Influence of lipase under ultrasonic microwave assisted extraction on changes of triacylglycerol distribution and melting profiles during lipolysis of milk fat
Khamis Ali Omarab,
Mahamadou Elhadji Goungac,
Ruijie Liua,
Waleed Aboshoraa,
Nabil Qaid M. Al-Hajja,
Qingzhe Jina and
Xingguo Wang*a
aState Key Laboratory of Food Science and Technology, Synergetic Innovation Center of Food Safety and Nutrition, School of Food Science and Technology, Jiangnan University, Wuxi 214122, Jiangsu, China. E-mail: wxg1002@qq.com; Fax: +86-510-85876799; Tel: +86-510-85876799
bDepartment of Food Safety and Quality, Zanzibar Food and Drugs Board, P. O. Box 3595, Zanzibar, Tanzania
cDépartement des Sciences et Techniques de Productions Végétales, Faculté d’Agronomie et des Sciences de l’Environnement, Université Dan Dicko Dan Koulodo de Maradi, BP 465 Maradi, Niger
First published on 12th October 2016
Abstract
In this study, the use of low irradiation ultrasonic microwave assisted extraction (UMAE) on lipases mixed with anhydrous milk fat (AMF) or anhydrous buffalo milk fat (ABF), with the aim of hydrolyzing triacylglycerols (TAGs), especially those containing short chain fatty acids, is reported. Lipozyme-435, Novozyme-435 and Thermomyces lanuginosus were mixed with phosphate buffer and either AMF or ABF in a jacketed flask. The reaction mixtures were exposed to low irradiation UMAE under temperature control (50 °C ± 2 °C) for 45 minutes. An ultra-performance liquid chromatography (UPLC) system coupled with quadrupole time-of-flight mass spectrometry (Qu-ToF-MS) was used to analyze TAG in AMF and ABF after lipolysis. The re-distribution was evidenced by the presence of a higher percentage of TAGs with at least two medium-chain fatty acids and one long-chain fatty acid, and a high percentage of TAGs with long-chain fatty acids as a consequence of a decreased percentage of TAGs with short-chain fatty acids. Furthermore, the melting and crystallization profiles were modified in both AMF and ABF treated with lipases in comparison to just AMF or ABF.
Introduction
Milk fat is a natural product with excellent organoleptic properties, which make it an important ingredient in the bakery and confectionery industry. Milk fat is composed of different fatty acids which form numerous heterogeneous triacylglycerol (TAG) molecules, resulting in a very broad melting range of approximately −40 °C to 40 °C.1–6 The presence of both free short-chain fatty acids (C4:0 to C8:0) and short-chain fatty acids as part of TAG molecules contributes to a desirable flavor in butter.7 Lipases that hydrolyze TAG molecules, especially those that contain short-chain fatty acids, with the aim of producing elevated levels of short-chain fatty acids, can serve as bases for the production of food flavor ingredients.7Knowledge of TAG composition is very important due to the fact that TAGs do not only contribute to nutritional value, but also contribute to the properties of fats and oils, for instance mouth feel and melting properties.8 TAGs are a large class of neutral lipids that naturally occur in both oil and fats, and consist of an esterified glycerol backbone with three fatty acid residues.9,10 Separation and identification of TAGs, especially in milk fat, still poses a challenge to scientists and therefore, there are many strategies applied for their investigation. Mass spectrometry (MS) is well known as a powerful technology for analyzing TAGs,8 especially when combined with efficient separation techniques.11 Quadrupole time-of-flight mass spectrometry (Qu-ToF-MS) has been recently reported to serve as a powerful tool for identifying TAGs in lipids, due to its excellent ability to accurately detect MS/MS fragment ions produced.12
The study of crystallization is also important from both technological and academic points of view for understanding the effects of formulation and processing factors on the kinetics of crystallization for the purpose of product quality control.13 Many authors who have studied the thermal behavior of milk fat by differential scanning calorimetry showed that the melting and crystallization behaviors in several steps correspond to a separate group of TAG molecules.14
Many scientists working in this area have reported the use of mechanical shaking for the production of a pleasant flavor of butter by using different lipases. The use of mechanical shaking is time consuming since it needs at least four hours15–18 for production of the desired flavor. The use of enzymes in organic media with the application of ultrasound irradiation, either alone or in combination with other thermal processing, is gaining popularity due to its efficiency, with a variety of enzymes used in industry.19,20 In addition, the system is also effective in the enzymatic heterogeneous mixtures of immiscible substrates and catalysts by using ultrasound irradiation to accelerate their activities.21 In the dairy industry, ultrasonic processing is a new domain which, in addition to other processing technologies, is expected to add value to dairy products by aiming to modify the functionality of milk components.22 Ultrasonic irradiation has a greater impact on the enzymatic activities23 by taking into account that the energy input must not be excessive, so as to protect enzyme functionality.19
Emerging new technologies can help to reduce the time constraint by producing the flavor in a short time. One such technology is the use of ultrasonic microwave assisted extraction (UMAE) under low irradiation with the aim of boosting the enzyme activity to produce the desired flavor in a short time. The use of UMAE under low continuous irradiation is promising due to the fact that lipases are biologically active, and higher irradiation may lead to their destruction. The low continuous irradiation maintains a temperature that allows the lipase to work efficiently. UMAE has been used in different applications for the extraction of bioactive compounds, with either the presence or absence of an enzyme. However, for the production of milk fat, flavor enrichment with short-chain fatty acids to our knowledge has not yet been explored. In the present study anhydrous milk fat (AMF) and anhydrous buffalo milk fat (ABF) were treated with Lipozyme-435, Novozyme-435 and Thermomyces lanuginosus (T. lanuginosus) under low UMAE irradiation to hydrolyze the short chain fatty acids to increase the buttery flavor. The aim of the study was to investigate the TAG changes and the melting profiles of the hydrolyzed products.
Materials and methods
Materials
Fresh unsalted buffalo butter (83.48% fat, solids not fat 2.91%, moisture 13.61% and peroxide value 0.145 meq. kg−1) was collected from the Department of Dairy Science, Faculty of Agriculture, Suez Canal University (Ismailia, Egypt). AMF with a moisture content of 0.14% was a gift from Shanghai Kerry Oils & Grains Industrial Co., Ltd. Immobilized lipases (Lipozyme-435 (≥20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1), obtained from Rhizomucor miehei, and Novozyme-435 (≥5000 U g−1), obtained from Candida antarctica), as well as free lipase (≥100
000 U g−1), obtained from Rhizomucor miehei, and Novozyme-435 (≥5000 U g−1), obtained from Candida antarctica), as well as free lipase (≥100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1), obtained from T. lanuginosus, were gifts from Novozyme (Shandong) Innovation & Business Center, China. The OPO (1,3-dioleoyl-2-palmitoylglycerol) and OOO (tri-olein) (≥95%) TAG standards were obtained from Sigma-Aldrich Chemical Co. Ltd. (St. Louis, MO, USA). n-Hexane, acetonitrile and iso-propanol were of HPLC grade. All other solvents were of analytical or HPLC grade. Phosphate salts were of analytical grade.
000 U g−1), obtained from T. lanuginosus, were gifts from Novozyme (Shandong) Innovation & Business Center, China. The OPO (1,3-dioleoyl-2-palmitoylglycerol) and OOO (tri-olein) (≥95%) TAG standards were obtained from Sigma-Aldrich Chemical Co. Ltd. (St. Louis, MO, USA). n-Hexane, acetonitrile and iso-propanol were of HPLC grade. All other solvents were of analytical or HPLC grade. Phosphate salts were of analytical grade.
Preparation of anhydrous buffalo milk fat
The ABF was prepared as described by Omar et al.16 The ABF obtained was stored in a freezer under nitrogen at −20 °C before further experimental use.Performance of milk fat hydrolysis reactions
The hydrolyses of both AMF and ABF were conducted at 50 °C ± 2 °C in a 125 mL jacketed flask. In each experimental set-up, 10 grams of ABF or AMF was used. The enzyme to substrate ratio was set at 5%. Throughout the study, 0.2 M phosphate buffer at pH 7.5 was used. The phosphate buffer with ABF or AMF was kept at a constant ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Before lipase was added to the reaction vessel, AMF or ABF was first warmed for 15 minutes, and then the appropriate amount of lipase was added. The contents of the reaction vessel were subjected to a low continuous microwave emission for 45 minutes at a constant ultrasonic irradiation of 50 W, with a temperature control mode set at (50 °C ± 2 °C) by using CW-2000 UMAE. The temperature control mode at this set temperature allowed a low ultrasonic shock to be introduced periodically in the reaction vessel. The low ultrasonic shock helps to maintain the enzyme stability and reduces the possibility of enzyme destruction. The hydrolyzed product (Lipozyme-435 treated AMF or ABF, Novozyme-435 treated AMF or ABF, T. lanuginosus treated AMF or ABF) was separated from the lipase by using Whatman filter paper no. 1 for the two immobilized lipases after every 45 minutes of hydrolysis. For T. lanuginosus, 10 minutes were used to inactivate the lipase at 85 °C after every 45 minutes of hydrolysis. The hydrolyzed products were immediately centrifuged at 2147 × g for 10 minutes. The top layer was separated and stored at −20 °C until further analysis. The immobilized lipase was washed several times with hexane and then dried at room temperature (25 °C ± 2 °C) before its next use. Each experiment was done in triplicate.
2). Before lipase was added to the reaction vessel, AMF or ABF was first warmed for 15 minutes, and then the appropriate amount of lipase was added. The contents of the reaction vessel were subjected to a low continuous microwave emission for 45 minutes at a constant ultrasonic irradiation of 50 W, with a temperature control mode set at (50 °C ± 2 °C) by using CW-2000 UMAE. The temperature control mode at this set temperature allowed a low ultrasonic shock to be introduced periodically in the reaction vessel. The low ultrasonic shock helps to maintain the enzyme stability and reduces the possibility of enzyme destruction. The hydrolyzed product (Lipozyme-435 treated AMF or ABF, Novozyme-435 treated AMF or ABF, T. lanuginosus treated AMF or ABF) was separated from the lipase by using Whatman filter paper no. 1 for the two immobilized lipases after every 45 minutes of hydrolysis. For T. lanuginosus, 10 minutes were used to inactivate the lipase at 85 °C after every 45 minutes of hydrolysis. The hydrolyzed products were immediately centrifuged at 2147 × g for 10 minutes. The top layer was separated and stored at −20 °C until further analysis. The immobilized lipase was washed several times with hexane and then dried at room temperature (25 °C ± 2 °C) before its next use. Each experiment was done in triplicate.
Fatty acid analysis
Fatty acids from AMF or ABF were converted to free fatty acids released as fatty acid methyl esters (FAMEs). 50 mg of AMF or ABF was dissolved in 2 mL of n-hexane, thereafter 0.5 mL 2 N KOH in methanol solution was added. The mixture was vigorously shaken and vortexed for 2 minutes. The excess water was removed by the addition of sodium thiosulfate to the mixture before centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes. A supernatant of AMF or ABF was passed through a 0.45 μm membrane filter before a 0.5 μL sample was injected into a gas chromatograph (Shimadzu GC-2010, Tokyo Japan) equipped with an FID detector, and a capillary column, PEG-20M 30M i.d. 0.32 mm × 1.0 μm. The initial column temperature was held at 100 °C for 3 minutes and ramped up to 180 °C for 8 minutes at a rate of 10 °C min−1, then maintained at this temperature for 1 minute. The second ramp was programmed to reach 240 °C in 20 minutes at a rate of 3 °C min−1 and maintained at this temperature for 9 minutes. The injector and detector temperatures were both set at 250 °C. The pressure of the carrier gas (N2) was maintained at 110.0 kPa at a rate of 3 mL min−1 and H2 gas was maintained at a flow rate of 47 mL min−1. The air flow rate was set at 400 mL min−1 and the split ratio was set at 1
000 rpm for 10 minutes. A supernatant of AMF or ABF was passed through a 0.45 μm membrane filter before a 0.5 μL sample was injected into a gas chromatograph (Shimadzu GC-2010, Tokyo Japan) equipped with an FID detector, and a capillary column, PEG-20M 30M i.d. 0.32 mm × 1.0 μm. The initial column temperature was held at 100 °C for 3 minutes and ramped up to 180 °C for 8 minutes at a rate of 10 °C min−1, then maintained at this temperature for 1 minute. The second ramp was programmed to reach 240 °C in 20 minutes at a rate of 3 °C min−1 and maintained at this temperature for 9 minutes. The injector and detector temperatures were both set at 250 °C. The pressure of the carrier gas (N2) was maintained at 110.0 kPa at a rate of 3 mL min−1 and H2 gas was maintained at a flow rate of 47 mL min−1. The air flow rate was set at 400 mL min−1 and the split ratio was set at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12. The fatty acids from AMF or ABF were identified by a comparison of the retention times to the 40 fatty acid standards.
