence of sulfuric acid and peroxide hydrogen. This conclusion also could be supported by the results of GPC analysis of the lignin samples. As shown in Fig. 6, the peak of GPC curve of lignin samples moved apparently to the small molecular weight with the increased dissolution temperature.
 | ||
| Fig. 6 Molecular weight distribution for the extracted lignin (L100 refers to the lignin extracted at 100 °C). | ||
To further elucidate the structural features of WML and the lignin samples obtained at various dissolution temperatures, 13C NMR analysis was also carried out, and the spectra were gathered in Fig. 7. Most of the observed characteristic signals have been precisely assigned in Table 5 according to the literatures.29,31 Changes in the characteristic signals in the spectra can be divided into three regions. (1) The signals between 104.4 and 168.2 ppm represented the aromatic region of the lignin gradually weakened with the increasing of temperature, suggesting that G, S, H units were partially degraded during the dissolution process. (2) The signals between 50 and 86 ppm attributed to lignin interunit linkages abated, showing that these linkages (such as β-O-4 bond) and –OCH3 were easily cleaved during mixture liquid treatment at the condition given. (3) The characteristic signal region between 10.0 and 40.0 ppm was assigned to the groups of methyl and methylene in the aliphatic side-chains of lignin, which intensified gradually with dissolution temperature. It is likely that these types of side-chain carbons are probably formed as a result of degradation of phenolic β-aryl ethers and possibly also through condensation with formaldehyde.32 Apparently, the degradation of phenolic β-aryl ethers and condensation with formaldehyde were favored at higher temperature.33
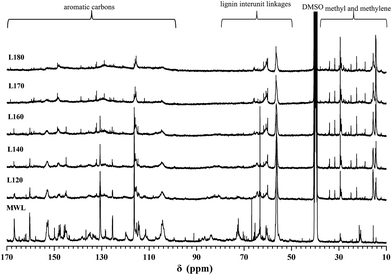 | ||
| Fig. 7 13C NMR spectra of the extracted lignin at 100–180 °C (L100 refers to the lignin extracted at 100 °C). | ||
| Chemical shift region (ppm) | Proposed dominant type of carbon | Representative resonance peak |
|---|---|---|
| a The major regions and characteristic signals were assigned according to the literatures.29,30 | ||
| 100–170 | Aromatics in lignin | S units: 152.3 ppm (C-3/C-5, etherified), 147.1 ppm (C-3/C-5, nonetherified), 138.2 ppm (C-4, etherified), 134.3 ppm (C-1, etherified), 106.8 (C-2/C-6, oxidized), and 104.4 ppm (C-2/C-6). G units: 149.7 ppm (C-3, etherified), 145.4 ppm (C-4, nonetherified), 134.3 ppm (C-1, etherified), 133.1 ppm (C-1, nonetherified), 119.4 ppm (C-6, nonetherified), 114.8 ppm (C-5, nonetherified), and 111.1 ppm (C-2, nonetherified). H units: 127.2 ppm (C-2/C-6) |
| 50–90 | Lignin interunit linkages | 86.2 ppm (S units)/84.6 ppm (G and H units) (C-β, β-O-4 linkage), 72.3 ppm (C-α, β-O-4 linkage), 60.2 ppm (C-γ, β-O-4 linkage). 56.0 ppm (–OCH3 groups in G and S units) |
| 10–40 | Methyl and methylene groups | 30.4 ppm (carbon in –CH–), 25.7 ppm (carbon in –CH2–) and 10.4 ppm (carbon in –CH3–) |
Dissolution mechanism and potential applications
Based on the detailed analysis of the above-mentioned dissolution process, products, resides and recovered lignin, a possible dissolution mechanism for lignocellulose was proposed. As shown in Fig. 8, under the catalysis of H+ and H2O2, the LCC (lignin–carbohydrate complexes) structures, such as phenyl glycosides, which connect the lignin and polysaccharide, are first cleaved. The released lignin with large molecular weight is insoluble in ethanol and water. However, part of ether bonds (such as β-O-4), which connect the hydroxyphenylpropane units in lignin, can be further cracked under the catalysis of H+,34 obtaining the fractured lignin with relatively lower molecular weight, which could be dissolved into the liquid.During the course of lignin removal, hemicellulose is also released and dissolved into the liquid due to its hydrophilicity. Accompanied with the release of lignin and hemicellulose, the peripheral amorphous cellulose fiber is also dissolved into liquid. Subsequently, crystal cellulose is gradually dissolved into the liquid in the form of water-soluble oligosaccharides. Finally, lignocellulose biomass would be completely dissolved into the mixture solution. H+ plays a key role in the entire dissolution process. For example, glycosidic bond is attacked by H+ to form polysaccharide, oligosaccharides and monosaccharides (such as glucose, xylose and arabinose) through hydrolysis.35 Furthermore, saccharides can be hydrolyzed into and low-boiling products (5-HMF, FF, LA and EL) under the catalysis of H+.36,37
Using the dissolution process as a pretreatment method, lignocellulose biomass is solubilized, and the dissolved products could be controlled by changing reaction temperatures for the following applications.
