Effect of fuel content on luminescence and antibacterial properties of zinc oxide nanocrystalline powders synthesized by the combustion method†
Trilok K. Pathak*a,
Ashwini Kumara,
C. W. Swartbc,
H. C. Swarta and
R. E. Kroon *a
*a
aDepartment of Physics, University of the Free State, Bloemfontein, South Africa. E-mail: tpathak01@gmail.com; PathakTK@ufs.ac.za; KroonRE@ufs.ac.za
bDepartment of Microbial, Biochemical and Food Biotechnology, University of the Free State, Bloemfontein, South Africa
cLaboratory for Microscopy, University of the Free State, Bloemfontein, South Africa
First published on 3rd October 2016
Abstract
Nanoscale ZnO powders were synthesized via the combustion method using zinc nitrate hexahydrate as the source (oxidant) material and urea, and glycine or citric acid monohydrate as fuels. The effect of the relative fuel to oxidant ratio on the characteristics of ZnO particles produced with urea as the fuel was also investigated. X-ray diffraction analysis revealed that the ZnO nanocrystalline particles were successfully synthesized by combustion and the crystallite size was different depending on the fuel. The surface morphology showed a dramatic change as the fuel changed in the synthesis process. The chemical states of the synthesized ZnO powders were investigated using X-ray photoelectron spectroscopy, which allowed an assessment of the Zn and O related defect concentrations. The optical bandgap varied from 3.12 to 3.14 eV for different fuels and it decreased to 3.10 eV in the fuel rich case for urea. The maximum defect level photoluminescence emission was observed for the ZnO powder synthesized using urea as the fuel, for the stoichiometric fuel mix. All products exhibited similar antibacterial effects; however they had a greater effect on Staphylococcus aureus compared to Escherichia coli.
1. Introduction
Nanoscale ZnO powder has attracted great attention due to its excellent physical and chemical properties. It is widely used in nanoscale devices such as nanogenerators,1 ultraviolet photodetectors,2 gas sensors,3 solar cells,4 field emission displays,5 electrical and optical devices,6,7 photocatalysis,8,9 medical10 and environmental applications.11 These nanomaterials have novel electronic, structural and thermal properties which have potential interest in basic and applied research. ZnO is a wide band gap (Eg = 3.37 eV) semiconductor with a large free excitation binding energy (60 meV).12 Semiconductor nanocrystals or nanoparticles may have superior optical and antibacterial properties than bulk crystals due to quantum confinement effects and the large surface to volume ratio. The synthesis and properties of ZnO nanostructure such as nanowires,13 nanotubes,14 nanorods15 and nanoparticles16 have been reported. The nanoparticles have great significance as three dimensional confined systems bridging the gap between bulk materials and molecular compounds. A variety of techniques have been employed for the synthesis of ZnO nanoparticles such as sol–gel synthesis,17 the hydrothermal method,18 the solution combustion method19 and solid state reactions.20 Among these, the combustion technique is noteworthy as a fast method to synthesize nanocrystalline materials in as-synthesized form with large surface area without the further need of heat treatment. Nanocrystalline oxides are produced through the redox reaction between an oxidizer containing the metal precursor and an organic fuel at a moderately low initiation temperature of around 350–600 °C within a few minutes.21 The main advantage of this method is that the high temperature of the exothermic reaction assures high purity and well crystallized powder. In combustion synthesis, the type of fuel and the fuel to oxidizer ratio (F/O) play critical roles in influencing the nature of combustion reaction and the flame temperature. Potti and Srivastava22 synthesised ZnO powder by the combustion method using different fuels in solution (citric acid, dextrose, glycine, oxalyl dihydrazide, oxalic acid, and urea) and investigated structural, optical and textural properties. They observed that crystallite size and bandgap of ZnO was influenced by different fuels. Different modifications made to conventional combustion approaches for preparation of nanomaterials are critically analyzed by Aruna et al.23 They described that the combustion process involves a self-sustained reaction in a homogeneous solution of different oxidizers (metal nitrates) and fuels (urea, glycine, hydrazides). It is concluded that fuels in the combustion synthesis process play an important role to get nanosize oxide materials. Selection of a suitable fuel and the F/O ratio influences the combustion process and the properties of the product. Noori et al.24 reported that a stoichiometric fuel-oxidizer mix (F/O = 1) is known to produce the highest exothermicity with complete combustion. An arbitrary F/O ratio sometimes leads to formation of intermediate phases or raw materials in the final product. In this regard, various fuels have been tested to synthesize nanocrystalline ZnO. Sousa et al.25 used metal nitrate and urea to synthesis ZnO nanopowder with a size about 400–500 nm for various applications. Hwang et al.26 worked on ZnO nanopowder synthesized by a combustion method with glycine as a fuel and metal nitrate mixed in a stoichiometric ratio.In the present work, we report the synthesis of nanocrystalline ZnO powders by the combustion technique using a variety of organic fuels, namely urea, glycine and citric acid monohydrate. The effect of the type of fuel, and the F/O ratio in the case of urea, on the properties of the final product has been studied. The structure, morphology, optical and luminescence as well as antibacterial properties of the resulting ZnO nanoparticles are reported.
