Reduced graphene oxide modified TiO2 semiconductor materials for dye-sensitized solar cells
Abstract
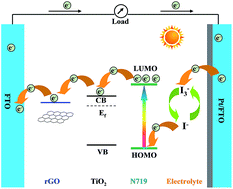
Reduced graphene oxide (rGO) was prepared by reduction of graphene oxide (GO) under mild conditions and rGO/TiO2 composite semiconductor materials were prepared by mixing rGO into TiO2 paste and were used to deposit the photoanode films of dye sensitized solar cells (DSSCs). After preparation, the successful synthesis of rGO was confirmed using X-ray diffraction (XRD) and Raman spectroscopy analysis. The effect of rGO content on the performance of dye-sensitized solar cells was also investigated. After the addition of rGO, the photoanodes displayed enhanced dye adsorption properties with lower internal resistances, faster electron transport and lower charge recombination rate, which resulted in a high current density. At the optimum rGO concentration, the DSSC exhibited a Jsc of 15.23 mA cm−2, a Voc of 0.71 V, and a FF of 0.62 with the energy conversion efficiency (η) of 6.69%, indicating a increase in Jsc and η respectively with respect to that of a DSSC based on an unmodified TiO2 photoanode, which gives a Jsc of 13.56 mA cm−2, a Voc of 0.70 V, and a FF of 0.63 with a η of 5.97%. However, the addition of excess rGO weakened the crystallization of particles on the surface of the photoelectrodes, which led to the enhancement of charge recombination, the reduction of dye adsorption and the decrease of photoelectric conversion efficiency of DSSCs. The rGO modified TiO2 semiconductor materials could really enhance the efficiency of DSSCs after the optimal amount of rGO was successfully determined.

 Please wait while we load your content...
Please wait while we load your content...