(1) Hemicellulose and lignin were removed from lignocellulose biomass by the dissolution process. Obtained cellulose can be hydrolyzed to glucose and further fermented to ethanol with high efficient.16,17 In addition, the hemicellulose (xylan) can be converted to xylose (for xylitol production) and valuable chemicals (such as furfural) during this dissolution process. The fractured lignin can be further degraded to phenolic compounds38 or used directly as low graded fuel and materials.3,39
(2) Hemicellulose is converted into furfural and 5-HMF at 160 °C while part of cellulose is converted into levulinate, ethyl levulinate at 180 °C during the dissolution process. Aldol condensation occurs in the mixture of levulinate, ethyl levulinate, furfural and HMF, and produces a combination of oxygenates with a carbon chain of 10 to 17 carbon atoms. Then the C10–C17 long chain alkane-based aviation fuel can be obtained via a series of hydrogenation and hydrodeoxygenation processes.22,23 That is to say, FF, 5-HMF, LA and EL can be obtained from lignocellulose biomass by adjusting the dissolution temperature, which can be used as platform chemicals for the production of intermediates with long carbon chain by condensation and hydrodeoxygenation.
Conclusions
Complete dissolution of corn straw under mild conditions was achieved in a solution of ethanol, water, sulfuric acid, and hydrogen peroxide. The effect of temperature on the dissolution of corn straw was investigated carefully. The result showed that dissolution of corn straw increased with temperature. Hemicellulose was completely dissolved at 140 °C, and further converted to monosaccharide and chemicals (such as HMF and FF) during the course of dissolution. Lignin was also almost dissolved completely when the dissolution temperature further increased to above 150 °C. The dissolved lignin was recovered as organosolv lignin and was intensively characterized by elemental analysis, FT-IR, NMR and GPC. Results showed that the recovered lignin has lower molecular weight and fewer lignin interunit linkages, suggesting the depolymerization of lignin in the course of dissolution. Cellulose was also completely dissolved in a solution of water and ethanol at 170 °C, and part of cellulose was converted to oligosaccharides and monosaccharide, even chemicals such as EL and LA. It can be said that cellulose, hemicellulose and lignin can be separated roughly from lignocellulose biomass during the course of dissolution by adjusting dissolution temperature, and obtaining chemicals, sugars and fractured lignin. This process is favourable for the downstream-integrated utilization of lignocellulose biomass.Experimental
Materials
Corn straw, obtained from Liaoning province of China, was used as feedstocks in this work. The corn straw was dried at 80 °C for 24 h after washed with distilled water to clean the biomass surface. Then, the corn straw was milled and sieved to 40–60 meshes. The corn straw is composed of 37.68% cellulose, 16.59% hemicellulose, 23.42% lignin and 22.31% other components according to the National Renewable Energy Laboratory (NREL) analytical methods.24 And its average molecular formula is C10H16.3O7.833N0.0005 determined by ultimate analysis. Milled wood lignin (MWL) was used to investigate the changes of lignin chemical characteristics after the dissolution process, which was obtained from corn straw by solvent extraction according to the Björkman method.40 All regents used in this work were of analytical grade.Experimental procedures and methods
The dissolution experiments were conducted in a 100 mL Teflon lined autoclave with an electromagnetic stirrer. For each run, 2.0 g corn straw and 60 g liquid mixtures (composed of 75.0% ethanol, 21.5% water, 1.5% peroxide hydrogen and 2.0% sulfuric acid) were loaded into the autoclave. The reactor was purged with N2 for three times and then was heated to a designated temperature at a rate of 5 °C min−1. The time of dissolution at designated temperatures was 120 min.After the reaction was finished, the reactor was cooled to room temperature by using flowing water. The reaction mixture was filtrated with sand core funnel, and the obtained insoluble residue was washed with 60 mL distilled water for three times before oven-dried at 80 °C. The filtrate was diluted with five-fold distilled water to precipitate the dissolved lignin. The recovered lignin was freeze-dried prior to weighing and off-line analysis. And the major water-soluble products were quantified by High Performance Liquid Chromatograph (HPLC). The detailed separation procedure is shown in Fig. 9.
Since gaseous fractions of less than 20 mg were collected by capsules during each run, the gaseous products generated during the dissolution of corn straw were treated as negligible and was not taken into account in the mass balance.
The dissolution degree of corn straw and the yield of extracted lignin were calculated according to the following equations:
| Dissolution degree (%) = (1 − WR/Wc) × 100% | (1) |
| Extracted lignin (%) = WRL/WL × 100% | (2) |
Characterization of raw corn straw, residue and extracted lignin
FT-IR spectra were obtained on a Nicolet iS50 FT-IR spectrophotometer (Thermo Scientific, USA). The instrument was worked with a mercury cadmium telluride (MCT) detector and the spectrum was recorded in the range of 4000–400 cm−1.The molecular weight distribution of sample was analyzed by Gel Permeation Chromatography (GPC) on a Waters 2695 HPLC apparatus (Separations Module) with Waters 2998 Photodiode Array detector (PDA) and Styragel HR4E columns. THF was used as eluent with the flow rate of 1.0 mL min−1. The injected sample volume was 20 μL, and the temperature of the column was kept at 30 °C. The average molecular weight of the sample was measured through extranet standard method wherein polystyrene was used as a standard compound.
The 13C NMR spectra of lignin samples were recorded on a Bruker NMR spectrometer at 100 MHz.
The elemental analysis of sample was carried out with a Vario EL III element analyzer (Elementar Analysensysteme Gmbh, Germany). Oxygen content was estimated by the conservation of mass based on the assumption that the samples only contain the elements of C, H, N, S and O. The Higher Heating Value (HHV) of the sample was calculated based on the Dulong formula:41
| HHV (MJ kg−1) = 0.3383C + 1.442 × (H–O/8) | (3) |
Water-soluble products analysis
The concentration of sugars and chemical products (5-hydroymethylfurfural (5-HMF), levulinic acid (LA), ethyl levulinate (EL) and furfural (FF)) in the liquid were determined by HPLC (Waters 2695S) with Shodex SH1011 Sugar column (Shodex, Japan) and Waters e2695 Reflective Index Detector (RID). 0.01 mol L−1 sulfuric acid solution was used as mobile phase with the flow rate of 0.5 mL min−1. The column temperature and detector temperature were set to 50 °C.The yields of sugars and chemical products were estimated by eqn (4).
| Yield (%) = C × V/Wc × 100% | (4) |
Acknowledgements
The authors gratefully acknowledge the financial support of National Natural Science Foundation of China (No. 51576198), the National Key Technology R&D Program (No. 2015BAD15B06), and the Youth Innovation Promotion Association of CAS (No. 2015288).Notes and references
- P. McKendry, Bioresour. Technol., 2002, 83, 47 CrossRef CAS PubMed.
- Z. Chen and J. Long, Bioresour. Technol., 2016, 214, 16 CrossRef CAS PubMed.
- X. Zhang, Q. Zhang, T. Wang, L. Ma, Y. Yu and L. Chen, Bioresour. Technol., 2013, 134, 73 CrossRef PubMed.
- M. Z. Jacobson, Energy Environ. Sci., 2009, 2, 148 CAS.
- M. Stöcker, Angew. Chem., Int. Ed., 2008, 47, 9200 CrossRef PubMed.
- P. Mäki-Arvela, I. Anugwom, P. Virtanen, R. Sjoholm and J. P. Mikkola, Ind. Crops Prod., 2010, 32, 175 CrossRef.
- S. Cheng, I. D’cruz, M. Wang, M. Leitch and C. Xu, Energy Fuels, 2010, 24, 4659 CrossRef CAS.
- J. D'Souza and N. Yan, ACS Sustainable Chem. Eng., 2013, 1, 534 CrossRef.
- H. Pan, Renewable Sustainable Energy Rev., 2011, 15, 3454 CrossRef CAS.
- Z. Fang and C. Fang, AIChE J., 2008, 54, 2751 CrossRef CAS.
- A. Emmel, A. L. Mathias, F. Wypych and L. P. Ramos, Bioresour. Technol., 2003, 86, 105 CrossRef CAS PubMed.
- R. Hu, L. Lin, T. Liu and S. Liu, Bioresour. Technol., 2010, 101, 3586 CrossRef CAS PubMed.
- A. O. Ayeni, F. K. Hymore, S. N. Mudliar, S. Deshmukh, D. Satpute, J. A. Omoleye and R. A. Pandey, Fuel, 2013, 106, 187 CrossRef CAS.
- J. A. da Costa Correia, J. E. M. Júnior, L. R. B. Gonçalves and M. V. P. Rocha, Bioresour. Technol., 2013, 139, 249 CrossRef PubMed.
- Z. Yuan, J. Long, X. Zhang, T. Wang, R. Shu and L. Ma, RSC Adv., 2016, 6, 41032 RSC.
- J. M. Hernández-Salas, M. S. Villa-Ramírez, J. S. Veloz-Rendón, K. N. Rivera-Hemández, R. A. González-César, M. A. Plascencia-Espinosa and S. R. Trejo-Estrada, Bioresour. Technol., 2009, 100, 1238 CrossRef PubMed.
- Y. Lin and S. Tanaka, Appl. Microbiol. Biotechnol., 2006, 69, 627 CrossRef CAS PubMed.
- M. Sasaki, T. Adschiri and K. Arai, AIChE J., 2004, 50, 192 CrossRef CAS.
- M. E. Himmel, S. Y. Ding, D. K. Johnson, W. S. Adney, M. R. Nimlos, J. W. Brady and T. D. Foust, Science, 2007, 315, 804 CrossRef CAS PubMed.
- Z. Yuan, J. Long, T. Wang, R. Shu, Q. Zhang and L. Ma, Energy Convers. Manage., 2015, 101, 481 CrossRef CAS.
- L. Jurasek, Y. Sun and D. S. Argyropoulos, ACS Symp. Ser., 2009, 130–148 Search PubMed.
- A. S. Amarasekara, T. B. Singh, E. Larkin, M. A. Hasan and H. J. Fan, Ind. Crops Prod., 2015, 65, 546 CrossRef CAS.
- Q. N. Xia, Q. Cuan, X. H. Liu, X. Q. Gong, G. Z. Lu and Y. Q. Wang, Angew. Chem., Int. Ed., 2014, 53, 9755 CrossRef CAS PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Laboratory analytical procedure, 2008 Search PubMed.
- X. Pan, J. F. Kadla, K. Ehara, N. R. Gilkes and J. N. Saddler, J. Agric. Food Chem., 2006, 54, 5806 CrossRef CAS PubMed.
- H. M. Liu and Y. L. Liu, Bioresour. Technol., 2014, 151, 424 CrossRef CAS PubMed.
- J. Long, B. Guo, J. Teng, Y. Yu, L. Wang and X. Li, Bioresour. Technol., 2011, 102, 10114 CrossRef CAS PubMed.
- R. El Hage, N. Brosse, L. Chrusciel, C. A. Sanchez, P. Sannigraphi and A. J. Ragauskas, Polym. Degrad. Stab., 2009, 94, 1632 CrossRef CAS.
- N. E. EI Mansouri, Q. Yuan and F. Huang, BioResources, 2011, 6, 2647 Search PubMed.
- D. She, F. Xu, Z. Geng, R. Sun, G. L. Jones and M. S. Baird, Ind. Crops Prod., 2010, 32, 21 CrossRef CAS.
- R. Sun, J. Tomkinson and G. L. Jones, Polym. Degrad. Stab., 2000, 68, 111 CrossRef CAS.
- C. Heitner, D. Dimmel and J. Schmidt, Lignin and Lignans: Advances in Chemistry, CRC Press, New York, 2010 Search PubMed.
- T. Rauhala, A. W. T. King, G. Zuckerstätter, S. Suuronen and H. Sixta, Nord. Pulp Pap. Res. J., 2011, 26, 386 CAS.
- C. W. Lahive, P. J. Deuss, C. S. Lancefield, Z. Sun, D. B. Cordes, C. Young, F. Tran, A. M. Z. Slawin, J. G. De Vries, P. C. J. Kamer, N. J. Westwood and K. Barta, J. Am. Chem. Soc., 2016, 138, 8900 CrossRef CAS PubMed.
- J. F. G. Martín, S. Sánchez and M. Cuevas, Renewable Energy, 2013, 51, 382 CrossRef.
- X. Tong, Y. Ma and Y. Li, Appl. Catal., A, 2010, 385, 1 CrossRef CAS.
- B. Girisuta, L. P. B. M. Janssen and H. J. Heeres, Green Chem., 2006, 8, 701 RSC.
- J. Long, W. Lou, L. Wang, B. Yin and X. Li, Chem. Eng. Sci., 2015, 122, 24 CrossRef CAS.
- J. N. Chheda, G. W. Huber and J. A. Dumesic, Angew. Chem., Int. Ed., 2007, 46, 7164 CrossRef CAS PubMed.
- A. Björkman, Sven. Papperstidn., 1956, 59, 477 Search PubMed.
- T. Zhang, Y. Zhou, D. Liu and L. Petrus, Bioresour. Technol., 2007, 98, 1454 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |


