Silica-supported ultra small gold nanoparticles as nanoreactors for the etherification of silanes†
Abstract
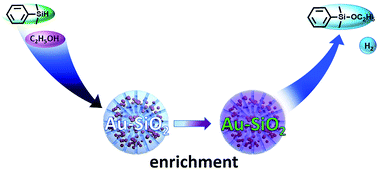
Ultra small gold nanoparticles supported by porous silica (Au–SiO2) were successfully synthesized. Due to enrichment of reactants by silica, the Au–SiO2 particles functioned as nanoreactors for catalytic etherification of silanes with high selectivity and reusability. The reaction kinetics indicated that the catalysis operated by a zero order reaction mechanism, which is contrary to previously reported homogeneous catalysts, as well as Au–Al2O3 and Au–FeOx prepared by the same method. The mechanism of the reaction was described by the Langmuir–Hinshelwood model, with the rate determining step being the surface reaction on the gold nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...