2. Experimental details
2.1 Preparation of ZnO nanocrystalline powders
For synthesis of ZnO nanoparticles, zinc nitrate hexahydrate, i.e. Zn(NO3)2·6H2O, was used as the precursor (oxidant) while different materials, namely urea, glycine, or citric acid monohydrate, were used as the fuel. All the chemicals were purchased from Sigma Aldrich and used as received. Table 1 shows the combustion reactions for the different fuels used. The F/O ratio for a combustion reaction is determined by
 | (1) |
| Fuel | Combustion reaction |
|---|---|
| Urea | 3Zn(NO3)2 + 5CO(NH2)2 → 3ZnO + 5CO2 + 10H2O + 8N2 |
| Glycine | Zn(NO3)2 + 2CH2(NH2)(COOH) + 2O2 → ZnO + 4CO2 + 5H2O + 2N2 |
| Citric acid | Zn(NO3)2 + C3H5O(COOH)3 + 2O2 → ZnO + 6CO2 + 4H2O + N2 |
The zinc nitrate hexahydrate and fuel were dissolved in 5 ml of double distilled water and stirred thoroughly to obtain a transparent solution, which was placed inside a pre-heated muffle furnace at 600 °C to initiate the combustion process. Within a short time (less than 5 min) the mixture ignited with a flame and the rapid evolution of enormous amounts of gases produced a voluminous foamy product (ash). This was ground using an agate pestle and mortar to produce the final powder, without any additional heat treatment. The synthesis process is illustrated in Fig. 1.
2.2 Characterization methods
The prepared ZnO was characterized by X-ray diffraction (XRD) using a Bruker D8 Advance diffractometer with Cu Kα radiation to assess the crystal structure. A PHI 5000 Versaprobe system was used to examine the surface chemistry with X-ray photoelectron spectroscopy (XPS) analysis using Al Kα X-rays of energy 1486.6 eV. The surface morphology and elemental analysis of the samples was investigated by scanning electronic microscopy (SEM) (Jeol JSM-7800F microscope with a field emission electron gun, equipped with an energy dispersive X-ray spectroscopy (EDS) detector from Oxford Instruments), while the ZnO nanoparticles were imaged using a Philips CM100 transmission electron microscope (TEM). Diffuse reflectance (DR) spectra were collected using a Lambda 950 UV-vis spectrophotometer from PerkinElmer equipped with a spectral on integrating sphere. The photoluminescence (PL) data was excited using a 325 nm He–Cd laser and the emission was detected by a photomultiplier tube after being dispersed in a Horiba iHR320 spectrometer. For the antibacterial tests, Staphylococcus aureus and Escherichia coli were cultivated for 48 h at 37 °C to obtain cell growth. Cells were then suspended in distilled water and used for the steps to follow. S. aureus and E. coli were cultivated as lawn cultures on nutrient agar plates by spreading 100 μl of the respective bacterial suspensions onto the agar using a sterile bent glass rod. The lawn was left to dry, after which a small amount of the various ZnO test samples were placed in the middle of each test plate. This allows for a concentration gradient to form towards the periphery of the plate (plate diffusion method). Plates were incubated at 37 °C for 48 h where after the radius of the zone of inhibition for each plate (if present, indicating antibacterial activity) was measured.3. Results and discussion
3.1 X-ray diffraction
The XRD patterns of the ZnO powders synthesized using a stoichiometric F/O ratio of one is depicted in Fig. 2 and are typical of ZnO powders having the hexagonal wurtzite structure (JCPDS 01-075-0576). This indicates that the ZnO was formed directly by the self-propagating high temperature exothermic combustion reaction initiated at moderate temperature, without the need of subsequent heat treatment.None of the samples showed extra peaks from the reactants or impurity phases. The crystallite size may be estimated from the Scherrer equation27
 | (2) |
For urea as the fuel, Fig. 3 shows the XRD patterns for different F/O ratios. The (101) peak in the XRD patterns shows a small shift towards higher angle as the F/O ratio was increased, which might be caused by the strain produced on the surface. Fig. 4 shows Williamson–Hall (W–H) plots for the different fuel ratios, from which the crystallite size as well as microstrain were calculated according to β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = Kλ/D + 4ε
θ = Kλ/D + 4ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where β is the 2θ-FWHM (in radians) of the diffraction peaks, λ is the X-ray wavelength, K is a shape factor taken as 0.9, D is the crystallite size and ε is the microstrain.28 The W–H plots have slope equal to the microstrain and an intercept of Kλ/D from which the crystallite size can be determined. The crystallite size and microstrain for samples prepared using urea as the fuel for different fuel ratio during combustion synthesis are shown in Table 2.
θ, where β is the 2θ-FWHM (in radians) of the diffraction peaks, λ is the X-ray wavelength, K is a shape factor taken as 0.9, D is the crystallite size and ε is the microstrain.28 The W–H plots have slope equal to the microstrain and an intercept of Kλ/D from which the crystallite size can be determined. The crystallite size and microstrain for samples prepared using urea as the fuel for different fuel ratio during combustion synthesis are shown in Table 2.
| F/O | Crystallite size Scherrer's equation (nm) | Crystallite size W–H plots (nm) | Strain (%) |
|---|---|---|---|
| 0.8 | 29 | 30 | 0.02 |
| 0.9 | 27 | 29 | 0.05 |
| 1.0 | 27 | 28 | 0.02 |
| 1.1 | 37 | 38 | 0.12 |
The crystallite size of the F/O = 1 sample is 28 nm, while the microstrain has a very small value of 0.02%, indicating only a small amount of microstrain in this material produced with the combustion method. Srinatha et al.29 have made a study of ZnO prepared using L-glutamine, leucine and L-valine fuels and different F/O ratios which were much further from stoichiometric than in the present study. While they reported that the crystallite size decreased consistently with increasing F/O ratio, in the present study the minimum crystallite size was obtained with the stoichiometric F/O ratio of one.
3.2 X-ray photoelectron spectroscopy
Fig. 5 and 6 shows the XPS results obtained for the ZnO samples produced with different fuels. Photoelectron peaks corresponding to Zn, O, and C were detected. The C is due to adventitious hydrocarbons adsorbed on the surface during the exposure of the sample to the ambient atmosphere. All binding energies were corrected for the shift caused by charging using the C 1s peak (284.6 eV) as a ref. 30. The high resolution XPS spectra of the Zn 2p with binding energy is shown in Fig. 5(a) and contain the doublet corresponding to 2p3/2 and 2p1/2 transitions whose binding energies are 1021.2 and 1044.3 eV. The binding energy difference between the two lines is 23.1 eV, which corresponds well to the standard reference value of ZnO. The values of the binding energies and binding energy difference, obtained from the XPS study, show that the Zn atoms are in the Zn2+ oxidation state.31 The Zn peaks for the sample prepared with urea show some broadening and are deconvoluted in Fig. 5(b). It is clear that two distinct peaks are present, which are attributed to the ZnO matrix and to Zn related defects.32 Different native point defect such as oxygen vacancy (VO), zinc vacancy (VZn), interstitial zinc (Zni) and interstitial oxygen (Oi) have been proposed to be responsible for various defects in ZnO.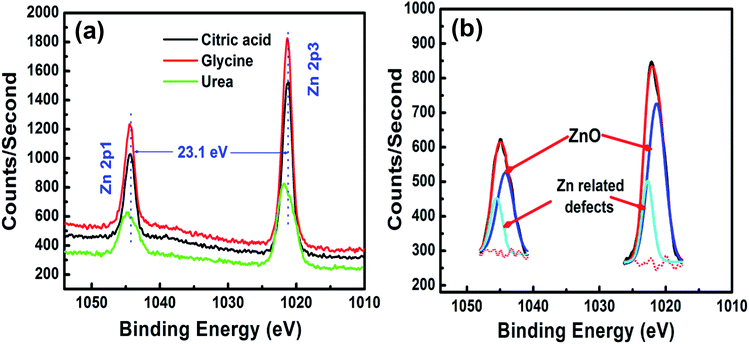 | ||
| Fig. 5 (a) High resolution XPS spectra of the Zn 2p peaks, with (b) deconvoluted Zn peak for the sample produced using urea. | ||
 | ||
| Fig. 6 O 1s peak in ZnO synthesized with (a–c) different fuels and (c–e) urea with different F/O ratios. | ||
Kumar et al.33 reported that the ZnO, O 1s peak may be de-convoluted into three peaks, namely O1 at 530.2 eV, O2 at 531.2 eV and O3 at 532.6 eV. They attributed the O1 peak to O2− ions in the perfect hexagonal wurtzite structure of the ZnO lattice, while the O2 peak is associated with O2− ions associated with defects within the matrix of ZnO. The O3 peak originates from chemisorbed species (such as CO3, H2O or O2) on the surface of the ZnO.34 The high resolution O 1s peaks for the ZnO samples prepared with different fuels and different F/O are shown in Fig. 6. The binding energy position and area under the different peak are shown in Table 3. The position of the O1, O2 and O3 peaks change only with the different fuels and this is not believed to be significant. The area under the O2 peak is large for all the samples, suggesting intrinsically high defect concentrations for such samples prepared by the combustion method, and it is surprising that the maximum value occurs for the sample prepared using a stoichiometric proportion of urea (F/O = 1.0).
| Fuel | Peak position (±0.3 eV) | Area (%) | ||||
|---|---|---|---|---|---|---|
| O1 | O2 | O3 | O1 | O2 | O3 | |
| Citric acid | 530.1 | 530.8 | 532.1 | 21 | 57 | 22 |
| Glycine | 530.0 | 530.7 | 532.4 | 18 | 65 | 17 |
| F/O = 1.0 urea | 530.1 | 531.2 | 532.6 | 23 | 69 | 8 |
| F/O = 0.8 urea | 530.2 | 531.3 | 532.3 | 48 | 37 | 15 |
| F/O = 1.1 urea | 530.1 | 531.4 | 532.6 | 29 | 56 | 15 |
3.3 Electron microscopy
The microstructure of ZnO samples prepared with different types of fuel for a stoichiometric reaction are shown by SEM images in Fig. 7. When citric acid was used as the fuel (Fig. 7(a)) it resulted in small spherical nanoparticles of ZnO approximately 30 nm in diameter that were weakly agglomerated. The glycine fuel produced individual monodispersed nanoparticles ranging from about 30 to 300 nm in size (Fig. 7(c)), the larger ones having irregular structure and facets suggesting they consist of several fused grains. Using urea as the fuel resulted in monolithic growth of much larger particles, which Fig. 7(e) shows to have a faceted external surface which indicates a multicrystalline nature. The finest particles were obtained when urea was used as the fuel because no oxygen was required with urea in the combustion process and the formation temperature of the ZnO material was easily achieved. Fig. 7(b), (d) and (f) show EDS spectra that confirm the formation of ZnO nanomaterial by different fuels as citric acid, glycine and urea respectively. Although some C contamination is generally expected to be observed during EDS, the amount of C varied significantly and was most prominent for the sample prepared with citric acid as fuel, which also had the greatest C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn atom ratio in the precursor mix (see Table 1).
Zn atom ratio in the precursor mix (see Table 1).
 | ||
| Fig. 7 Morphology and EDS results of ZnO synthesized by (a, b) citric acid (c, d) glycine (e, f) urea. | ||
Fig. 8 shows the impact of the F/O ratio on the ZnO growth using urea as the fuel. The fuel lean (F/O = 0.8) ZnO material exhibited cauliflower morphology. After increasing the fuel content to stoichiometric (F/O = 1.0) conditions the minimum particle size (roughness) is obtained on the growth surface, while large plate-like surfaces were formed under fuel rich (F/O = 1.1) conditions. In the fuel rich condition, due to insufficient oxygen for complete combustion, the particles are weakly agglomerated. Nanoflower ZnO structures were also obtained by Sharma et al.35 using the sol–gel method and considered as promising antibacterial agents. The surface area and morphology of samples which depends on the F/O ratio may be an important consideration in this application of ZnO material. EDS results confirm the formation of ZnO nanomaterial by different F/O ratios. The carbon peak also obtained in EDS spectra is only significant in the fuel rich sample (F/O) of Fig. 8, but varies in different positions for the same sample (compare Fig. 7(f) and 8 for F/O = 1.0) and may be due to contamination or carbon present in the fuels.
Although XRD (and the Scherrer equation) is widely used to determine the crystallite size, with TEM besides directly measuring the particle size the morphology of the particles can also be observed. Therefore TEM is an excellent complementary way to determine the particle and crystallite size of nanoparticles. The TEM results are presented in Fig. 9 for ZnO prepared with combustion using citric acid and glycine as fuels. As already revealed by the SEM results, the ZnO synthesized using urea has large cauliflower structure agglomeration of crystallites compared to these two fuels, making it hard to measure by TEM because there are no individual small nanoparticles, and therefore no useful results were obtained. For both citric acid and glycine cases, the TEM shows the ZnO nanoparticles to have hexagonal faceted shapes, consistent with their crystal structure. Zak et al.36 used TEM to examine ZnO nanoparticles synthesized by the combustion method and reported hexagonal ZnO nanoparticles. The particle size distribution analysis showed quite a wide distribution in particle size with an average of about 24 nm for citric acid (Fig. 9(a)) and 44 nm for glycine (Fig. 9(b)). These show the same trend as for the crystallite sizes determined by XRD (19 nm for citric acid and 22 nm for glycine) but the value for glycine is significantly larger. This suggests that many of the ZnO nanoparticles produced with glycine consist of two or more crystallites, as also seen from the SEM data. Note that the Scherrer equation assumes a single particle size rather than a range of value and that the XRD broadening can be influenced by the smallest crystallites, while a drawback of TEM is the limited sampling from the entire sample.
3.4 Optical studies
 | (3) |
 | ||
| Fig. 10 (a) Absorbance with reflectance and (b) Tauc plots to determine the optical bandgap of the ZnO synthesis by different fuel. | ||
 | ||
| Fig. 11 (a) Absorbance with reflectance and (b) Tauc plots to determine the optical bandgap of ZnO synthesised by different F/O ratios using urea. | ||
ZnO has a direct transition and the corresponding bandgaps for different fuels and different contents of urea shown in Fig. 10(b) and 11(b) respectively are calculated from a Tauc plot of (αhν)2 versus the photon energy (hν).39 The optical bandgap varies from 3.12 to 3.14 eV when using different fuels and it decreased to 3.10 eV in the fuel rich case as the urea content increased as shown in Fig. 11(b). Such changes in the ZnO bandgap energy are in good agreement with the corresponding shift seen in the absorption edge.
 | ||
| Fig. 12 PL spectra for ZnO nanoparticles with (a) different fuels and (b) different F/O ratios using urea. | ||
The PL spectra of ZnO nanoparticles synthesised with different fuel ratios of urea are shown in Fig. 12(b). Only deep level defect emission was obtained which has a broad range of 450–800 nm. The position of the PL peak changes from 641, 628, 632, 620 nm with respect to fuel content F/O = 0.8, 0.9, 1.0, 1.1. In the stoichiometric condition (F/O = 1) the defect level was a maximum compared to fuel lean (F/O = 0.8, 0.9) and fuel rich (F/O = 1.1) conditions. The red shift in PL emission from lean fuel to stoichiometric conditions may be due to the higher density of defects and hence an interactions between them. Considering these interesting optical properties, ZnO nano material may be used in photodetectors, transparent electrodes, solar cells and other optical devices.
3.5 Antibacterial studies
The ZnO produced with the different fuels had significantly different microstructures and were tested for antibacterial activity against S. aureus and E. coli using the plate diffusion technique. As expected, the control plates did not exhibit any antibacterial effect. However, the ZnO produced with all three fuels displayed antibacterial activity as indicated by the inhibition zone surrounding the compounds on the plate. The inhibition zone indicates that bacterial cells could not survive at a high concentration of the compound. These experiments were repeated in triplicate resulting in an average inhibition zone (measuring the radius) of 8.48 mm for ZnO with urea, 8.25 mm with glycine and 9.47 mm with citric acid when testing against S. aureus. Since the measurements have an accuracy of about ±0.1 mm, it can be concluded that the ZnO produced with citric acid was slightly more effective, but the other two samples showed no significant difference. When testing against E. coli an inhibition zone of 5.25 mm was measured for ZnO produced with urea, 5.00 mm with glycine and 3.25 mm with citric acid. In general the effectivity against E. coli was lower than against S. aureus, and the ZnO sample produced using citric acid was significantly less effective than the other two samples. These results are summarized in Fig. 13.Similar results were investigated by Ekthammathat et al.43 where the maximum antibacterial activity of ZnO was against S. aureus. This is because of the attachment of ZnO particle to the outer cell wall membrane of the bacteria. ZnO with defects have different morphology and could be activated by photon to create electron–hole (e−–h+) pairs; the hole split the H2O molecules on the ZnO particles into OH and H+.44 The electron reacted with oxygen molecules and hydrogen ions to produce molecules of H2O2. The mechanism of the inhibitory effects of ZnO nanoparticles on bacteria may be explained on the basis of low tolerance for oxygen. Due to the lack of some important oxidative stress response genes and global stationary-phase stress response gene bacteria are extremely sensitive to oxidative stress as well as to other environmental stresses.45 Dutta et al.46 explained that the hydroxyl radicals generated in suspensions of ZnO nanomaterials in culture media could extract the hydrogen atoms from the allylic position of the unsaturated fatty acid. The allyl free radical that is formed might react with the oxygen molecule to form lipid peroxide radical. The radicals penetrate the inner cell membranes and created serious disruption of their internal contents. Thus ZnO particle may inhibit the growth of the cell and finally the death of the cell.
4. Conclusions
ZnO powders were successfully synthesized by the combustion method using different fuels and different F/O ratios. The XRD patterns were consistent with polycrystalline ZnO having the hexagonal wurtzite structure, and the micro strain of the samples was found to be very low. The ZnO NP size changed for different fuels with the minimum crystallite size of 19 nm obtained by using citric acid as the fuel. When urea was used as the fuel with different F/O ratios, the stoichiometric condition produced the minimum crystallite size due to complete combustion compared to the fuel lean and fuel rich cases. The morphology of ZnO also changed with different fuels. XPS results allowed an assessment of the defect concentrations on the surface of the ZnO powder, both in terms of Zn and O related defects. In the ZnO powder synthesized with urea the near-band-edge PL peak intensity was negligible compared to the defect emission. Maximum defect peak broadening between 450–800 nm was obtained with urea in stoichiometric condition. The inhibition zone radii for S. aureus and E. coli were also affected by oxygen vacancies, morphology or defects in the ZnO powder. The ZnO powder synthesized by urea showed the maximum inhibition zone radius value of 8.48 mm and 5.25 mm for S. aureus and E. coli respectively. These materials may be used in other antibacterial and antifungal studies.Acknowledgements
This work is supported both by the South African Research Chairs Initiative of the Department of Science and Technology, the National Research Foundation of South Africa (84415). The PL system used in this study is supported both technically and financially by rental pool programme of the national laser centre (NCL) (Grant No. NLCLREGM00-CON-001). Dr Liza Coetsee-Hugo is acknowledged for XPS measurements. The University of the Free State is acknowledged for financial support.References
- X. Wang, J. Song and Z. L. Wang, Nanowire and nanobelt arrays of zinc oxide from synthesis to properties and novel devices, J. Mater. Chem., 2007, 17, 711–720 RSC.
- J. H. Jun, H. Seong, K. Cho, B. M. Moon and S. Kim, Ultraviolet photodetectors based on ZnO nanoparticles, Ceram. Int., 2009, 35, 2797–2801 CrossRef CAS.
- H. M. Lin, S. J. Tzeng, P. J. Hsiau and W. L. Tsai, Electrode effects on gas sensing properties of nanocrystalline zinc oxide, Nanostruct. Mater., 1998, 10, 465–477 CrossRef CAS.
- Z. S. Wang, C. H. Huang, Y. Y. Huang, Y. J. Hou, P. H. Xie, B. W. Zhang and H. M. Cheng, A highly efficient solar cell made from a dye-modified ZnO-covered TiO2 nanoporous electrode, Chem. Mater., 2001, 13, 678–682 CrossRef CAS.
- M. Kitano and M. Shiojiri, Benard convection ZnO/resin lacquer coating—a new approach to electrostatic dissipative coating, Powder Technol., 1997, 93, 267–273 CrossRef CAS.
- M. J. Zheng, L. D. Zhang, G. H. Li and W. Z. Shen, Fabrication and optical properties of large-scale uniform zinc oxide nanowire arrays by one-step electrochemical deposition technique, Chem. Phys. Lett., 2002, 363, 123–128 CrossRef CAS.
- R. Wu and C. S. Xie, Formation of tetrapod ZnO nanowhiskers and its optical properties, Mater. Res. Bull., 2004, 39, 637–645 CrossRef CAS.
- M. L. Curridal, R. Comparelli, P. D. Cozzli, G. Mascolo and A. Agostiano, Colloidal oxide nanoparticles for the photocatalytic degradation of organic dye, Mater. Sci. Eng., C, 2003, 23, 285–289 CrossRef.
- V. P. Kamat, R. Huehn and R. Nicolaescu, A sense and shoot approach for photocatalytic degradation of organic contaminants in water, J. Phys. Chem. B, 2002, 106, 788–794 CrossRef.
- J. W. Rasmussen, E. Martinez, P. Louka and D. G. Wingett, Zinc Oxide Nanoparticles for Selective Destruction of Tumor Cells and Potential for Drug Delivery Applications, Expert Opin. Drug Delivery, 2010, 7(9), 1063–1077 CrossRef CAS PubMed.
- I. Udom, M. K. Ram, E. K. Stefanakos, A. F. Hepp and D. Y. Goswami, One dimensional-ZnO nanostructures: Synthesis, properties and environmental applications, Mater. Sci. Semicond. Process., 2013, 16(6), 2070–2083 CrossRef CAS.
- A. Janotti and C. G. Van de walle, Fundamental of Zinc oxide as a semiconductor, Rep. Prog. Phys., 2009, 72, 126501–126529 CrossRef.
- Y. C. Kong, D. P. Yu, B. Zhang, W. Fang and S. Q. Feng, Ultraviolet emitting ZnO nanowires synthesis by a chemical vapour deposition approach, Appl. Phys. Lett., 2001, 78(4), 407–409 CrossRef CAS.
- Y. J. Xing, Z. H. Xi, Z. Q. Xue, X. D. Zhang, J. H. Song, R. M. Wang, J. Xu, Y. Song, S. L. Zhang and D. P. Yu, Optical properties of the ZnO nanotubes synthesized via vapor phase growth, Appl. Phys. Lett., 2003, 83, 1689–1691 CrossRef CAS.
- B. P. Zhang, N. T. Binh, Y. Segawa, Y. Kashiwaba and K. Haga, Photoluminescence study of ZnOnanorodsepitaxially grown on sapphire (1120) substrates, Appl. Phys. Lett., 2004, 84, 586–588 CrossRef CAS.
- L. Guo, S. Yang, C. Yang and P. Yu, Highly monodisperse polymer-capped ZnO nanoparticles: Preparation and optical properties, Appl. Phys. Lett., 2000, 76, 2901–2903 CrossRef CAS.
- S. Y. Chu, T. M. Yan and S. L. Chen, Characteristics of sol–gel synthesis of ZnO based powders, J. Mater. Sci. Lett., 2000, 19, 349–352 CrossRef CAS.
- B. Liu and H. C. Zeng, Hydrothermal synthesis of ZnO nanorods in the diameter regime of 50 nm, J. Am. Chem. Soc., 2003, 125, 4430–4431 CrossRef CAS PubMed.
- A. Aimable, M. T. Buscaglia and P. Bowen, Polymer-assisted precipitation of ZnO nanoparticles with narrow particle size distribution, J. Eur. Ceram. Soc., 2010, 30, 591–598 CrossRef CAS.
- Z. P. Sun, L. Liu, L. Zhang and D. Z. Jia, Rapid synthesis of ZnO nano-rods by one step, room-temperature, solid-state reaction and their gas-sensing properties, Nanotechnology, 2006, 17, 2266–2270 CrossRef CAS.
- K. C. Patil, S. T. Aruna and T. Mimani, Combustion synthesis: an update, Curr. Opin. Solid State Mater. Sci., 2002, 6, 507–512 CrossRef CAS.
- P. R. Potti and V. C. Srivastava, Comparative Studies on Structural, Optical, and Textural Properties of Combustion Derived ZnO Prepared Using Various Fuels and Their Photocatalytic Activity, Ind. Eng. Chem. Res., 2012, 51, 7948–7956 CrossRef CAS.
- S. T. Aruna and A. S. Mukasyan, Combustion synthesis and nanomaterials, Curr. Opin. Solid State Mater. Sci., 2008, 12, 44–50 CrossRef CAS.
- N. Riahi-Noori, R. Sarraf-Mamooryb, P. Alizadehb and A. Mehdikhani, Synthesis of ZnO nano powder by a gel combustion method, J. Ceram. Process. Res., 2008, 9(3), 246–249 Search PubMed.
- V. C. de Sousa, M. R. Morelli and R. H. G. Kiminami, Combustion process in the synthesis of ZnO–Bi2O3, Ceram. Int., 2000, 26, 561–564 CrossRef CAS.
- C. C. Hwang and T. Y. Wu, Synthesis and characterization of nanocrystalline ZnO powders by a novel combustion synthesis method, Mater. Sci. Eng., B, 2004, 111, 197–206 CrossRef.
- R. E. Kroon, Nanoscience and the Scherrer equation versus the ‘Scherrer–Gottingen equation’, S. Afr. J. Sci., 2013, 109(5/6), a0019 Search PubMed , 2 pages.
- W. A. I. Tabaza, H. C. Swart and R. E. Kroon, Optical properties of Bi and energy transfer from Bi to Tb in MgAl2O4 phosphor, J. Lumin., 2014, 148, 192–197 CrossRef CAS.
- N. Srinatha, V. Dinesh Kumar, K. G. M. Nair and B. Angadi, The effect of fuel and fuel-oxidizer combinations on ZnO nanoparticles synthesized by solution combustion technique, Adv. Powder Technol., 2015, 26, 1355–1363 CrossRef CAS.
- J. F. Moulder, W. F. Strickle, P. E. Sobol and K. D. Bomben, Handbook of X-rayPhotoelectron Spectroscopy, ULVAC-PHI Inc, 1995 Search PubMed.
- M. N. Islam, T. B. Ghosh, K. L. Chopra and H. N. Acharya, XPS and X-ray diffraction studies of aluminum-doped zinc oxide transparent conducting films, Thin Solid Films, 1996, 280, 20–25 CrossRef CAS.
- V. Kumar, F. Singh, O. M. Ntwaeaborwa and H. C. Swart, Effect of Br+6 ions on the structural, morphological and luminescent properties of ZnO/Si thin films, Appl. Surf. Sci., 2013, 279, 472–478 CrossRef CAS.
- V. Kumar, H. C. Swart, O. M. Ntwaeaborwa, R. E. Kroon, J. J. Terblans, S. K. K. Shaat, A. Yousif and M. M. Duvenhage, Origin of the red emission in zinc oxide nanophosphors, Mater. Lett., 2013, 101, 57–60 CrossRef CAS.
- R. Al-Gaashani, S. Radiman, A. R. Daud, N. Tabet and Y. Al-Douri, XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods, Ceram. Int., 2013, 39, 2283–2292 CrossRef CAS.
- V. Sharma, A. Mohammad, V. Mishra, A. Chaudhary, K. Kapoor and S. M. Mobin, Fabrication of innovative ZnO nanoflowers showing drastic biological activity, New J. Chem., 2016, 40, 2145–2155 RSC.
- A. Khorsand Zak, M. Ebrahimizadeh Abrishami, W. H. A. Majid, R. Yousefi and S. M. Hosseini, Effects of annealing temperature on some structural and optical properties of ZnO nanoparticles prepared by a modified sol–gel combustion method, Ceram. Int., 2011, 37, 393–398 CrossRef.
- H. Yu, J. Yu, B. Cheng and M. Zhou, Effects of hydrothermal post-treatment on microstructures and morphology of titanate nanoribbons, J. Solid State Chem., 2006, 179, 349–354 CrossRef CAS.
- M. Dressel and G. Gruner, Electrodynamics of Solids, Optical Properties of Electron in Matter, Cambridge University Press, Cambridge, 2002, pp. 159–165 Search PubMed.
- J. Yu, C. Li and S. Liu, Effect of PSS on morphology and optical properties of ZnO, J. Colloid Interface Sci., 2008, 326, 433–438 CrossRef CAS PubMed.
- M. H. Huang, Y. Wu, H. Feick, N. Tran, E. Weber and P. Yang, Catalytic growth of zinc oxide nanowires by vapor transport, Adv. Mater., 2001, 13(2), 113–116 CrossRef CAS.
- B. Srinivasa Rao, B. Rajesh Kumar, V. Rajagopal Reddy and T. Subba Rao, Preparation and characterization of CdS nanoparticles by chemical co-precipitation technique, Chalcogenide Lett., 2011, 8(3), 177–185 Search PubMed.
- J. R. Heath and J. J. Shiang, Covalency in semiconductor quantum dots, Chem. Soc. Rev., 1998, 27(1), 65–71 RSC.
- N. Ekthammathat, S. Thogtem, T. Thogtem and A. Phuruangrat, Characterization and antibacterial activity of nanostructure ZnO thin films synthesized through a hydrothermal method, Powder Technol., 2014, 254, 199–205 CrossRef CAS.
- S. Dwivedi, R. Wahab, F. Khan, Y. K. Mishra, J. Musarrat and A. A. Al-Khedhairy, Reactive Oxygen Species Mediated Bacterial Biofilm Inhibition via Zinc Oxide Nanoparticles and Their Statistical Determination, PLoS One, 2014, 9(11), e111289 Search PubMed.
- Y. Xie, Y. He, P. L. Irwin, T. Jin and X. Shi, Antibacterial Activity and Mechanism of Action of Zinc Oxide Nanoparticles against Campylobacter jejuni, Appl. Environ. Microbiol., 2011, 77(7), 2325–2331 CrossRef CAS PubMed.
- R. K. Dutta, B. P. Nenavathu, M. K. Gangishetty and A. V. R. Reddy, Studies on antibacterial activity of ZnO nanoparticles by ROS induced liquid peroxidation, Colloids Surf., B, 2012, 97, 143–150 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22341a |
| This journal is © The Royal Society of Chemistry 2016 |