12. The fatty acids from AMF or ABF were identified by a comparison of the retention times to the 40 fatty acid standards.
Ultra pressure liquid chromatography analysis
The TAGs in hydrolyzed milk fat from AMF or ABF were analyzed by a Waters Acquity Ultra Pressure Liquid Chromatography (UPLC) system coupled to a Waters Qu-ToF Premier mass spectrometer using an ACQUITY UPLC BEH C18 analytical column (i.d. 2.1 × 50 mm, 1.9 μm). The mobile phase was added with 10 mM ammonium acetate which served as an electrolyte. The TAG separation was carried out by using two mobile phases, (A) acetonitrile/isopropyl alcohol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) and (B) 40% acetonitrile, at 300 μL min−1. Initially, 70% of A was used and maintained for 1 min, then increased to 87% for 30 min before being held for 1 min, and then was again returned to the initial 70% for 1 min before reaching equilibrium for 4 min. The column temperature was kept at 45 °C and the sample chamber was maintained at 20 °C. 1.0 μL was set as the dead volume and each sample was analyzed in triplicate.
9, v/v) and (B) 40% acetonitrile, at 300 μL min−1. Initially, 70% of A was used and maintained for 1 min, then increased to 87% for 30 min before being held for 1 min, and then was again returned to the initial 70% for 1 min before reaching equilibrium for 4 min. The column temperature was kept at 45 °C and the sample chamber was maintained at 20 °C. 1.0 μL was set as the dead volume and each sample was analyzed in triplicate.
Mass spectrometry conditions
The positive ion mode with a mass range of 200 to 1500 m/z for 1 s duration was used on a Waters Qu-ToF Premier mass spectrometer. High-purity nitrogen was used as a nebulizer and drying gas was set at a constant flow rate of 1.5 mL min−1. The source parameters were fixed as follows: 400 °C (desolvation temperature), 100 °C (source temperature), 3.5 V (capillary voltage) and 20 V (sampling cone voltage). The collision energy was set at 35 V for MS/MS analysis. The sodium format was used previously to calibrate the instrument and the lock mass spray for precise mass determination was set by leucine enkephalin in the positive ion mode. All the data were acquired and processed by Mass Lynx V4.1 software for the identification of different TAGs contained in hydrolyzed AMF or ABF.Melting profiles conditions
Differential scanning calorimetry (DSC Q2000 V4.7A Build 121, TA Instruments, New Castle, DE, USA) was used to analyze the melting and crystallization profiles of AMF, ABF and the hydrolyzed products. During the analysis the system was purged with nitrogen gas at 20 mL min−1, and at the same time nitrogen was used to serve as a refrigerant for cooling the system. The calibration was carried out by using indium, eicosane, and dodecane standards. 5–8 mg of sample was hermetically sealed in an aluminum pan, then heated to 80 °C, and held for 5 min to destroy completely the previous crystal structure, before the samples were allowed to cool at −40 °C and maintained for 5 minutes. This allowed the melting profiles to be obtained by heating the samples to 80 °C at a rate of 10 °C min−1. The melting and crystallization profiles were recorded from −40 °C to 80 °C. An empty aluminum pan was used as a reference and each sample was analyzed in duplicate.Solid fat content analysis
The solid fat content (SFC) analyses of AMF, ABF and the hydrolyzed products were performed by using AM4000MQC (Oxford, Oxfordshire, UK) low-resolution nuclear magnetic resonance (NMR). Approximately 2.5 mL of melted sample was introduced in NMR tubes then kept at 80 °C for 30 min, and then tempered at 0 °C for 90 min, and finally 30 minutes at each 5 °C interval. The SFC was determined in the 0–40 °C temperature range. All samples were analyzed in triplicate.Statistical analysis
SPSS statistical software (v. 19.0, IBM SPSS, Chicago, IL, USA) was used during data analysis. All analyses were carried out in triplicate, and were reported as means ± standard deviations. The box-plots were treated using Origin 8.5 software (Origin Lab, Northampton, MA, USA). One way analysis of variance (ANOVA) was used to establish the presence or absence of significant differences (p < 0.05) between Lipozyme-435, Novozyme-435 and T. lanuginosus treated AMF or ABF in terms of the TAGs obtained after 45 minutes of lipolysis with UMAE. Duncan multiple range tests were used (p < 0.05).Results and discussion
Fatty acid composition
The fatty acid compositions of both AMF and ABF were determined and their compositions are shown in Table 1. In general all fatty acids identified were found in both milk fats although in different percentages. There were no significant differences observed between the two milk fats for total short-chain, saturated long-chain and un-saturated fatty acids. However, for total medium-chain fatty acids, AMF was found to contain a higher percentage compared to ABF (Table 1).| Name | % AMF | % ABF |
|---|---|---|
| Butanoic acid | 1.96 | 2.29 |
| Caproic acid | 1.58 | 1.15 |
| Caprylic acid | 1.04 | 0.58 |
| Capric acid | 2.67 | 1.27 |
| Lauric acid | 5.04 | 1.82 |
| Myristic acid | 13.31 | 11.10 |
| Pentadecanoic acid | 1.07 | 1.46 |
| Palmitic acid | 34.74 | 39.05 |
| Palmitoleic acid | 1.69 | 2.76 |
| Margaric acid | 0.40 | 0.79 |
| Stearic acid | 10.42 | 10.55 |
| Oleic acid | 22.43 | 24.75 |
| Linoleic acid | 1.21 | 0.97 |
| Linolenic acid | 0.51 | 0.18 |
| Gamma-linolenic acid | 0.61 | 0.19 |
| Stearidonic acid | 1.00 | 0.64 |
| Arachidic acid | 0.13 | 0.23 |
| Gadoleic acid | 0.19 | 0.26 |
| Total short chain fatty acids | 4.58 | 4.02 |
| Total medium chain fatty acids | 7.71 | 3.09 |
| Total saturated long chain fatty acids | 60.07 | 63.18 |
| Total un-saturated fatty acids | 27.64 | 29.75 |
Triacylglycerol identification and distribution
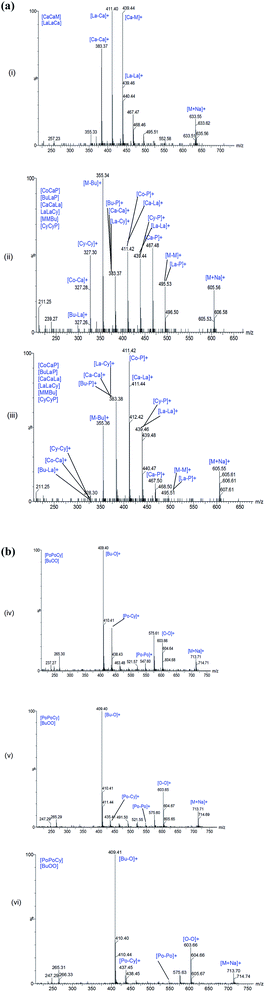
In this study three different types of lipase were used on two types of milk fat to assess their impact on re-distribution of TAGs under low irradiation ultrasonic microwave assisted extraction. The lipases used were Lipozyme-435, Novozyme-435 and T. lanuginosus, on anhydrous cow or buffalo milk fat. Different exposure time intervals of low irradiation ultrasonic microwave assisted extraction were tested, however, 45 minutes was found to be the best in our experiments for the targeted products. Therefore, in this paper 45 minutes under low irradiation ultrasonic microwave assisted extraction is discussed in detail.Fig. 1a(i–iii) shows the identification of some mass spectra of Lipozyme-435, Novozyme-435 and T. lanuginosus treated AMF. Fig. 1a(i–iii) shows mass spectra with multiple identified TAGs and higher percentage TAGs found in Lipozyme-435, Novozyme-435 and T. lanuginosus treated AMF. The identification of a TAG was carried out by using the parent ion along with the loss of daughter ions presented on the mass spectrum. For instance, the identification of m/z 605 with multiple TAGs was carried out as follows: the daughter ions for CoCaP were [Co–Ca]+, [Ca–P]+ and [Co–P]+ and they formed due to the neutral loss of caproic, capric and palmitic acids along with ammonia; the daughter ions for BuLaP were [Bu–La]+, [Bu–P]+ and [La–P]+ which formed due to the neutral loss of butanoic, lauric and palmitic acids along with ammonia; the daughter ions for CaCaLa were [Ca–Ca]+ and [Ca–La]+ which formed due to the neutral loss of capric and lauric acids along with ammonia; the daughter ions for LaLaCy were [La–La]+ and [La–Cy]+ which formed due to the neutral loss of caprylic and lauric acids along with ammonia; the daughter ions for MMBu were [M–Bu]+ and [M–M]+ which formed due to the neutral loss of butanoic and myristic acids along with ammonia; the daughter ions for CyCyP were [Cy–Cy]+ and [Cy–P]+ which formed due to the neutral loss of caprylic and palmitic acids along with ammonia.
Fig. 1b(iv–vi) shows the highest percentage (18.89 ± 0.80, 21.35 ± 0.97 and 21.64 ± 0.59) TAGs that were identified in Lipozyme-435, Novozyme-435 and T. lanuginosus treated ABF, respectively. The TAGs identified were PoPoCy/BuOO in all three samples. The daughter ions of PoPoCy were [Po–Po]+ and [Po–Cy]+ which formed due to the neutral loss of caprylic and palmitoleic acids along with ammonia; while the daughter ions of BuOO were [Bu–O]+ and [O–O]+ which formed due to the neutral loss of butanoic and oleic acids along with ammonia.
Table 2 shows the identified TAGs and their re-distribution when AMF was treated with Lipozyme-435, Novozyme-435 and T. lanuginosus. In general the TAGs with two short-chain fatty acids and one other fatty acid were found in low percentages compared to AMF (data not shown). A high percentage (15.61 ± 0.89) of a TAG (CaCaO) with two medium-chain fatty acids and other long chain fatty acids was identified when Lipozyme-435 was added to AMF. This shows that Lipozyme-435 was regio-specific when hydrolyzing the AMF and preferred more short-chain fatty acids. As a consequence the TAG which contained medium and long-chain fatty acids was increased in percentage. However, for Novozyme-435 and T. lanuginosus treated AMF, the same TAG was not found and instead the TAG (BuOO/PoPoCy) with two long-chain fatty acids and one short-chain fatty acid was identified. No significant difference was observed (p < 0.05) on the percentage of BuOO/PoPoCy produced in Novozyme-435 and T. lanuginosus treated AMF. Novozyme-435 treated AMF was found to contain higher percentages of TAGs with long-chain fatty acids, followed by Lipozyme-435 treated AMF (Table 2). This showed that Novozyme-435 was selective for TAGs with short-chain fatty acids and less selective for TAGs which contained long-chain fatty acids in AMF. The TAGs which contained long-chain fatty acids increased in percentage as a consequence of a decreasing amount of TAGs with short-chain or medium-chain fatty acids during lipolysis. The results suggest that the degree of hydrolysis was dependent on the number of carbon atoms of the TAGs, with hydrolysis preferentially affecting TAGs containing esterified short-chain fatty acids, hence there was an increase in the relative percentage of TAGs with long-chain fatty acids.24
| Rt | [M + Na]+ | TAG identified | TAG ND, 1= Lipozyme-435, 2= Novozyme-435, 3 = T. lanuginosus | AMF treated with lipase (% TAG obtained) | ||
|---|---|---|---|---|---|---|
| Lipozyme-435 | Novozyme-435 | T. lanuginosus | ||||
| a Note: Rt (retention time), TAG (triacylglycerol), ND (not detected), Bu (butanoic acid), Co (caproic acid), Cy (caprylic acid), Pg (pelargonic acid), Ca (capric acid), La (lauric acid), M (myristic acid), P (palmitic acid), Po (palmitoleic acid), S (stearic acid), O (oleic acid), L (linoleic acid), Ln (linolenic acid). | ||||||
| 2.06 | 605 | CoCaP/BuLaP/CaCaLa/LaLaCy/MMBu/CyCyP | NDa | 2.33 ± 0.26 | 3.43 ± 0.22 | |
| 2.78 | 629 | CyCyL | 3.26 ± 0.17 | NDa | NDa | |
| 3.65 | 631 | BuLaO/CyCyO | 6.31 ± 0.17 | 4.90 ± 0.13a | 6.55 ± 0.78 | |
| 4.78 | 633 | CaCaM/LaLaCa/CoLaP/CyCyS/MMCo | 1:CoLaP/CyCyS/MMCo, 2:CyCyS, 3:BuMP | 9.86 ± 0.44a | 11.48 ± 0.40 | 11.09 ± 0.18 |
| 5.41 | 661 | BuPP/CoMP/CaCaP/MMCy/LaLaLa | 1:LaLaCa | 0.27 ± 0.07a | 0.66 ± 0.09 | 0.46 ± 0.06 |
| 6.21 | 687 | CaCaO | 15.61 ± 0.89 | NDa | NDa | |
| 6.28 | 713 | BuOO/PoPoCy | NDa | 19.12 ± 0.96 | 18.26 ± 0.90 | |
| 6.96 | 689 | CoPP/BuPS/CaCaS/LaLaM/MMCa | 1:BuPS | 0.38 ± 0.07 | 0.33 ± 0.07 | 0.19 ± 0.05a |
| 7.56 | 715 | CoPO/LaLaPo | 8.38 ± 0.56 | 5.41 ± 0.23a | 8.52 ± 0.36 | |
| 7.83 | 717 | LaLaP/MMLa/PPCy/CoSP | 1,2:CoSP | 5.02 ± 0.46a | 5.67 ± 0.21 | 4.51 ± 0.23a |
| 8.41 | 731 | PPPg | 0.20 ± 0.03a | 0.32 ± 0.07 | 0.15 ± 0.04a | |
| 9.14 | 743 | PCyO/LaLaO | 7.24 ± 0.35 | 3.19 ± 0.33a | 7.62 ± 0.32 | |
| 9.35 | 769 | OOCy/PoPoLa | NDa | 2.25 ± 0.14 | NDa | |
| 10.05 | 745 | PPCa/LaLaS/MMM | 0.24 ± 0.04a | 0.66 ± 0.11 | 0.14 ± 0.03a | |
| 11.12 | 797 | MML/OOCa/PoPoM | 1:POPoM | 7.60 ± 0.38 | 4.59 ± 0.15a | 6.90 ± 0.24 |
| 12.13 | 823 | PoPoPo | 0.24 ± 0.04 | NDa | NDa | |
| 13.31 | 825 | PML/PoPoP/OOLa | 8.81 ± 0.50 | 6.35 ± 0.29a | 8.62 ± 0.31 | |
| 14.42 | 851 | PoPoO/PPLn | NDa | 0.37 ± 0.06 | NDa | |
| 15.56 | 853 | OOM/PPL | 10.00 ± 0.52 | 8.69 ± 0.39a | 9.40 ± 0.52a | |
| 16.20 | 879 | OOPo/OPL | NDa | 0.42 ± 0.06 | NDa | |
| 16.67 | 855 | OPP | NDa | 0.74 ± 0.10 | NDa | |
| 17.87 | 907 | OOO | 8.63 ± 0.39a | 13.17 ± 0.42 | 8.69 ± 0.42a | |
| 20.10 | 909 | OOS/SSL | 4.46 ± 0.32 | 6.58 ± 0.27 | 3.61 ± 0.38a | |
| 22.13 | 911 | SSO | 0.63 ± 0.13a | 1.21 ± 0.24 | 0.44 ± 0.09a | |
Table 3 shows different types of TAG identified when three different lipases were applied to ABF. Lipozyme-435 and T. lanuginosus treated ABF were found to contain very low percentages of TAGs with at least two short-chain fatty acids compared with Novozyme-435 treated ABF. High percentages were found for TAGs which contained at least two medium-chain fatty acids for all three lipases mixed with ABF. However, the percentage differed from one lipase treated ABF to another. For instance, for m/z 633 the highest percentage (13.02 ± 0.59) was found for Novozyme-435 treated ABF, while for Lipozyme-435 and T. lanuginosus treated ABF, no significant difference was observed (p < 0.05). But for m/z 713 the highest percentage was produced in Novozyme-435 and T. lanuginosus treated ABF compared to Lipozyme-435 treated ABF. This showed that these lipases were specific for hydrolyzing buffalo milk fat with specific fatty acids. Furthermore, Table 3 shows that there is a close relationship between Lipozyme-435 and T. lanuginosus for hydrolyzing buffalo milk fat compared with Novozyme-435. The highest percentages of OOO and OOS/SSL were found in Lipozyme-435 treated ABF and then followed by T. lanuginosus treated ABF. The increase in TAGs with long-chain fatty acids was a consequence of decreasing TAGs with at least two short-chain fatty acids.24
| Rt | [M + Na]+ | TAG identified | TAG ND, 1= Lipozyme-435, 2= Novozyme-435, 3 = T. lanuginosus | ABF treated with lipase (% TAG obtained) | ||
|---|---|---|---|---|---|---|
| Lipozyme-435 | Novozyme-435 | T. lanuginosus | ||||
| a Note: Rt (retention time), TAG (triacylglycerol), ND (not detected), Bu (butanoic acid), Co (caproic acid), Cy (caprylic acid), Pg (pelargonic acid), Ca (capric acid), La (lauric acid), M (myristic acid), P (palmitic acid), Po (palmitoleic acid), S (stearic acid), O (oleic acid), L (linoleic acid), G (gadoleic acid). | ||||||
| 2.78 | 605 | CoCaP/BuLaP/CaCaLa/LaLaCy/MMBu/CyCyP | 1.87 ± 0.21 | NDa | 0.57 ± 0.10 | |
| 3.10 | 629 | CyCyL | NDa | 3.36 ± 0.24 | NDa | |
| 3.64 | 631 | BuLaO/CyCyO | NDa | 0.75 ± 0.09 | NDa | |
| 4.78 | 633 | BuMP/CaCaM/LaLaCa/MMCo | 2:BuMP | 11.98 ± 0.81a | 13.02 ± 0.59a | 11.87 ± 0.35a |
| 5.44 | 661 | BuPP/CoMP/CaCaP/MMCy/LaLaLa | 1.37 ± 0.26 | NDa | 1.55 ± 0.31 | |
| 6.24 | 713 | BuOO/PoPoCy | 18.89 ± 0.80a | 21.35 ± 0.97 | 21.64 ± 0.59 | |
| 6.93 | 689 | CoPP/BuPS/CaCaS/LaLaM/MMCa | 1,3:BuPS | 0.72 ± 0.17 | 0.28 ± 0.07a | 0.77 ± 0.16 |
| 7.56 | 715 | CoPO/LaLaPo | 7.13 ± 0.37 | 8.03 ± 0.28 | 4.77 ± 0.18a | |
| 7.78 | 717 | CoSP/LaLaP/MMLa/PPCy | 2:CoSP | 6.76 ± 0.35 | 5.62 ± 0.28a | 9.20 ± 0.60 |
| 8.43 | 731 | PPPg | 0.21 ± 0.06 | NDa | NDa | |
| 9.20 | 743 | PCyO/LaLaO | 3.26 ± 0.31 | 3.83 ± 0.37 | 1.98 ± 0.21a | |
| 9.33 | 769 | OOCy/PoPoLa | NDa | 2.40 ± 0.17 | 2.21 ± 0.13 | |
| 9.83 | 745 | PPCa/LaLaS/MMM | 0.48 ± 0.10 | NDa | NDa | |
| 11.06 | 797 | MML/OOCa/PoPoM | 4.26 ± 0.18 | 5.84 ± 0.34 | 2.20 ± 0.13a | |
| 13.27 | 825 | PML/PoPoP/OOLa | 5.39 ± 0.25 | 5.64 ± 0.31 | 4.20 ± 0.24a | |
| 15.62 | 853 | OOM/PPL | 8.45 ± 0.30 | 5.79 ± 0.39a | 8.05 ± 0.47 | |
| 16.15 | 879 | OPL | NDa | NDa | 0.21 ± 0.05 | |
| 16.25 | 879 | OOPo | 0.47 ± 0.13 | NDa | NDa | |
| 16.62 | 855 | OPP | 0.57 ± 0.13 | NDa | 0.56 ± 0.09 | |
| 17.75 | 881 | OOP | NDa | 6.74 ± 0.21 | NDa | |
| 17.92 | 907 | OOO | 11.14 ± 0.32 | NDa | 9.81 ± 0.26 | |
| 18.95 | 883 | SPO/SSPo/PPG | 0.34 ± 0.10 | NDa | NDa | |
| 20.09 | 909 | OOS/SSL | 5.59 ± 0.18 | 2.59 ± 0.28a | 4.57 ± 0.25 | |
| 22.11 | 911 | SSO | 0.66 ± 0.09 | NDa | 0.25 ± 0.04 | |
Melting and crystallization profiles
Melting and crystallization profiles of Lipozyme-435, Novozyme-435 and T. lanuginosus treated AMF or ABF were investigated and compared with those of just AMF or ABF. Fig. 2a shows the melting profiles of AMF and Lipozyme-435, Novozyme-435 and T. lanuginosus treated AMF. There was a great change, made by individual lipase, in the melting profile of hydrolyzed cow milk fat compared to AMF. The lowest temperatures were observed at around −19 °C for both Lipozyme-435 and Novozyme-435 treated AMF while for T. lanuginosus treated AMF, the temperature was observed at −17.63 °C and AMF at 8.08 °C. This clearly showed that there were some short-chain fatty acids obtained after hydrolysis since fatty acids with short chains tend to melt at low temperature due to their lower number of carbon atoms. Another interesting point to note was that at 3.60 °C for Lipozyme-435 treated AMF, 3.92 °C for Novozyme-435 treated AMF and 7.28 °C for T. lanuginosus treated AMF, these represented the middle melting points (MMP).25 The long arms for both Lipozyme-435 and Lipozyme-435 treated AMF were found at 30.26 °C which is associated with long chain fatty acids, both saturated and un-saturated, since they melt at high temperature due to their long chains and degree of un-saturation. However, for T. lanuginosus treated AMF the long arm was preceded to 35.05 °C, the temperature that was even much high compared to AMF which was found at 32.09 °C. According to Lopez & Ollivon and van Aken et al.26,27 the DSC melting curve of milk fat showed the three typical endothermic peaks which correspond to the low melting point (LMP), MMP and high melting point (HMP) fractions. The fractions may demarcate the temperature ranges during the heating of milk fat with an LMP fraction: −40 °C ≤ temperature ≤ 8 °C; MMP fraction: 8 °C < temperature ≤ 22 °C; HMP fraction: 22 °C < temperature ≤ 37.5 °C.26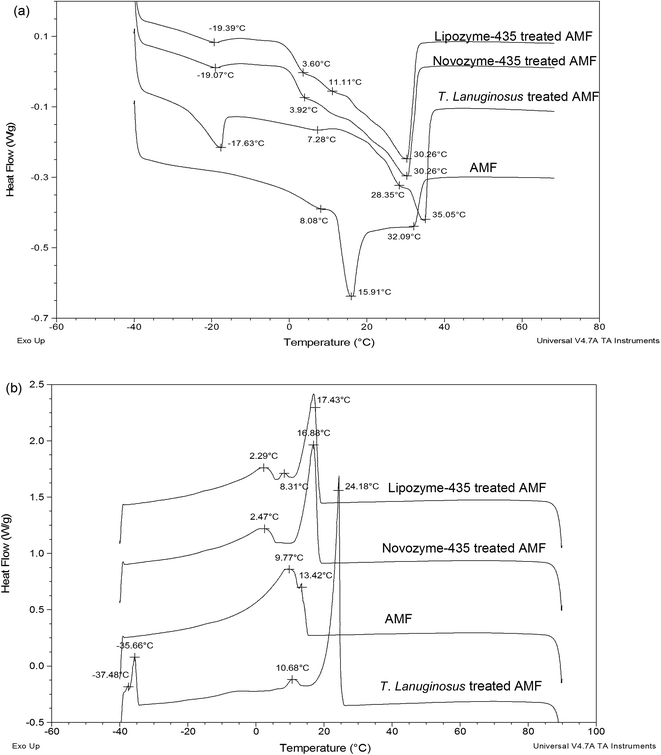 | ||
| Fig. 2 Melting and crystallization profiles of cow milk fat and when three lipases were added to cow milk fat. Note: (a) melting profiles and (b) crystallization profiles. | ||
Another great change was also observed in the crystallization profiles when Lipozyme-435, Novozyme-435 and T. lanuginosus treated AMF were compared with AMF (Fig. 2b). However, there was no significant difference observed for Lipozyme-435 and Novozyme-435 treated AMF; while T. lanuginosus treated AMF was characterized with four crystallization arms. Another point to note was that the long arm was again observed at a higher crystallization temperature for T. lanuginosus treated AMF. Those crystallization curves may relate to the existence of three types of fatty acids having high (long-chain and saturated fatty acids), middle (including medium-chain saturated and long-chain unsaturated fatty acids) and low (short-chain fatty acids) melting points.28
Fig. 3a shows melting profiles for ABF and Lipozyme-435, Novozyme-435 and T. lanuginosus treated ABF. Short arms at −18.59 °C, −16.67 °C and −14.76 °C were observed for T. lanuginosus, Novozyme-435 and Lipozyme-435 treated ABF respectively. The long arms were approximately the same for Novozyme-435 and Lipozyme-435 treated ABF while those of ABF and T. lanuginosus treated ABF were also approximately the same (Fig. 3a). The melting point of the fats decreases with decreasing chain length and increases with the degree of un-saturation of the fatty acids in the milk fat.29
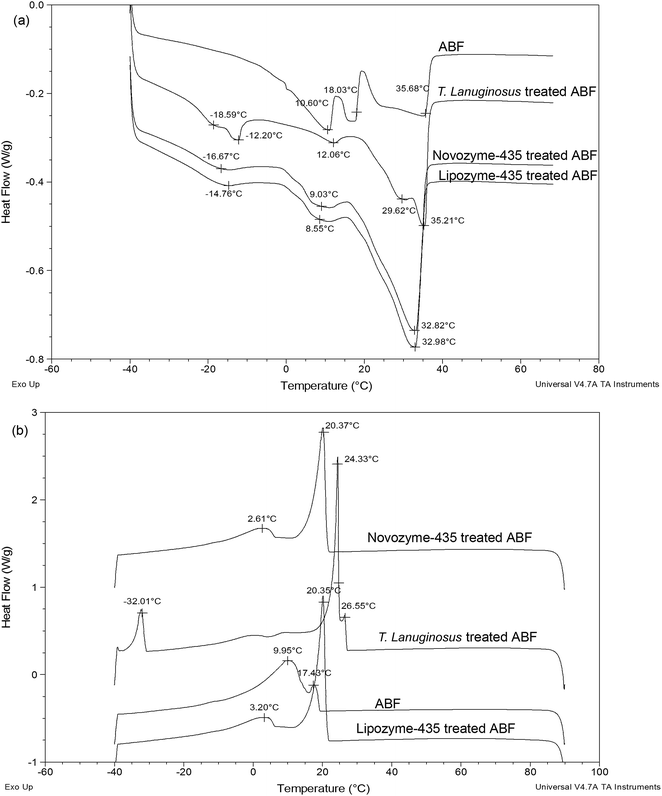 | ||
| Fig. 3 Melting and crystallization profiles of buffalo milk fat and when three lipases were added to buffalo milk fat. Note: (a) melting profiles and (b) crystallization profiles. | ||
Fig. 3b shows crystallization profiles for ABF, Lipozyme-435, Novozyme-435 and T. lanuginosus treated ABF. Clear evidence is shown for the changes of Lipozyme-435, Novozyme-435 and T. lanuginosus treated ABF when compared with ABF. No significant difference was again observed for Lipozyme-435 and Novozyme-435 treated ABF for both short and long arm crystallization curves (Fig. 3b). However, for T. lanuginosus treated ABF the long arm curve was brought to a higher temperature of around 24 °C and the short arm around 26 °C.
Solid fat content
A SFC study of AMF and Lipozyme-435, Novozyme-435 and T. lanuginosus treated AMF, and ABF and Lipozyme-435, Novozyme-435 and T. lanuginosus treated ABF was conducted to investigate their behaviors when exposed at different temperatures as shown in Fig. 4(a and b). In general, Lipozyme-435 and Novozyme-435 treated AMF were found to follow the same AMF solid fat content at almost all investigated temperature ranges. The temperature ranges of 0 °C to 10 °C and then 25 °C to 35 °C for the SFC values of Lipozyme-435 and Novozyme-435 treated AMF were almost the same as those observed for AMF. However, for T. lanuginosus treated AMF, its SFC values were lower in all tested temperature ranges compared to AMF (Fig. 4a). The SFC values of T. lanuginosus treated AMF, which were close to the SFC values of AMF, were at 20 °C and 35 °C. This showed that the product obtained after hydrolysis by this lipase under ultrasonic microwave assistance extraction was softer than the AMF.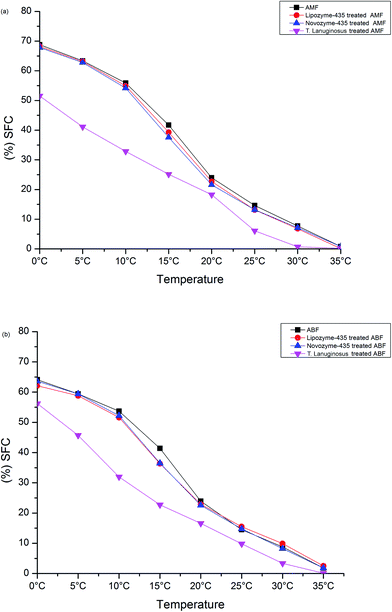 | ||
| Fig. 4 Solid fat content of cow and buffalo milk fat and when three lipases were added to cow/buffalo milk fat. | ||
Fig. 4b shows the SFC for ABF and Lipozyme-435, Novozyme-435 and T. lanuginosus treated ABF. In general, the SFC values of Lipozyme-435 and Novozyme-435 treated ABF behaved the same as observed for AMF in the different investigated temperature ranges, except in the 10 °C to 20 °C temperature range. However, the SFC values of T. lanuginosus treated AMF differed significantly in the 0 °C to 15 °C temperature range of the investigation.
Conclusions
The use of low ultrasonic microwave assisted extraction for the lipolysis of both AMF and ABF milk fat has been shown to influence the re-distribution of TAGs after 45 minutes of exposure. In general, the re-distribution has resulted in higher percentages of TAGs with at least two medium-chain fatty acids and one long-chain fatty acid, followed by a high percentage of TAGs with long-chain fatty acids. The increase in TAGs with medium-chain and long-chain fatty acids was the consequence of decreasing percentages of TAGs with at least two short-chain fatty acids. The melting and crystallization profiles were modified in both AMF and ABF treated lipases which may allow further processing in dairy and dairy applications. The results of this study give insight for both scientists and industrial technology into new ways (emerging technologies) of treating milk fat with lipases, aiming to elevate milk fat flavors by hydrolyzing TAGs with short chain fatty acids over a short time period.Conflict of interest
The authors have declared no conflict of interest.Acknowledgements
We are grateful to the National Natural Science Foundation of China for the financial support (Grant No. 131401525). We are also grateful to Novozyme (Shandong) Innovation & Business Center and Shanghai Kerry Oils & Grains Industrial Co., Ltd. for their provision of related raw materials.References
- J. Arul, A. Boudreau, J. Makhlouf, R. Tardi and T. Bellavia, J. Am. Oil Chem. Soc., 1988, 65, 1642–1646 CrossRef CAS.
- D. Büyükbeşe, E. E. Emre and A. Kaya, J. Am. Oil Chem. Soc., 2014, 91, 169–177 CrossRef.
- A. E. Fatouh, R. K. Singh, P. E. Koehler, G. A. Mahran and A. E. Metwally, Food Chem., 2005, 89, 243–252 CrossRef CAS.
- C. Lopez, C. Bourgaux, P. Lesieur, S. Bernadou, G. Keller and M. Ollivon, J. Colloid Interface Sci., 2002, 254, 64–78 CAS.
- M. S. Queirós, R. Grimaldi and M. L. Gigante, Food Res. Int., 2016, 84, 69–75 CrossRef.
- P. Ramel and A. G. Marangoni, RSC Adv., 2016, 6, 41189–41194 RSC.
- R. W. Lencki, N. Smink, H. Snelting and J. Arul, J. Am. Oil Chem. Soc., 1998, 75, 1195–1200 CrossRef CAS.
- J. Svensson and P. Adlercreutz, Eur. J. Lipid Sci. Technol., 2008, 110, 1007–1013 CrossRef CAS.
- P. Dugo, T. Kumm, M. Crupi, A. Cotroneo and L. Mondello, J. Chromatogr. A, 2006, 1112, 269–275 CrossRef CAS PubMed.
- D. Hmida, M. Abderrabba, A. Tchapla, S. Heron and F. Moussa, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2015, 990, 45–51 CrossRef CAS PubMed.
- E. Kofroňová, J. Cvačka, P. Jiroš, D. Sýkora and I. Valterová, Eur. J. Lipid Sci. Technol., 2009, 111, 519–525 CrossRef.
- Q. Zhou, B. Gao, X. Zhang, Y. Xu, H. Shi and L. L. Yu, Food Chem., 2014, 143, 199–204 CrossRef CAS PubMed.
- M. L. Herrera, M. de Leon Gatti and R. W. Hartel, Food Res. Int., 1999, 32, 289–298 CrossRef CAS.
- C. Lopez, C. Bourgaux, P. Lesieur, A. Riaublanc and M. Ollivon, Chem. Phys. Lipids, 2006, 144, 17–33 CrossRef CAS PubMed.
- I. Kurtovic, S. N. Marshall, M. R. Miller and X. Zhao, Food Chem., 2011, 127, 1562–1568 CrossRef CAS.
- K. A. Omar, M. E. Gounga, R. Liu, E. Mlyuka and X. Wang, J. Food Sci. Technol., 2016, 53, 1035–1046 CrossRef CAS PubMed.
- M. A. Regado, B. M. Cristóvao, C. G. Moutinho, V. M. Balcao, R. Aires-Barros, J. P. M. Ferreira and M. F. Xavier, Int. J. Food Sci. Technol., 2007, 42, 961–968 CrossRef CAS.
- B. Wang and S. Xu, Flavour Fragrance J., 2009, 24, 335–340 CrossRef CAS.
- B. Kwiatkowska, J. Bennett, J. Akunna, G. M. Walker and D. H. Bremner, Biotechnol. Adv., 2011, 29, 768–780 CrossRef CAS PubMed.
- C. P. O’donnell, B. K. Tiwari, P. Bourke and P. J. Cullen, Trends Food Sci. Technol., 2010, 21, 358–367 CrossRef.
- P. Adewale, M. J. Dumont and M. Ngadi, Chem. Eng. J., 2016, 284, 158–165 CrossRef CAS.
- P. Juliano, A. E. Torkamani, T. Leong, V. Kolb, P. Watkins, S. Ajlouni and T. K. Singh, Ultrason. Sonochem., 2014, 21, 2165–2175 CrossRef CAS PubMed.
- M. Bashari, A. Eibaid, J. Wang, Y. Tian, X. Xu and Z. Jin, Ultrason. Sonochem., 2013, 20, 155–161 CrossRef CAS PubMed.
- J. Fontecha, I. Mayo, G. Toledano and M. Juárez, Int. Dairy J., 2006, 16, 1498–1504 CrossRef CAS.
- C. Lopez and V. Briard-Bion, Le Lait, 2007, 87, 317–336 CrossRef CAS.
- C. Lopez and M. Ollivon, Chem. Phys. Lipids, 2009, 159, 1–12 CrossRef CAS PubMed.
- G. van Aken, E. Ten Grotenhuis, A. van Langevelde and H. Schenk, J. Am. Oil Chem. Soc., 1999, 76, 1323–1331 CrossRef CAS.
- S. I. Martínez-Monteagudo, M. Khan, F. Temelli and M. D. Saldaña, Int. Dairy J., 2014, 36, 29–37 CrossRef.
- M. A. Smiddy, T. Huppertz and S. M. van Ruth, Int. Dairy J., 2012, 24, 64–69 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |